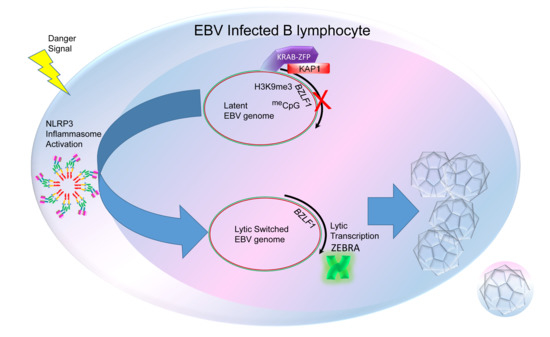
Inflammasome, the Constitutive Heterochromatin Machinery, and Replication of an Oncogenic Herpesvirus
Abstract
1. A Two-Population Problem
2. Epigenetic Regulation of the Lytic Phase
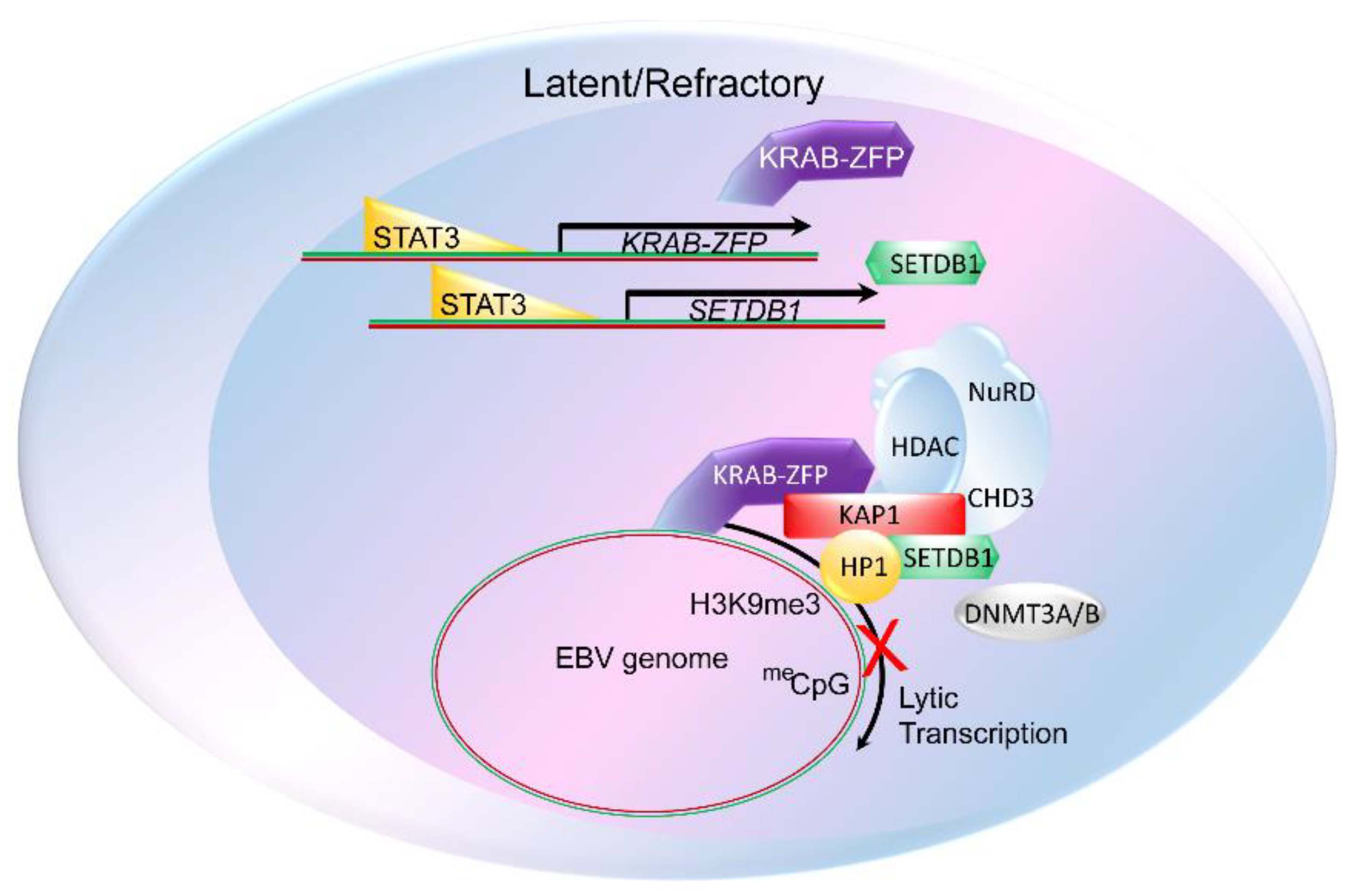
3. The Constitutive Heterochromatin Machinery Silences Herpesvirus Lytic Genes
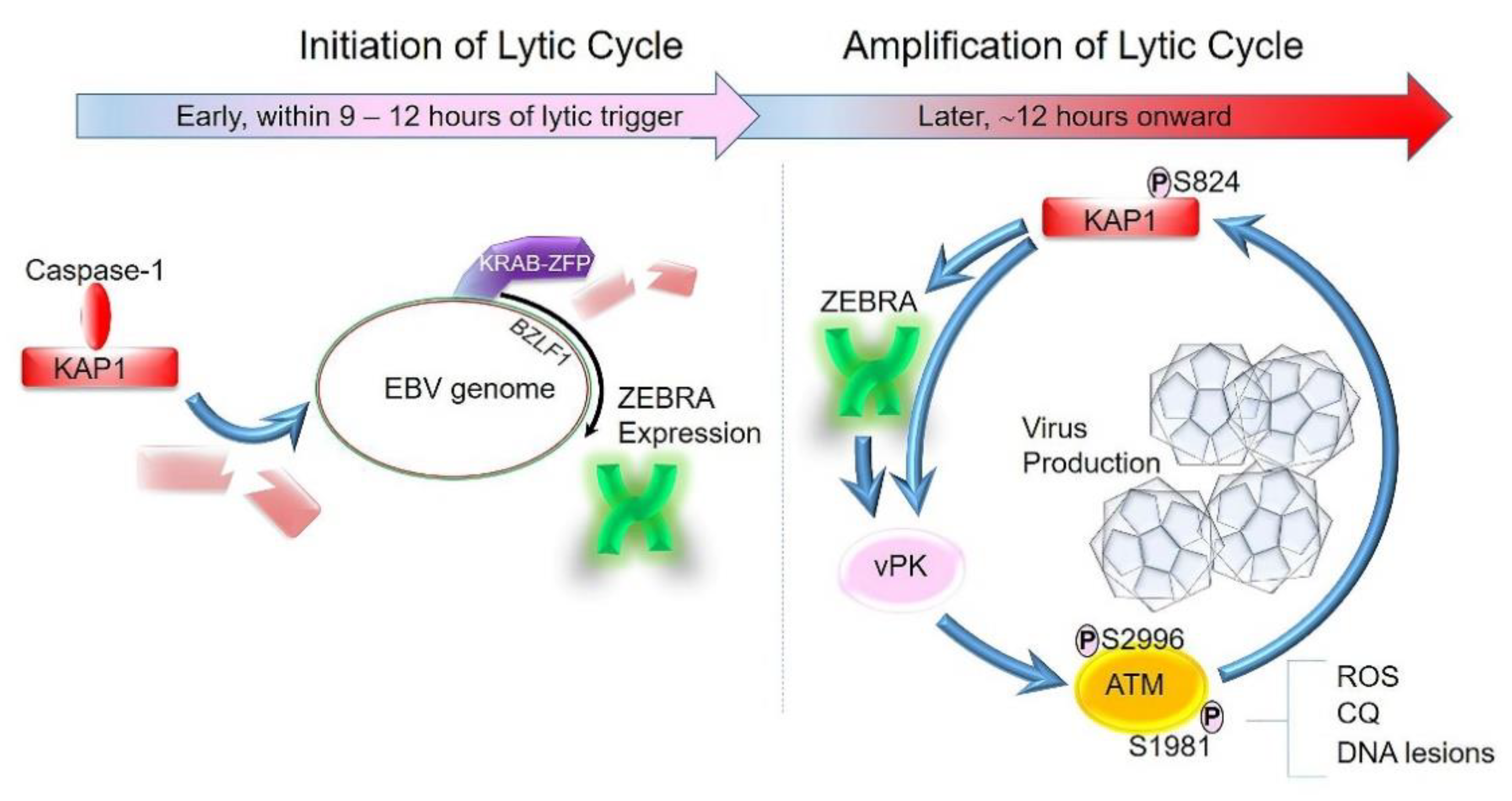
4. Lytic Cycle of EBV Can Be Broadly Divided into Two Phases: Initiation and Amplification
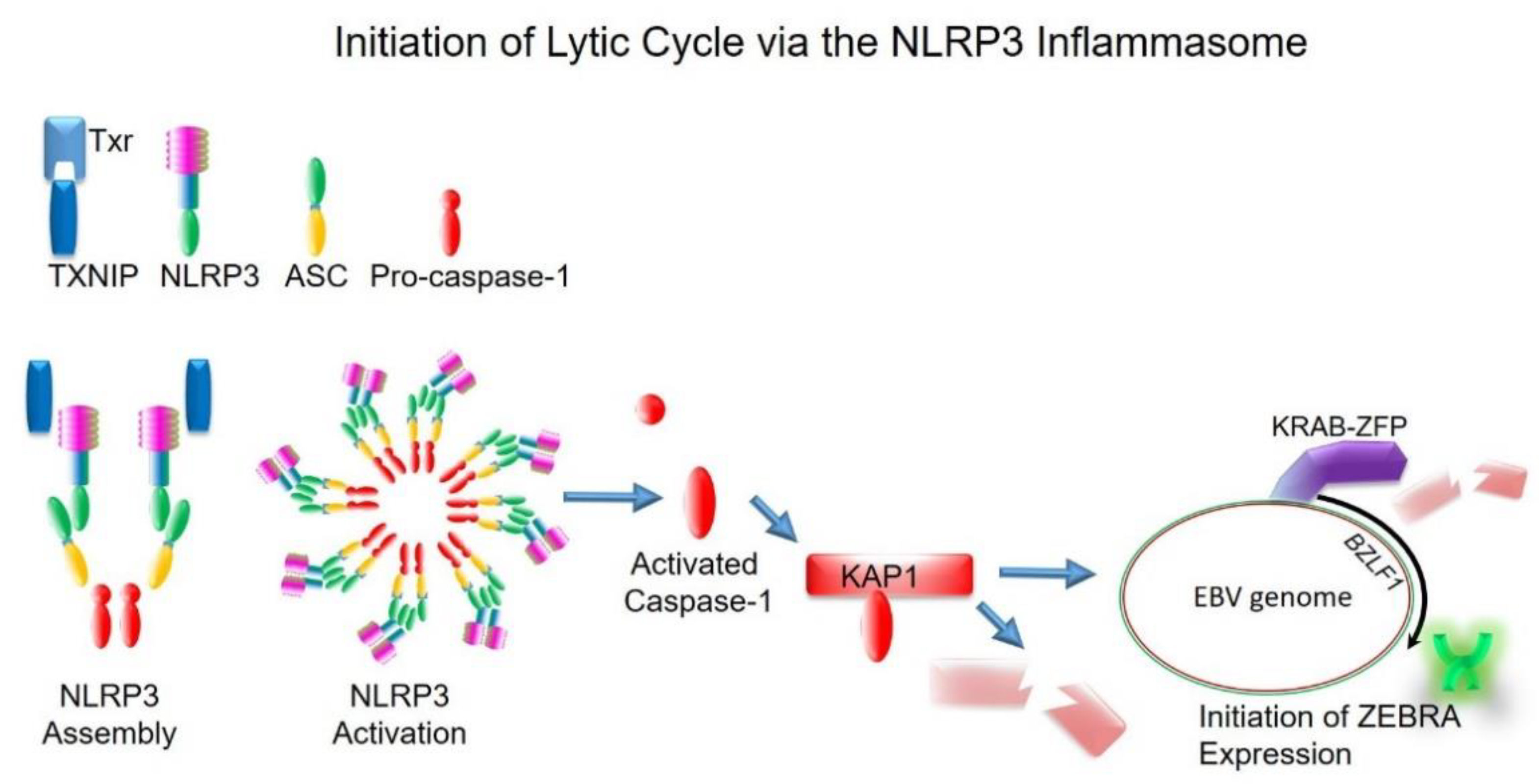
5. The Inflammasome as a Common Upstream Mechanism That Disrupts Heterochromatin-Mediated Silencing to Initiate the Lytic Cycle
6. The Constitutive Heterochromatin Machinery Is Able to Differentiate between Self and Foreign Genomes
7. Insights into Genetic Immune Disorders, Metabolic Syndromes, and Herpesvirus Diseases
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farrell, P.J. Epstein-Barr Virus and Cancer. Annu. Rev. Pathol. 2019, 14, 29–53. [Google Scholar] [CrossRef]
- Young, L.S.; Rickinson, A.B. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer 2004, 4, 757–768. [Google Scholar] [CrossRef]
- Munz, C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat. Rev. Microbiol. 2019, 17, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Rosemarie, Q.; Sugden, B. Epstein-Barr Virus: How Its Lytic Phase Contributes to Oncogenesis. Microorganisms 2020, 8, 1824. [Google Scholar] [CrossRef]
