Foliar Application of Nanoclay Promotes Potato (Solanum tuberosum L.) Growth and Induces Systemic Resistance against Potato Virus Y
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Source of the Viral Isolate
2.2. Source of Nanoclay
2.3. Nanoclays Characterization
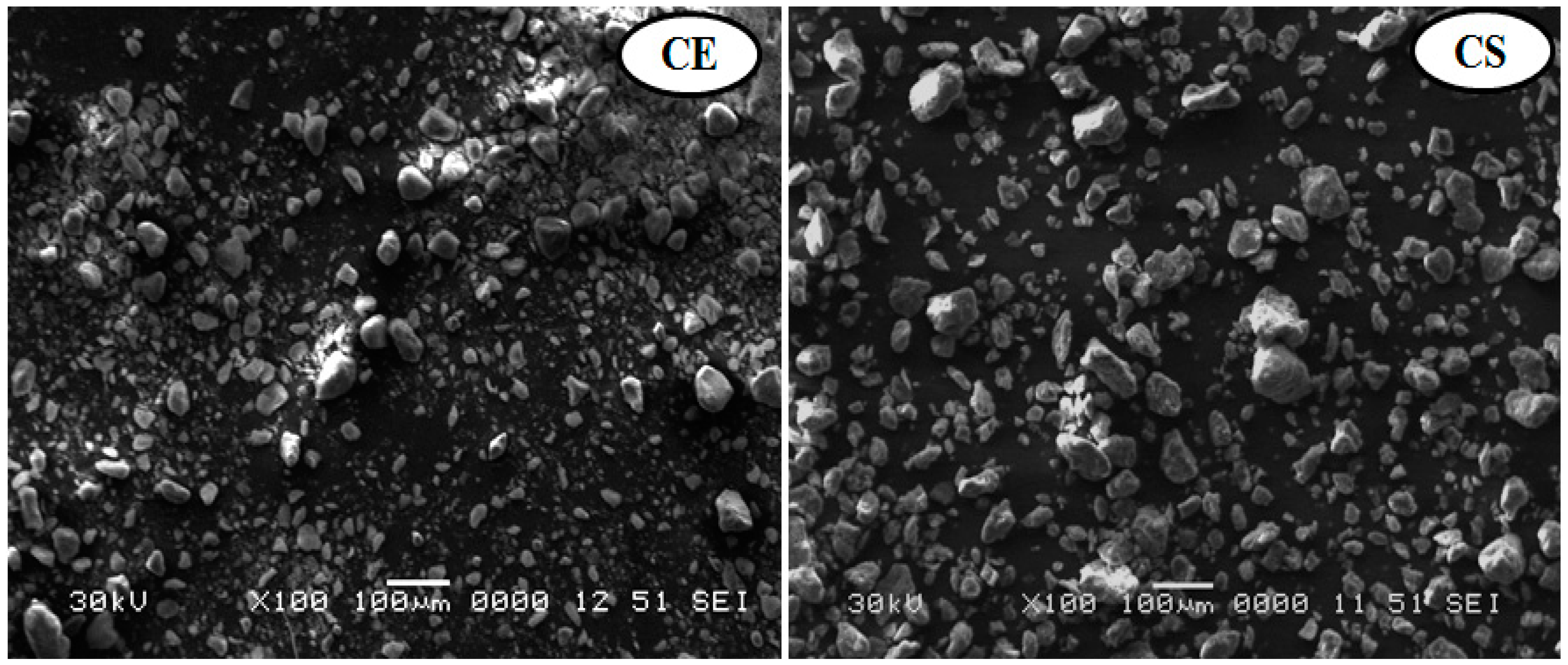
2.3.1. SEM
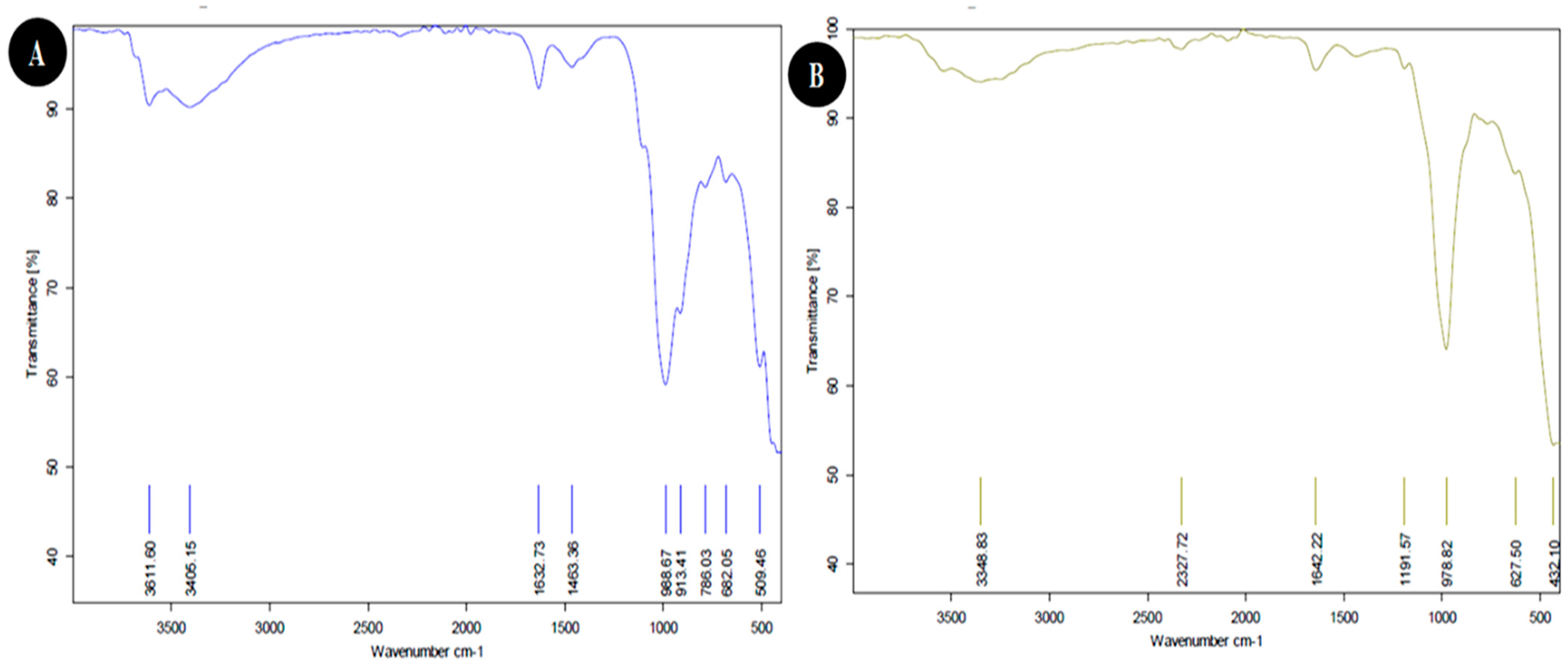
2.3.2. FTIR
2.3.3. TEM
2.3.4. EDS
2.4. Greenhouse Experimental Design
2.5. Determination of Oxidative Stress Markers
2.5.1. Malondialdehyde (MDA)
2.5.2. Hydrogen Peroxide (H2O2)
2.6. Evaluation of Antioxidant Enzymes Activity
2.6.1. Peroxidase (POX)
2.6.2. Polyphenol Oxidase (PPO)
2.7. Analysis of Defense-Related Gene Expression Levels
2.7.1. Extraction of Total RNA and cDNA Synthesis
2.7.2. Quantitative Real-Time PCR (qPCR)Assay and Data Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Nanoclay Characterization
3.1.1. Scanning Electron Microscopy Analysis
3.1.2. Transmission Electron Microscopy Analysis
3.1.3. FTIR Analysis
3.1.4. EDS Analysis
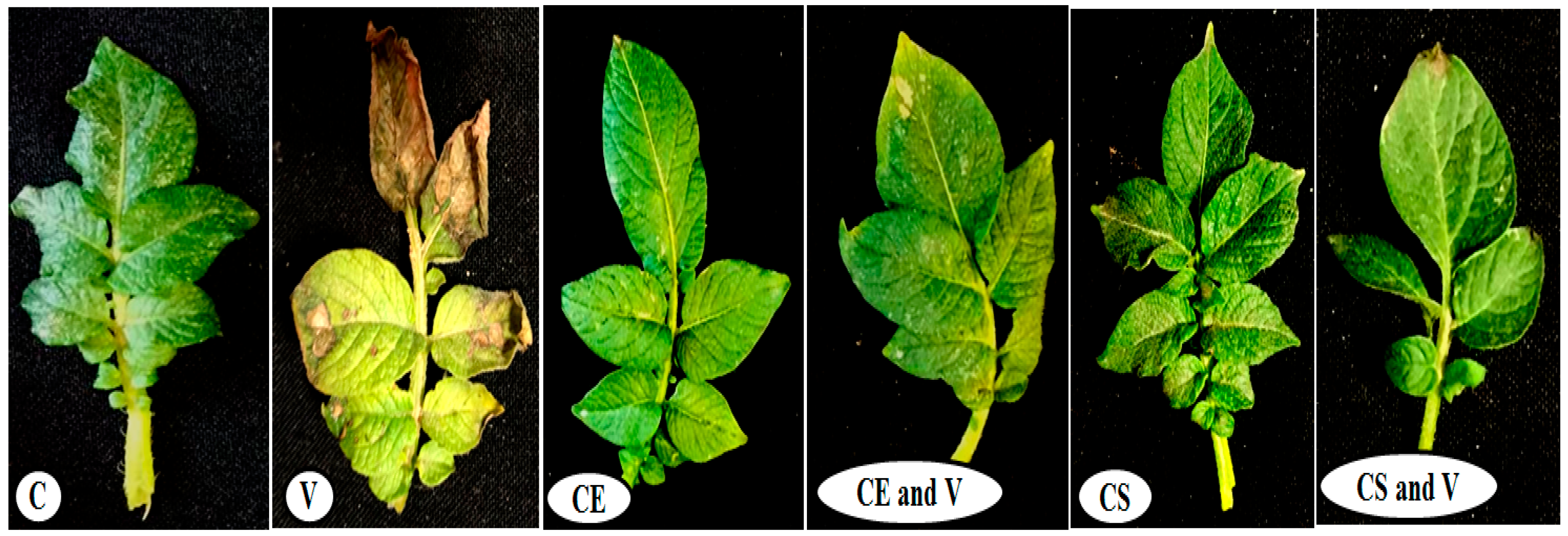
3.2. Plant Growth Evaluation
3.3. Effect of Nanoclays on Oxidative Stress Markers
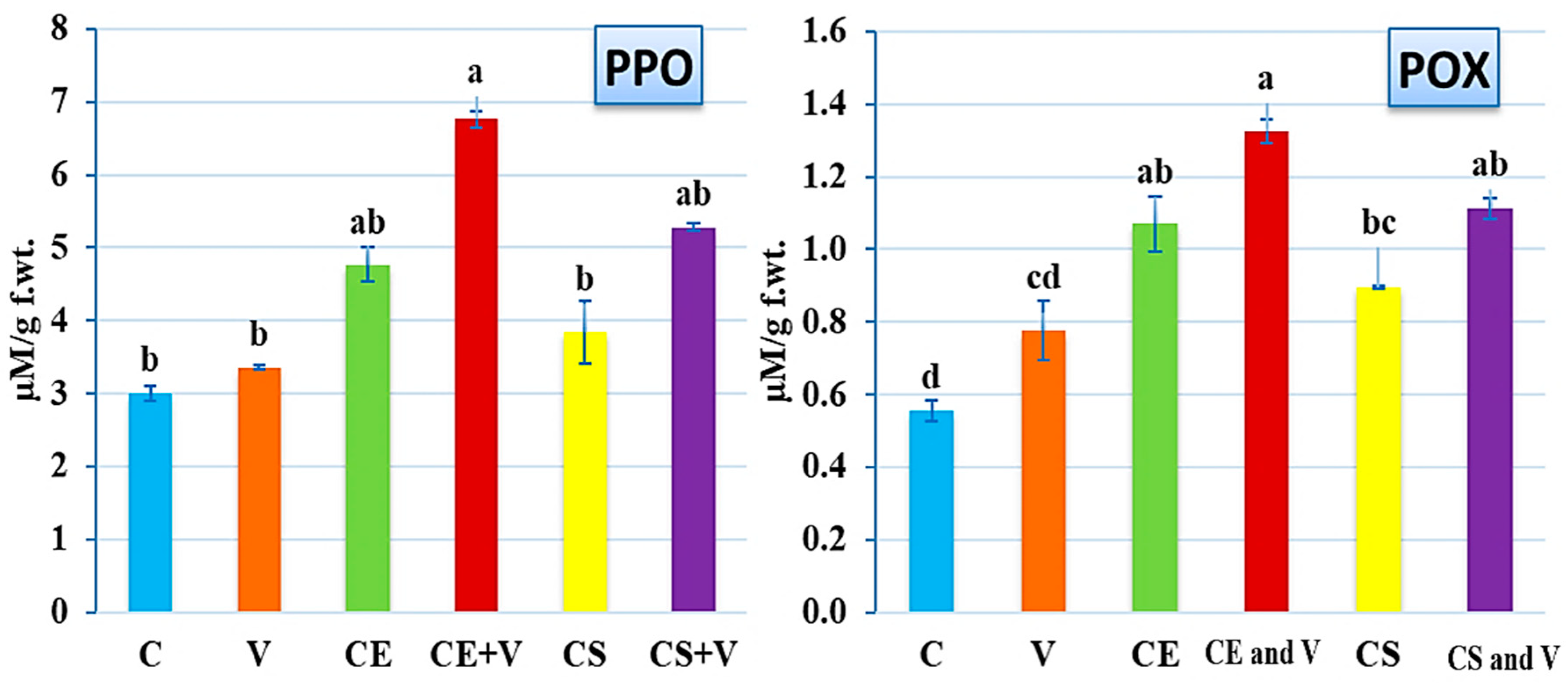
3.4. Effect of Nanoclays on Antioxidant Enzymes Activity
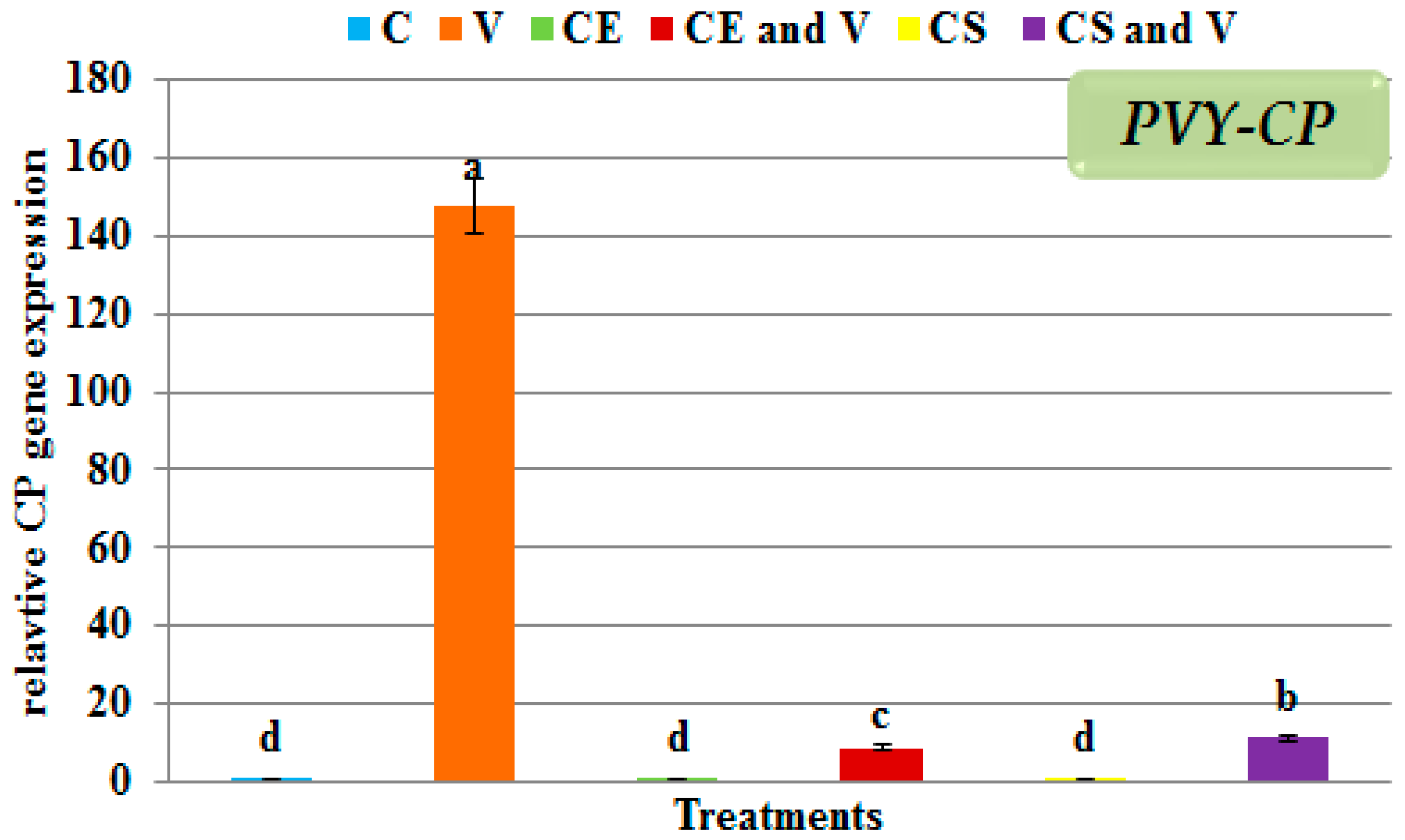
3.5. Effect of Nanoclays on the Accumulation Level of PVY-CP
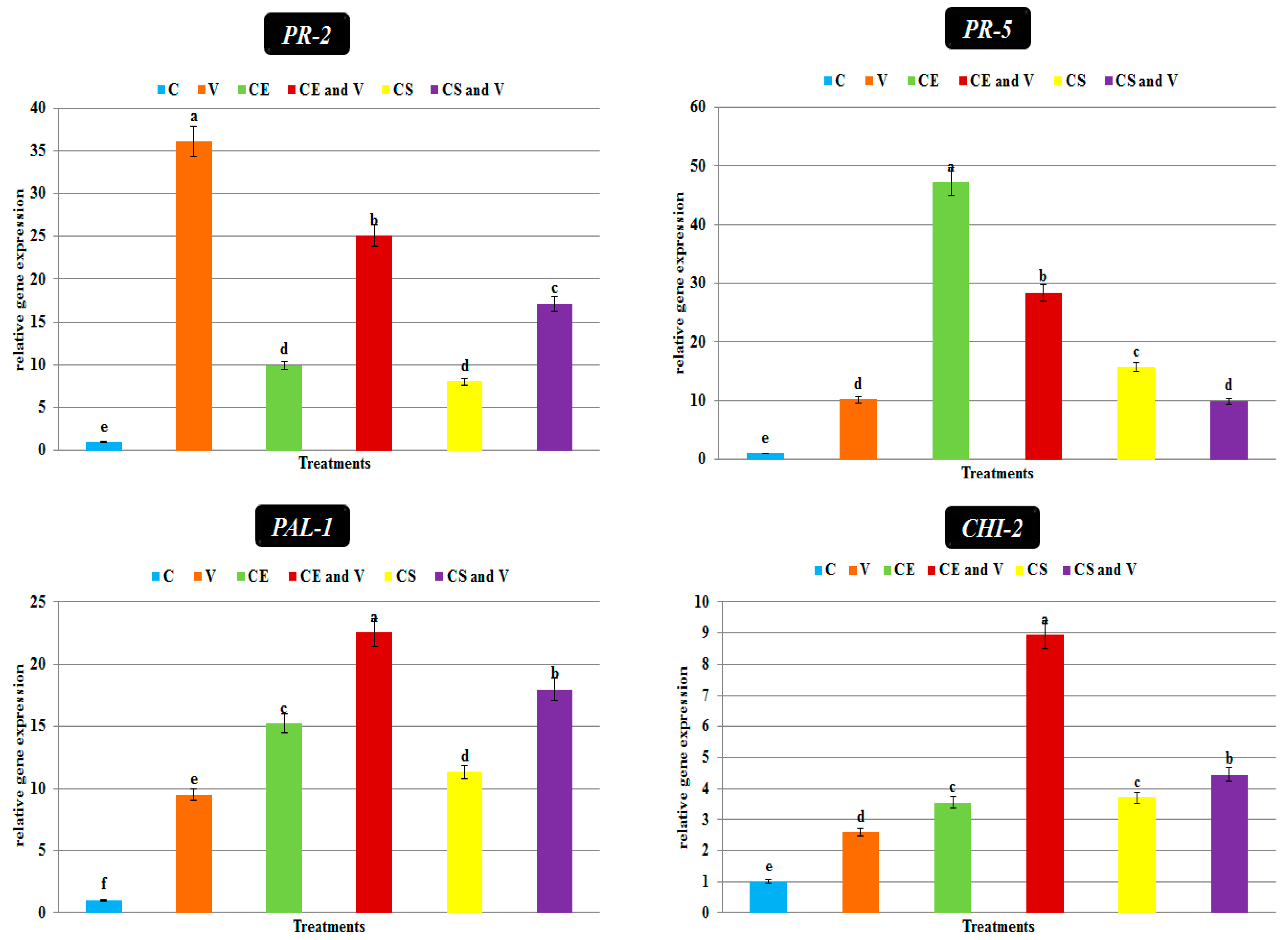
3.6. Transcriptional Expression Levels of Defense-Related Genes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrios, G.N. Plant Pathology; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 0080473784. [Google Scholar]
- Abdelkhalek, A.; Hafez, E. Plant Viral Diseases in Egypt and Their Control. In Cottage Industry of Biocontrol Agents and Their Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 403–421. [Google Scholar]
- Worrall, E.A.; Hamid, A.; Mody, K.T.; Mitter, N.; Pappu, H.R. Nanotechnology for plant disease management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 16. [Google Scholar] [CrossRef]
