Temporal Dynamics of Influenza A(H5N1) Subtype before and after the Emergence of H5N8
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Spatial Analysis
2.2. Correlation Analysis
3. Results
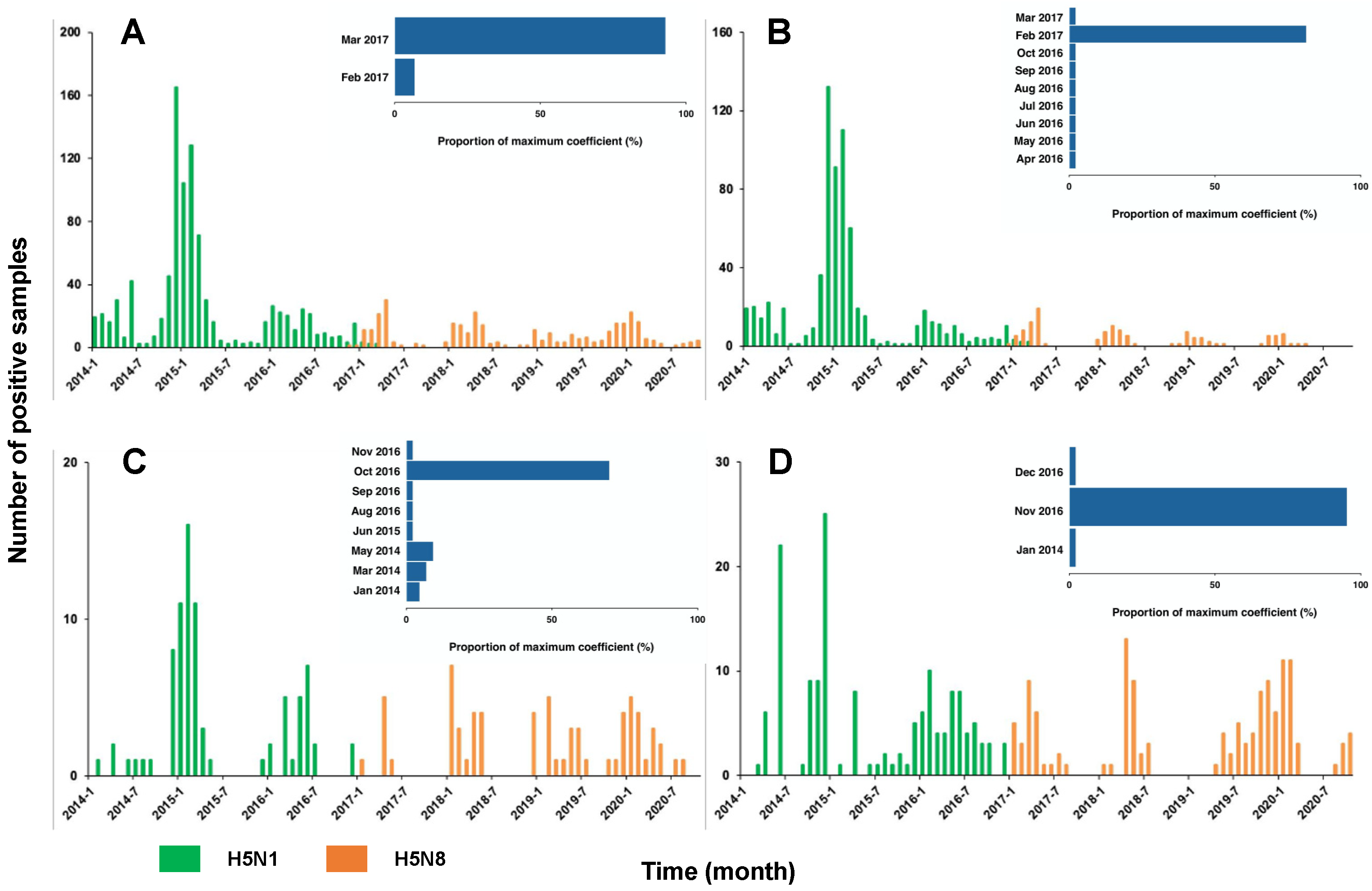
3.1. Surveillance Outcomes
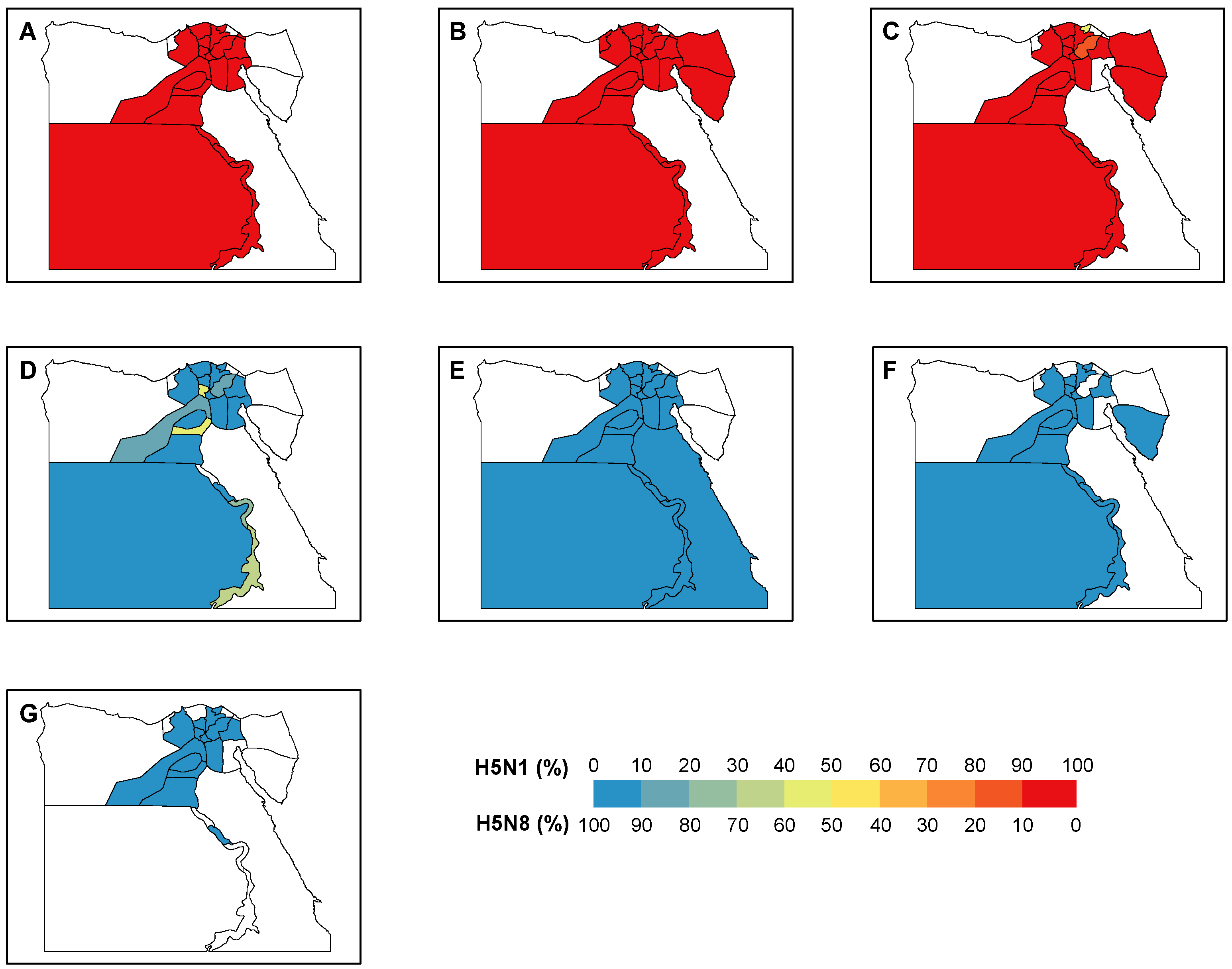
3.2. Changing Temporal and Spatial Distribution of H5N1 and H5N8
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- Monto, A.S.; Fukuda, K. Lessons From Influenza Pandemics of the Last 100 Years. Clin. Infect. Dis. 2020, 70, 951–957. [Google Scholar] [CrossRef]
- Dunning, J.; Thwaites, R.S.; Openshaw, P.J.M. Seasonal and pandemic influenza: 100 years of progress, still much to learn. Mucosal. Immunol. 2020, 13, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Boni, M.F.; Galvani, A.P.; Wickelgren, A.L.; Malani, A. Economic epidemiology of avian influenza on smallholder poultry farms. Theor. Popul. Biol. 2013, 90, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Richard, M.; Verhagen, J.H.; van Riel, D.; Schrauwen, E.J.; van den Brand, J.M.; Mänz, B.; Bodewes, R.; Herfst, S. One health, multiple challenges: The inter-species transmission of influenza A virus. One Health 2015, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.F. Lessons from emergence of A/goose/Guangdong/1996-like H5N1 highly pathogenic avian influenza viruses and recent influenza surveillance efforts in southern China. Zoonoses Public Health 2012, 59 (Suppl. 