- Chiu, Y.F.; Sugden, B. Epstein-Barr Virus: The Path from Latent to Productive Infection. Annu. Rev. Virol. 2016, 3, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Kenney, S.C.; Mertz, J.E. Regulation of the latent-lytic switch in Epstein-Barr virus. Semin. Cancer Biol. 2014, 26, 60–68. [Google Scholar] [CrossRef]
- Gao, X.; Ikuta, K.; Tajima, M.; Sairenji, T. 12-O-Tetradecanoylphorbol-13-acetate Induces Epstein–Barr Virus Reactivation via NF-κB and AP-1 as Regulated by Protein Kinase C and Mitogen-Activated Protein Kinase. Virology 2001, 286, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hagemeier, S.R.; Barlow, E.A.; Kleman, A.A.; Kenney, S.C. The Epstein-Barr Virus BRRF1 Protein, Na, Induces Lytic Infection in a TRAF2- and p53-Dependent Manner. J. Virol. 2011, 85, 4318–4329. [Google Scholar] [CrossRef]
- Iwakiri, D.; Takada, K. Phosphatidylinositol 3-Kinase Is a Determinant of Responsiveness to B Cell Antigen Receptor-Mediated Epstein-Barr Virus Activation. J. Immunol. 2004, 172, 1561–1566. [Google Scholar] [CrossRef]
- Lee, H.H.; Chang, S.S.; Lin, S.J.; Chua, H.H.; Tsai, T.J.; Tsai, K.; Lo, Y.C.; Chen, H.C.; Tsai, C.H. Essential role of PKCdelta in histone deacetylase inhibitor-induced Epstein-Barr virus reactivation in nasopharyngeal carcinoma cells. J. Gen. Virol. 2008, 89, 878–883. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.R.; Huang, S.Y.; Chen, J.Y.; Wang, L.H. Microtubule depolymerization activates the Epstein-Barr virus lytic cycle through protein kinase C pathways in nasopharyngeal carcinoma cells. J. Gen. Virol. 2013, 94, 2750–2758. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Chen, L.; Yang, L.; Li, L.; Tao, Y.; Li, W.; Li, Z.; Liu, H.; Tang, M.; et al. (-)-Epigallocatechin-3-gallate inhibition of Epstein-Barr virus spontaneous lytic infection involves ERK1/2 and PI3-K/Akt signaling in EBV-positive cells. Carcinogenesis 2013, 34, 627–637. [Google Scholar] [CrossRef]
- Gradoville, L.; Kwa, D.; El-Guindy, A.; Miller, G. Protein Kinase C-Independent Activation of the Epstein-Barr Virus Lytic Cycle. J. Virol. 2002, 76, 5612–5626. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Burton, E.M.; Bhaduri-McIntosh, S. Chloroquine triggers Epstein-Barr virus replication through phosphorylation of KAP1/TRIM28 in Burkitt lymphoma cells. PLoS Pathog. 2017, 13, e1006249. [Google Scholar] [CrossRef]
- Bhaduri-McIntosh, S.; Miller, G. Cells lytically infected with Epstein-Barr virus are detected and separable by immunoglobulins from EBV-seropositive individuals. J. Virol. Methods 2006, 137, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.M.; Akinyemi, I.A.; Frey, T.R.; Xu, H.; Li, X.; Su, L.J.; Zhi, J.; McIntosh, M.T.; Bhaduri-McIntosh, S. A heterochromatin inducing protein differentially recognizes self versus foreign genomes. PLoS Pathog. 2021, 17, e1009447. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.R.; Koganti, S.; Zhi, J.; Megyola, C.; Freeman, A.F.; Palendira, U.; Tangye, S.G.; Farrell, P.J.; Bhaduri-McIntosh, S. Signal Transducer and Activator of Transcription 3 Limits Epstein-Barr Virus Lytic Activation in B Lymphocytes. J. Virol. 2013, 87, 11438–11446. [Google Scholar] [CrossRef]