- Rykbost, K.A.; Hane, D.C.; Hamm, P.B.; Voss, R.; Kirby, D. Effects of seedborne potato virus Y on Russet Norkotah performance. Am. J. Potato Res. 1999, 76, 91–96. [Google Scholar] [CrossRef]
- Claude, M.; Fauquet, M. Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses; Elsevier Science & Technology: Amsterdam, The Nrtherlands, 2004; ISBN 1281763470. [Google Scholar]
- Visser, J.C.; Bellstedt, D.U.; Pirie, M.D. The recent recombinant evolution of a major crop pathogen, Potato virus Y. PLoS ONE 2012, 7, e50631. [Google Scholar] [CrossRef]
- Eraky, M.A.; Rashed, S.M.; Nasr, M.E.-S.; El-Hamshary, A.M.S.; Salah El-Ghannam, A. Parasitic contamination of commonly consumed fresh leafy vegetables in Benha, Egypt. J. Parasitol. Res. 2014, 2014, 122. [Google Scholar] [CrossRef]
- Legg, J.P.; Shirima, R.; Tajebe, L.S.; Guastella, D.; Boniface, S.; Jeremiah, S.; Nsami, E.; Chikoti, P.; Rapisarda, C. Biology and management of Bemisia whitefly vectors of cassava virus pandemics in Africa. Pest Manag. Sci. 2014, 70, 1446–1453. [Google Scholar] [CrossRef]
- Loebenstein, G.; Katis, N. Control of Plant Virus Diseases: Seed-Propagated Crops; Academic Press: Piscataway, NJ, USA, 2014; ISBN 0128012641. [Google Scholar]
- Abd El-Rahim, W.M.; Moawad, H.; Hashem, M.M.; Gebreil, G.M.M.; Zakaria, M. Highly efficient fungal pectinase and laccase producers among isolates from flax retting liquor. Biocatal. Agric. Biotechnol. 2020, 25, 101570. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.-C.; Yeam, I.; Jahn, M.M. Genetics of plant virus resistance. Annu. Rev. Phytopathol. 2005, 43, 581–621. [Google Scholar] [CrossRef]
- Ma, G.; Chen, P.; Buss, G.R.; Tolin, S.A. Genetics of resistance to two strains of Soybean mosaic virus in differential soybean genotypes. J. Hered. 2004, 95, 322–326. [Google Scholar] [CrossRef] [Green Version]