2), 32–42. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jiang, Z.; Xu, B. Global spatiotemporal and genetic footprint of the H5N1 avian influenza virus. Int. J. Health Geogr. 2014, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Sims, L.D.; Domenech, J.; Benigno, C.; Kahn, S.; Kamata, A.; Lubroth, J.; Martin, V.; Roeder, P. Origin and evolution of highly pathogenic H5N1 avian influenza in Asia. Vet. Rec. 2005, 157, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.J.; Donis, R.O. Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013-2014. Influenza Other Respir. Viruses 2015, 9, 271–276. [Google Scholar] [CrossRef]
- Chan, P.K. Outbreak of avian influenza A(H5N1) virus infection in Hong Kong in 1997. Clin. Infect. Dis. 2002, 34 (Suppl. 2), S58–S64. [Google Scholar] [CrossRef]
- World Health Organisation. Human Infection with Avian Influenza A (H5N8—The Russian Federation. World Health Organisation. 2021. Available online: https://www.euro.who.int/en/countries/poland/news/news/2021/3/avian-influenza-ah5n8-infects-humans-in-russian-federation (accessed on 20 June 2021).
- Chen, H.; Smith, G.J.; Zhang, S.Y.; Qin, K.; Wang, J.; Li, K.S.; Webster, R.G.; Peiris, J.S.; Guan, Y. Avian flu: H5N1 virus outbreak in migratory waterfowl. Nature 2005, 436, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Smith, G.J. The emergence and diversification of panzootic H5N1 influenza viruses. Virus Res. 2013, 178, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Nguyen, N.T. Evolutionary dynamics of highly pathogenic avian influenza A/H5N1 HA clades and vaccine implementation in Vietnam. Clin. Exp. Vaccine Res. 2014, 3, 117–127. [Google Scholar] [CrossRef]
- Parvin, R.; Nooruzzaman, M.; Kabiraj, C.K.; Begum, J.A.; Chowdhury, E.H.; Islam, M.R.; Harder, T. Controlling Avian Influenza Virus in Bangladesh: Challenges and Recommendations. Viruses 2020, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.E.; El-Kady, M.F.; EL-Sawah, A.A.A.; Luttermann, C.; Parvin, R.; Shany, S.; Beer, M.; Harder, T. Respiratory disease due to mixed viral infections in poultry flocks in Egypt between 2017 and 2018: Upsurge of highly pathogenic avian influenza virus subtype H5N8 since 2018. Transbound. Emerg. Dis. 2021, 68, 21–36. [Google Scholar] [CrossRef] [PubMed]
- CDC. Highly Pathogenic Asian Avian Influenza A(H5N1) Virus. Centers for Disease Control. and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD). 2018. Available online: https://www.cdc.gov/flu/avianflu/h5n1-virus.htm (accessed on 27 April 2021).