- Koganti, S.; Clark, C.; Zhi, J.; Li, X.; Chen, E.I.; Chakrabortty, S.; Hill, E.R.; Bhaduri-McIntosh, S. Cellular STAT3 Functions via PCBP2 To Restrain Epstein-Barr Virus Lytic Activation in B Lymphocytes. J. Virol. 2015, 89, 5002–5011. [Google Scholar] [CrossRef]
- Frey, T.R.; Brathwaite, J.; Li, X.; Burgula, S.; Akinyemi, I.A.; Agarwal, S.; Burton, E.M.; Ljungman, M.; McIntosh, M.T.; Bhaduri-McIntosh, S. Nascent Transcriptomics Reveal Cellular Prolytic Factors Upregulated Upstream of the Latent-to-Lytic Switch Protein of Epstein-Barr Virus. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- King, C.A.; Li, X.; Barbachano-Guerrero, A.; Bhaduri-McIntosh, S. STAT3 Regulates Lytic Activation of Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2015, 89, 11347–11355. [Google Scholar] [CrossRef]
- Li, X.; Burton, E.M.; Koganti, S.; Zhi, J.; Doyle, F.; Tenenbaum, S.A.; Horn, B.; Bhaduri-McIntosh, S. KRAB-ZFP Repressors Enforce Quiescence of Oncogenic Human Herpesviruses. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Daigle, D.; Megyola, C.; El-Guindy, A.; Gradoville, L.; Tuck, D.; Miller, G.; Bhaduri-McIntosh, S. Upregulation of STAT3 Marks Burkitt Lymphoma Cells Refractory to Epstein-Barr Virus Lytic Cycle Induction by HDAC Inhibitors. J. Virol. 2010, 84, 993–1004. [Google Scholar] [CrossRef]
- Lupo, A.; Cesaro, E.; Montano, G.; Zurlo, D.; Izzo, P.; Costanzo, P. KRAB-Zinc Finger Proteins: A Repressor Family Displaying Multiple Biological Functions. Curr. Genom. 2013, 14, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Emerson, R.O.; Thomas, J.H. Adaptive Evolution in Zinc Finger Transcription Factors. PLoS Genet. 2009, 5, e1000325. [Google Scholar] [CrossRef]