- Ichiki, T.U.; Nagaoka, E.N.; Hagiwara, K.; Uchikawa, K.; Tsuda, S.; Omura, T. Integration of mutations responsible for the attenuated phenotype of Pepper mild mottle virus strains results in a symptomless cross-protecting strain. Arch. Virol. 2005, 150, 2009–2020. [Google Scholar] [CrossRef]
- Parizad, S.; Bera, S. The effect of organic farming on water reusability, sustainable ecosystem, and food toxicity. Environ. Sci. Pollut. Res. 2021, 1–12. [Google Scholar] [CrossRef]
- Kamel, S.M.; Elgobashy, S.F.; Omara, R.I.; Derbalah, A.S.; Abdelfatah, M.; El-Shaer, A.; Al-Askar, A.A.; Abdelkhalek, A.; Abd-Elsalam, K.A.; Essa, T. Antifungal Activity of Copper Oxide Nanoparticles against Root Rot Disease in Cucumber. J. Fungi 2022, 8, 911. [Google Scholar] [CrossRef] [PubMed]
- Derbalah, A.; Abdelsalam, I.; Behiry, S.; Abdelkhalek, A.; Abdelfatah, M.; Ismail, S.; Elsharkawy, M.M. Copper oxide nanostructures as a potential method for control zucchini yellow mosaic virus in Squash. Pest Manag. Sci. 2022, 2, 7001. [Google Scholar] [CrossRef]
- Bapat, M.S.; Singh, H.; Shukla, S.K.; Singh, P.P.; Vo, D.V.N.; Yadav, A.; Goyal, A.; Sharma, A.; Kumar, D. Evaluating green silver nanoparticles as prospective biopesticides: An environmental standpoint. Chemosphere 2022, 286, 131761. [Google Scholar] [CrossRef]
- Stanley, S. Biological nanoparticles and their influence on organisms. Curr. Opin. Biotechnol. 2014, 28, 69–74. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer-layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Kagale, S.; Marimuthu, T.; Kagale, J.; Thayumanavan, B.; Samiyappan, R. Induction of systemic resistance in rice by leaf extracts of Zizyphus jujuba and Ipomoea carnea against Rhizoctonia solani. Plant Signal. Behav. 2011, 6, 919–923. [Google Scholar] [CrossRef]
- Liu, D.; He, X.; Li, W.; Chen, C.; Ge, F. Molecular cloning of a thaumatin-like protein gene from Pyrus pyrifolia and overexpression of this gene in tobacco increased resistance to pathogenic fungi. Plant Cell, Tissue Organ Cult. 2012, 111, 29–39. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, M.; Li, W.; Bai, G. Regulation of chlorogenic acid biosynthesis by hydroxycinnamoyl CoA quinate hydroxycinnamoyl transferase in Lonicera japonica. Plant Physiol. Biochem. 2017, 121, 74–79. [Google Scholar] [CrossRef]