- Saeed, N.M.; Rashid, P.M.A.; Dyary, H.O. Genetic characterization of highly pathogenic avian influenza A (H5N8) virus isolated from domestic geese in Iraq, 2018. BMC Vet. Res. 2021, 17, 124. [Google Scholar] [CrossRef]
- Verhagen, J.H.; Fouchier, R.A.M.; Lewis, N. Highly Pathogenic Avian Influenza Viruses at the Wild-Domestic Bird Interface in Europe: Future Directions for Research and Surveillance. Viruses 2021, 13, 212. [Google Scholar] [CrossRef]
- Fusaro, A.; Zecchin, B.; Vrancken, B.; Abolnik, C.; Ademun, R.; Alassane, A.; Arafa, A.; Awuni, J.A.; Couacy-Hymann, E.; Coulibaly, M.B.; et al. Disentangling the role of Africa in the global spread of H5 highly pathogenic avian influenza. Nat. Commun. 2019, 10, 5310. [Google Scholar] [CrossRef]
- Saito, T.; Tanikawa, T.; Uchida, Y.; Takemae, N.; Kanehira, K.; Tsunekuni, R. Intracontinental and intercontinental dissemination of Asian H5 highly pathogenic avian influenza virus (clade 2.3.4.4) in the winter of 2014–2015. Rev. Med. Virol. 2015, 25, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Torchetti, M.K.; Winker, K.; Ip, H.S.; Song, C.S.; Swayne, D.E. Intercontinental Spread of Asian-Origin H5N8 to North America through Beringia by Migratory Birds. J. Virol. 2015, 89, 6521–6524. [Google Scholar] [CrossRef]
- Lycett, S.J.; Pohlmann, A.; Staubach, C.; Caliendo, V.; Woolhouse, M.; Beer, M.; Kuiken, T. Genesis and spread of multiple reassortants during the 2016/2017 H5 avian influenza epidemic in Eurasia. Proc. Natl. Acad. Sci. USA 2020, 117, 20814–20825. [Google Scholar] [CrossRef] [PubMed]
- Napp, S.; Majó, N.; Sánchez-Gónzalez, R.; Vergara-Alert, J. Emergence and spread of highly pathogenic avian influenza A(H5N8) in Europe in 2016–2017. Transbound. Emerg. Dis. 2018, 65, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Banyard, A.C.; Whittard, E.; Karibayev, T.; Al Kafagi, T.; Chvala, I.; Byrne, A.; Meruyert Akberovna, S.; King, J.; Harder, T.; et al. Emergence and spread of novel H5N8, H5N5 and H5N1 clade 2.3.4.4 highly pathogenic avian influenza in 2020. Emerg. Microbes Infect. 2021, 10, 148–151. [Google Scholar] [CrossRef]
- Baek, Y.G.; Lee, Y.N.; Lee, D.H.; Shin, J.I.; Lee, J.H.; Chung, D.H.; Lee, E.K.; Heo, G.B.; Sagong, M.; Kye, S.J.; et al. Multiple Reassortants of H5N8 Clade 2.3.4.4b Highly Pathogenic Avian Influenza Viruses Detected in South Korea during the Winter of 2020–2021. Viruses 2021, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.M.; Abdelwhab, E.M.; Harder, T.C. Evolutionary features of influenza A/H5N1 virus populations in Egypt: Poultry and human health implications. Arch. Virol. 2016, 161, 1963–1967. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.A.; Erfan, A.M.; Hagag, N.; Zanaty, A.; Samir, A.H.; Samy, M.; Abdelhalim, A.; Arafa, A.A.; Soliman, M.A.; Shaheen, M.; et al. Highly Pathogenic Avian Influenza Virus (H5N8) Clade 2.3.4.4 Infection in Migratory Birds, Egypt. Emerg. Infect. Dis. 2017, 23, 1048–1051. [Google Scholar] [CrossRef]
- Naguib, M.M.; Harder, T. Endemic situation of multiple avian influenza strains in poultry in Egypt: A continuing nightmare. Zoonoses Public Health 2018, 65, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.A.; Sedeik, M.E.; Elbestawy, A.R.; Zain El-Abideen, M.A.; Ibrahim, H.H.; Kilany, W.H.; Ali, A. Co-infections, genetic, and antigenic relatedness of avian influenza H5N8 and H5N1 viruses in domestic and wild birds in Egypt. Poult. Sci. 2019, 98, 2371–2379. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 20 June 2021).