- Urrutia, R. KRAB-containing zinc-finger repressor proteins. Genome Biol. 2003, 4, 231. [Google Scholar] [CrossRef]
- Ecco, G.; Imbeault, M.; Trono, D. KRAB zinc finger proteins. Development 2017, 144, 2719–2729. [Google Scholar] [CrossRef]
- Quenneville, S.; Turelli, P.; Bojkowska, K.; Raclot, C.; Offner, S.; Kapopoulou, A.; Trono, D. The KRAB-ZFP/KAP1 System Contributes to the Early Embryonic Establishment of Site-Specific DNA Methylation Patterns Maintained during Development. Cell Rep. 2012, 2, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Rowe, H.M.; Friedli, M.; Offner, S.; Verp, S.; Mesnard, D.; Marquis, J.; Aktas, T.; Trono, D. De novo DNA methylation of endogenous retroviruses is shaped by KRAB-ZFPs/KAP1 and ESET. Development 2013, 140, 519–529. [Google Scholar] [CrossRef]
- Wiznerowicz, M.; Jakobsson, J.; Szulc, J.; Liao, S.; Quazzola, A.; Beermann, F.; Aebischer, P.; Trono, D. The Krüppel-associated Box Repressor Domain Can Trigger de Novo Promoter Methylation during Mouse Early Embryogenesis. J. Biol. Chem. 2007, 282, 34535–34541. [Google Scholar] [CrossRef]
- Sinclair, A.J. Epigenetic control of Epstein-Barr virus transcription—Relevance to viral life cycle? Front. Genet. 2013, 4, 161. [Google Scholar] [CrossRef]