- Tripathi, D.; Raikhy, G.; Kumar, D. Chemical elicitors of systemic acquired resistance—Salicylic acid and its functional analogs. Curr. Plant Biol. 2019, 17, 48–59. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, J.; Zhang, X.; Zhu, L.; Wang, X.; Guo, N.; Zhao, J.; Xing, H. Overexpression of chalcone isomerase (CHI) increases resistance against Phytophthora sojae in soybean. J. Plant Biol. 2018, 61, 309–319. [Google Scholar] [CrossRef]
- Aseel, D.G.; Makhlouf, A.; Riad, S.A.; Elmorsi, A.A.; Fegla, G. Two isolates of Potato virus Y (PVY) and the response of different potato cultivars against the viral infection. J. Virol. Antivir Res. 2015, 4, 2. [Google Scholar] [CrossRef]
- Kunze, G.W.; Dixon, J.B. Pretreatment for Mineralogical Analysis, Methods of Soil Analysis, Part 1—Physical and Mineralogical Methods, 2nd ed.; Agronomy Society of America and Soil Science of America: Madison, Wisconsin, 1989; p. 1181. [Google Scholar]
- Saleh, E.M.; Kishk, F.M.; Hedia, R.M.R.; El-Shafei, A.A.; El-Latif, A.; Mona, M. Efficacy of Nano-Clay Derived from Egyptian Alluvial Soils for Cu (II) Removal from Aqueous Solutions. Alexandria Sci. Exch. J. 2021, 42, 365–380. [Google Scholar] [CrossRef]
- Paul, R.; Datta, S.C.; Manjaiah, K.M.; Bhattacharyya, R. Characterization of nanoclay-phosphatase complex with IR spectroscopy and electron microscopy. Clay Res. 2015, 34, 99–109. [Google Scholar]
- Nguyen, H.; Tinet, E.; Chauveau, T.; Geinguenaud, F.; Lalatonne, Y.; Michel, A.; Aid-Launais, R.; Journé, C.; Lefèbvre, C.; Simon-Yarza, T. Bimodal fucoidan-coated zinc oxide/iron oxide-based nanoparticles for the imaging of atherothrombosis. Molecules 2019, 24, 962. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized Assay for Hydrogen Peroxide Determination in Plant Tissue Using Potassium Iodide. Am. J. Anal. Chem. 2014, 5, 730–736. [Google Scholar] [CrossRef]