- Nuñez, I.A.; Ross, T.M. A review of H5Nx avian influenza viruses. Ther. Adv. Vaccines Immunother. 2019, 7, 2515135518821625. [Google Scholar] [CrossRef]
- Samy, A.; Naguib, M.M. Avian Respiratory Coinfection and Impact on Avian Influenza Pathogenicity in Domestic Poultry: Field and Experimental Findings. Vet. Sci. 2018, 5, 23. [Google Scholar] [CrossRef]
- Naguib, M.M.; Verhagen, J.H.; Samy, A.; Eriksson, P.; Fife, M.; Lundkvist, Å.; Ellström, P.; Järhult, J.D. Avian influenza viruses at the wild-domestic bird interface in Egypt. Infect. Ecol. Epidemiol 2019, 9, 1575687. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.E.; Saad, N.; Abozeid, H.H.; Shany, S.; El-Kady, M.F.; Arafa, A.; El-Sawah, A.A.A.; Pfaff, F.; Hafez, H.M.; Beer, M.; et al. Genotyping and reassortment analysis of highly pathogenic avian influenza viruses H5N8 and H5N2 from Egypt reveals successive annual replacement of genotypes. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2020, 84, 104375. [Google Scholar] [CrossRef]
- Salaheldin, A.H.; El-Hamid, H.S.; Elbestawy, A.R.; Veits, J.; Hafez, H.M.; Mettenleiter, T.C.; Abdelwhab, E.M. Multiple Introductions of Influenza A(H5N8) Virus into Poultry, Egypt, 2017. Emerg. Infect. Dis. 2018, 24, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Yehia, N.; Naguib, M.M.; Li, R.; Hagag, N.; El-Husseiny, M.; Mosaad, Z.; Nour, A.; Rabea, N.; Hasan, W.M.; Hassan, M.K.; et al. Multiple introductions of reassorted highly pathogenic avian influenza viruses (H5N8) clade 2.3.4.4b causing outbreaks in wild birds and poultry in Egypt. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018, 58, 56–65. [Google Scholar] [CrossRef]
- Wang, R.H.; Jin, Z.; Liu, Q.X.; van de Koppel, J.; Alonso, D. A simple stochastic model with environmental transmission explains multi-year periodicity in outbreaks of avian flu. PLoS ONE 2012, 7, e28873. [Google Scholar] [CrossRef]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef]
- Tarek, M.; Naguib, M.M.; Arafa, A.-S.; Tantawy, L.A.; Selim, K.M.; Talaat, S.; Sultan, H.A. Epidemiology, Genetic Characterization, and Pathogenesis of Avian Influenza H5N8 Viruses Circulating in Northern and Southern Parts of Egypt, 2017–2019. Animals 2021, 11, 2208. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, F.; Li, R.; Rabie, N.; El-Husseiny, M.H.; Yehia, N.; Hagag, N.M.; Samy, M.; Selim, A.; Hassan, M.K.; Hassan, W.M.M.; et al. Temporal Dynamics of Influenza A(H5N1) Subtype before and after the Emergence of H5N8. Viruses 2021, 13, 1565. https://doi.org/10.3390/v13081565
Amer F, Li R, Rabie N, El-Husseiny MH, Yehia N, Hagag NM, Samy M, Selim A, Hassan MK, Hassan WMM, et al. Temporal Dynamics of Influenza A(H5N1) Subtype before and after the Emergence of H5N8. Viruses. 2021; 13(8):1565. https://doi.org/10.3390/v13081565
Chicago/Turabian StyleAmer, Fatma, Ruiyun Li, Neveen Rabie, Mohamed H. El-Husseiny, Nahed Yehia, Naglaa M. Hagag, Mohamed Samy, Abdullah Selim, Mohamed K. Hassan, Wafaa M. M. Hassan, and et al. 2021. "Temporal Dynamics of Influenza A(H5N1) Subtype before and after the Emergence of H5N8" Viruses 13, no. 8: 1565. https://doi.org/10.3390/v13081565
APA StyleAmer, F., Li, R., Rabie, N., El-Husseiny, M. H., Yehia, N., Hagag, N. M., Samy, M., Selim, A., Hassan, M. K., Hassan, W. M. M., Arafa, A.-S., Lundkvist, Å., Shahein, M. A., & Naguib, M. M. (2021). Temporal Dynamics of Influenza A(H5N1) Subtype before and after the Emergence of H5N8. Viruses, 13(8), 1565. https://doi.org/10.3390/v13081565