- Angelini, R.; Manes, F.; Federico, R. Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chick-pea stems. Planta 1990, 182, 89–96. [Google Scholar] [CrossRef]
- Cho, Y.K.; Ahn, H.Y.E.K. Purification and characterization of polyphenol oxidase from potato: II. Inhibition and catalytic mechanism. J. Food Biochem. 1999, 23, 593–605. [Google Scholar] [CrossRef]
- Rashad, Y.M.; Aseel, D.G.; Hafez, E.E. Antifungal potential and defense gene induction in maize against Rhizoctonia root rot by seed extract of Ammi visnaga (L.) Lam. Phytopathol. Mediterr. 2018, 57, 73–88. [Google Scholar]
- Rashad, Y.; Aseel, D.; Hammad, S.; Elkelish, A. Rhizophagus irregularis and Rhizoctonia solani differentially elicit systemic transcriptional expression of polyphenol biosynthetic pathways genes in sunflower. Biomolecules 2020, 10, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aseel, D.G.; Rashad, Y.M.; Hammad, S.M. Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against Tomato Mosaic Virus. Sci. Rep. 2019, 9, 179. [Google Scholar] [CrossRef]
- Heflish, A.A.; Hanfy, A.E.; Ansari, M.J.; Dessoky, E.S.; Attia, A.O.; Elshaer, M.M.; Gaber, M.K.; Kordy, A.; Doma, A.S.; Abdelkhalek, A. Green biosynthesized silver nanoparticles using Acalypha wilkesiana extract control root-knot nematode. J. King Saud Univ. 2021, 33, 101516. [Google Scholar] [CrossRef]
- Gholipour, V.; Shamanian, M.; Ashrafi, A.; Maleki, A. Development of aluminium-nanoclay composite by using powder metallurgy and hot extrusion process. Met. Mater. Int. 2021, 27, 3681–3694. [Google Scholar] [CrossRef]
- Yılmaz, B.; Irmak, E.T.; Turhan, Y.; Doğan, S.; Turhan, O. Synthesis, Characterization and Biological Properties of Intercalated Kaolinite Nanoclays: Intercalation and Biocompatibility. 2019. Available online: https://dspace.balikesir.edu.tr/xmlui/handle/20.500.12462/10389 (accessed on 26 September 2022).
- Wada, K. A structural scheme of soil allophane. Am. Mineral. J. Earth Planet. Mater. 1967, 52, 690–708. [Google Scholar]
- Wada, K.; Ballivet, M.; Boulter, J.; Connolly, J.; Wada, E.; Deneris, E.S.; Swanson, L.W.; Heinemann, S.; Patrick, J. Functional expression of a new pharmacological subtype of brain nicotinic acetylcholine receptor. Science 1988, 240, 330–334. [Google Scholar] [CrossRef]
- Lingegowda, D.C.; Kumar, J.K.; Prasad, A.G.D.; Zarei, M.; Gopal, S. FTIR spectroscopic studies on cleome gynandra-Comparative analysis of functional group before and after extraction. Rom. J. Biophys. 2012, 22, 137–143. [Google Scholar]
- Abidin, Z.; Matsue, N.; Henmi, T. Differential formation of allophane and imogolite: Experimental and molecular orbital study. J. Comput. Mater. Des. 2007, 14, 5–18. [Google Scholar] [CrossRef]
- He, L.; Inokuma, T.; Kurata, Y.; Hasegawa, S. Vibrational properties of SiO and SiH in amorphous SiOx: H films (0≤ x≤ 2.0) prepared by plasma-enhanced chemical vapor deposition. J. Non. Cryst. Solids 1995, 185, 249–261. [Google Scholar] [CrossRef]
- Musić, S.; Dragčević, Đ.; Popović, S. Hydrothermal crystallization of boehmite from freshly precipitated aluminium hydroxide. Mater. Lett. 1999, 40, 269–274. [Google Scholar] [CrossRef]
- Javad Nazarahari, M.; Khaksar Manshad, A.; Moradi, S.; Shafiei, A.; Abdulazez Ali, J.; Sajadi, S.M.; Keshavarz, A. Synthesis, characterization, and assessment of a CeO2@ nanoclay nanocomposite for enhanced oil recovery. Nanomaterials 2020, 10, 2280. [Google Scholar] [CrossRef]
- Manohar, H.S. Development of Nanoclay reinforced Aluminium Metal Matrix Composites for Enhanced Mechanical and Tribological Properties. Ph.D. Thesis, Mechanical Engineering, Jawaharlal Nehru Technological University Anantapur, Andhra Pradesh, India, 2014. [Google Scholar]
- El-Ramady, H.; Alshaal, T.; Elhawat, N.; Ghazi, A.; Elsakhawy, T.; Omara, A.E.-D.; El-Nahrawy, S.; Elmahrouk, M.; Abdalla, N.; Domokos-Szabolcsy, É. Plant nutrients and their roles under saline soil conditions. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 297–324. [Google Scholar]
- Abdelkhalek, A.; Al-Askar, A.A. Green Synthesized ZnO Nanoparticles Mediated by Mentha Spicata Extract Induce Plant Systemic Resistance against Tobacco Mosaic Virus. Appl. Sci. 2020, 10, 5054. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Qari, S.H.; Abu-Saied, M.A.A.-R.; Khalil, A.M.; Younes, H.A.; Nehela, Y.; Behiry, S.I. Chitosan Nanoparticles Inactivate Alfalfa Mosaic Virus Replication and Boost Innate Immunity in Nicotiana glutinosa Plants. Plants 2021, 10, 2701. [Google Scholar] [CrossRef]
- Noha, K.; Bondok, A.M.; El-Dougdoug, K.A. Evaluation of silver nanoparticles as antiviral agent against ToMV and PVY in tomato plants. Sciences 2018, 8, 100–111. [Google Scholar]
- Goggin, F.L.; Fischer, H.D. Reactive Oxygen Species in Plant Interactions With Aphids. Front. Plant Sci. 2021, 12, 811105. [Google Scholar] [CrossRef]
- Arena, G.D.; Ramos-González, P.L.; Nunes, M.A.; Ribeiro-Alves, M.; Camargo, L.E.A.; Kitajima, E.W.; Machado, M.A.; Freitas-Astúa, J. Citrus leprosis virus C infection results in hypersensitive-like response, suppression of the JA/ET plant defense pathway and promotion of the colonization of its mite vector. Front. Plant Sci. 2016, 7, 1757. [Google Scholar] [CrossRef]
- Jaiswal, N.; Singh, M.; Dubey, R.S.; Venkataramanappa, V.; Datta, D. Phytochemicals and antioxidative enzymes defence mechanism on occurrence of yellow vein mosaic disease of pumpkin (Cucurbita moschata). 3 Biotech 2013, 3, 287–295. [Google Scholar] [CrossRef]
- Madhusudhan, K.N.; Srikanta, B.M.; Shylaja, M.D.; Prakash, H.S.; Shetty, H.S. Changes in antioxidant enzymes, hydrogen peroxide, salicylic acid and oxidative stress in compatible and incompatible host-tobamovirus interaction. J. Plant Interact. 2009, 4, 157–166. [Google Scholar] [CrossRef]
- Radwan, D.E.M.; Ismail, K.S. The impact of hydrogen peroxide against cucumber green mottle mosaic virus infection in watermelon plants. Polish J. Environ. Stud. 2020, 29, 100. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Alsubaie, M.M.; Behiry, S.I. First Report of Protective Activity of Paronychia argentea Extract against Tobacco Mosaic Virus Infection. Plants 2021, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.Z.; Hamdy, E.; Hamed, E.A.; Hafez, E.; Abdelkhalek, A. The curative activity of some arylidene dihydropyrimidine hydrazone against Tobacco mosaic virus infestation. J. Saudi Chem. Soc. 2022, 12, 101504. [Google Scholar] [CrossRef]
- De Alché, D.J. A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef]
- El-Gendi, H.; Al-Askar, A.A.; Király, L.; Samy, M.A.; Moawad, H.; Abdelkhalek, A. Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Hortic. 2022, 8, 130. [Google Scholar] [CrossRef]
- Abouelsaad, I.; Renault, S. Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. J. Plant Physiol. 2018, 226, 136–144. [Google Scholar] [CrossRef]
- Bera, S.; Arena, G.D.; Ray, S.; Flannigan, S.; Casteel, C.L. The Potyviral Protein 6K1 Reduces Plant Proteases Activity during Turnip mosaic virus Infection. Viruses 2022, 14, 1341. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Donkersley, P.; Dong, Y.; Chen, X.; Zang, Y.; Xu, P.; Ren, G. Preference of the aphid Myzus persicae (Hemiptera: Aphididae) for tobacco plants at specific stages of potato virus Y infection. Arch. Virol. 2019, 164, 1567–1573. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Rubil, N.; Kalachova, T.; Hauser, T.P.; Burketová, L. Specialist aphid feeding causes local activation of salicylic and jasmonic acid signaling in Arabidopsis veins. Mol. Plant-Microb. Interact. 2022, 35, 119–124. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Aseel, D.G.; Király, L.; Künstler, A.; Moawad, H.; Al-Askar, A.A. Induction of Systemic Resistance to Tobacco mosaic virus in Tomato through Foliar Application of Bacillus amyloliquefaciens Strain TBorg1 Culture Filtrate. Viruses 2022, 14, 1830. [Google Scholar] [CrossRef]
- Li, L.; Steffens, J.C. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 2002, 215, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhao, P.; Wang, B.; Tariq, P.; Zhao, F.; Zhao, M.; Wang, Q.; Yang, T.; Fang, J. Overexpression of Polyphenol Oxidase Gene in Strawberry Fruit Delays the Fungus Infection Process. Plant Mol. Biol. Report. 2016, 34, 592–606. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Elbeaino, T.; Moawad, H.; El-Gendi, H. Protective and Curative Activities of Paenibacillus polymyxa against Zucchini yellow mosaic virus Infestation in Squash Plants. Biology 2022, 11, 1150. [Google Scholar]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Szerlauth, A.; Szalma, L.; Muráth, S.; Sáringer, S.; Varga, G.; Li, L.; Szilágyi, I. Nanoclay-based sensor composites for the facile detection of molecular antioxidants. Analyst 2022, 147, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- Jing, B.; Ma, Z.; Feng, J.; Liang, H.; Li, C.; Zhang, X. Evaluation of the antiviral activity of extracts from plants grown in the qinling region of China against infection by Tobacco mosaic virus (TMV). J. Phytopathol. 2012, 160, 181–186. [Google Scholar] [CrossRef]
- Waziri, H.M.A. Plants as antiviral agents. J. Plant Pathol. Microbiol. 2015, 6, 1. [Google Scholar]
- Doxey, A.C.; Yaish, M.W.F.; Moffatt, B.A.; Griffith, M.; McConkey, B.J. Functional divergence in the Arabidopsis β-1, 3-glucanase gene family inferred by phylogenetic reconstruction of expression states. Mol. Biol. Evol. 2007, 24, 1045–1055. [Google Scholar] [CrossRef]
- Sinha, M.; Singh, R.P.; Kushwaha, G.S.; Iqbal, N.; Singh, A.; Kaushik, S.; Kaur, P.; Sharma, S.; Singh, T.P. Current overview of allergens of plant pathogenesis related protein families. Sci. World J. 2014, 2014, 132. [Google Scholar] [CrossRef]
- Oide, S.; Bejai, S.; Staal, J.; Guan, N.; Kaliff, M.; Dixelius, C. A novel role of PR 2 in abscisic acid (ABA) mediated, pathogen-induced callose deposition in Arabidopsis thaliana. New Phytol. 2013, 200, 1187–1199. [Google Scholar] [CrossRef]
- Levy, A.; Erlanger, M.; Rosenthal, M.; Epel, B.L. A plasmodesmata-associated β-1, 3-glucanase in Arabidopsis. Plant J. 2007, 49, 669–682. [Google Scholar] [CrossRef]
- Beffa, R.; Meins Jr, F. Pathogenesis-related functions of plant β-1, 3-glucanases investigated by antisense transformation—A review. Gene 1996, 179, 97–103. [Google Scholar] [CrossRef]
- Li, X.-Y.; Gao, L.; Zhang, W.-H.; Liu, J.-K.; Zhang, Y.-J.; Wang, H.-Y.; Liu, D.-Q. Characteristic expression of wheat PR5 gene in response to infection by the leaf rust pathogen, Puccinia triticina. J. Plant Interact. 2015, 10, 132–141. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Teng, K.; Lai, J.; Zhang, Y.; Huang, Y.; Li, Y.; Liang, L.; Wang, Y.; Chu, C. Up-regulation of LSB1/GDU3 affects geminivirus infection by activating the salicylic acid pathway. Plant J. 2010, 62, 12–23. [Google Scholar] [CrossRef]
- Yang, L.; Xu, Y.; Liu, Y.; Meng, D.; Jin, T.; Zhou, X. HC-Pro viral suppressor from tobacco vein banding mosaic virus interferes with DNA methylation and activates the salicylic acid pathway. Virology 2016, 497, 244–250. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.; Reddy, M.S.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid pathway engineering: An emerging approach towards plant defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef]
- Kavil, S.; Otti, G.; Bouvaine, S.; Armitage, A.; Maruthi, M.N. PAL1 gene of the phenylpropanoid pathway increases resistance to the Cassava brown streak virus in cassava. Virol. J. 2021, 18, 1–10. [Google Scholar] [CrossRef]



| Gene | Direction | Primer Sequence (5′–3′) | Functional Annotation | Related Pathway |
|---|---|---|---|---|
| PR-2 | Forward | TATAGCCGTTGGAAACGAAG | β-1,3-glucanases | Pathogenesis-related proteins |
| Reverse | CAACTTGCCATCACATTCTG | |||
| PR-5 | Forward | ACCTCTTCCGCTGTCCTC | Thaumatin-like protein | Pathogenesis-related proteins |
| Reverse | GAAGACGACTTGGTAGTTGC | |||
| PAL-1 | Forward | ACGGGTTGCCATCTAATCTGACA | Phenylalanine ammonia-lyase | Phenylpropanoid biosynthetic |
| Reverse | CGAGCAATAAGAAGCCATCGCAAT | |||
| CHI-2 | Forward | GGCAGGCCATTGAAAAGTTCC | Chalcone isomerase 2 | Flavonoid/isoflavonoid biosynthesis |
| Reverse | CTAATCGTCAATGATCCAAGCGG | |||
| B-actin | Forward | ATGCCATTCTCCGTCTTGACTTG | βeta-actin | Housekeeping |
| Reverse | GAGTTGTATGTAGTCTCGTGGATT | |||
| PVY-CP | Forward | CAACTCCAGATGGAACAATTG | Potato Virus Y-coat protein | Virus replication |
| Reverse | CCATTCATCACAGTTGGC |
| Weave Number (cm−1) | Functional Group | |
|---|---|---|
| Egyptian Nanoclay | Standard Nanoclay | |
| 432.10 | Si-O-Si bending | |
| 509.46 | Fe-O Fe2O3 Si-O-Al stretching | |
| 627.50 | Si-O-Si of quartz | |
| 682.05 | Si-O-Si bending | |
| 786.03 | Si-O quartz | |
| 913.41 | C=C binding alkane | |
| 978.82 | OH deformation, linked to 2Al2 | |
| 988.67 | OH deformation, linked to 2Al2 | |
| 1191.57 | Al-O as Si cage (TO4) | |
| 1463.36 | C-H stretching | |
| 1632.73 | H-O-H deformation of water | |
| 1642.22 | H-O-H deformation of water | |
| 2327.72 | O=C=O Carbon dioxide | |
| 3348.83 | H-O-H stretching, Absorbed water | |
| 3611.60 | OH stretching of inner-surface hydroxyl groups | |
| 3405.15 | OH of water | |
| Elements | Egyptian Nanoclay | Standard Nanoclay | ||||
|---|---|---|---|---|---|---|
| Intensity | Weight % | Atomic % | Intensity | Weight % | Atomic % | |
| C | 722 | 47.34 | 63.67 | 698 | 31.43 | 45.58 |
| O | 225 | 21.42 | 21.63 | 292 | 31.16 | 33.93 |
| Na | 96 | 1.21 | 0.85 | |||
| Mg | 74 | 0.79 | 0.52 | 134 | 5.15 | 3.69 |
| Al | 136 | 5.29 | 3.16 | 134 | 2.42 | 1.56 |
| Si | 187 | 12.09 | 6.95 | 560 | 19.61 | 12.16 |
| S | 65 | 0.64 | 0.35 | |||
| K | 43 | 0.31 | 0.13 | 42 | 0.18 | 0.08 |
| Ti | 41 | 0.38 | 0.13 | 38 | 0.16 | 0.06 |
| Fe | 82 | 2.89 | 0.84 | 112 | 4.95 | 1.54 |
| Ni | 40 | 0.16 | 0.05 | |||
| Co | 44 | 0.20 | 0.05 | |||
| Cu | 118 | 8.09 | 2.06 | 88 | 3.34 | 0.92 |
| Ta | 94 | 0.80 | 0.08 | |||
| Treatment * | Plant Height (cm) | Shoot Length (cm) | Root Length (cm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) |
|---|---|---|---|---|---|
| C | 24.33 ± 1.53 c | 15.50 ± 1.8 bc | 07.66 ± 1.53 c | 3.53 ± 0.50 b | 0.99 ± 0.18 ab |
| V | 14.50 ± 1.32 d | 09.93 ± 1.04 d | 05.33 ± 1.52 c | 1.57 ± 0.60 c | 0.50 ± 0.20 b |
| CE | 37.66 ± 2.08 a | 15.00 ± 1.0b c | 22.66 ± 2.52 a | 5.27 ± 0.55 a | 0.93 ± 0.21 ab |
| CE and V | 30.33 ± 2.51 b | 16.33 ± 2.08 b | 13.66 ± 1.53 b | 3.77 ± 1.05 b | 0.78 ± 0.18 ab |
| CS | 35.66 ± 2.08 a | 20.50 ± 1.32 a | 15.17 ± 1.04 b | 4.67 ± 1.08 ab | 1.16 ± 0.56 a |
| CS and V | 29.83 ± 1.61 b | 13.67 ± 0.58 c | 16.66 ± 3.05 b | 2.23 ± 0.35 c | 0.81 ± 0.15 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aseel, D.G.; Abdelkhalek, A.; Alotibi, F.O.; Samy, M.A.; Al-Askar, A.A.; Arishi, A.A.; Hafez, E.E. Foliar Application of Nanoclay Promotes Potato (Solanum tuberosum L.) Growth and Induces Systemic Resistance against Potato Virus Y. Viruses 2022, 14, 2151. https://doi.org/10.3390/v14102151
Aseel DG, Abdelkhalek A, Alotibi FO, Samy MA, Al-Askar AA, Arishi AA, Hafez EE. Foliar Application of Nanoclay Promotes Potato (Solanum tuberosum L.) Growth and Induces Systemic Resistance against Potato Virus Y. Viruses. 2022; 14(10):2151. https://doi.org/10.3390/v14102151
Chicago/Turabian StyleAseel, Dalia G., Ahmed Abdelkhalek, Fatimah O. Alotibi, Marwa A. Samy, Abdulaziz A. Al-Askar, Amr A. Arishi, and Elsayed E. Hafez. 2022. "Foliar Application of Nanoclay Promotes Potato (Solanum tuberosum L.) Growth and Induces Systemic Resistance against Potato Virus Y" Viruses 14, no. 10: 2151. https://doi.org/10.3390/v14102151
APA StyleAseel, D. G., Abdelkhalek, A., Alotibi, F. O., Samy, M. A., Al-Askar, A. A., Arishi, A. A., & Hafez, E. E. (2022). Foliar Application of Nanoclay Promotes Potato (Solanum tuberosum L.) Growth and Induces Systemic Resistance against Potato Virus Y. Viruses, 14(10), 2151. https://doi.org/10.3390/v14102151