- Orzalli, M.H.; Conwell, S.E.; Berrios, C.; De Caprio, J.A.; Knipe, D.M. Nuclear interferon-inducible protein 16 promotes silencing of herpesviral and transfected DNA. Proc. Natl. Acad. Sci. USA 2013, 110, E4492–E4501. [Google Scholar] [CrossRef] [PubMed]
- Rauwel, B.; Jang, S.M.; Cassano, M.; Kapopoulou, A.; Barde, I.; Trono, D. Release of human cytomegalovirus from latency by a KAP1/TRIM28 phosphorylation switch. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.T.; Kuo, C.Y.; Ann, D.K. KAPtain in charge of multiple missions: Emerging roles of KAP1. World J. Biol. Chem. 2014, 5, 308–320. [Google Scholar] [CrossRef]
- Iyengar, S.; Farnham, P.J. KAP1 Protein: An Enigmatic Master Regulator of the Genome. J. Biol. Chem. 2011, 286, 26267–26276. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, A.A.; Noon, A.T.; Deckbar, D.; Ziv, Y.; Shiloh, Y.; Löbrich, M.; Jeggo, P.A. ATM Signaling Facilitates Repair of DNA Double-Strand Breaks Associated with Heterochromatin. Mol. Cell 2008, 31, 167–177. [Google Scholar] [CrossRef]
- White, D.; Rafalska-Metcalf, I.U.; Ivanov, A.V.; Corsinotti, A.; Peng, H.; Lee, S.C.; Trono, D.; Janicki, S.M.; Rauscher, F.J. The ATM substrate KAP1 controls DNA repair in heterochromatin: Regulation by HP1 proteins and serine 473/824 phosphorylation. Mol. Cancer Res. 2012, 10, 401–414. [Google Scholar] [CrossRef]
- Ziv, Y.; Bielopolski, D.; Galanty, Y.; Lukas, C.; Taya, Y.; Schultz, D.C.; Lukas, J.; Bekker-Jensen, S.; Bartek, J.; Shiloh, Y. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat. Cell Biol. 2006, 8, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-C.; Fitzgerald, L.D.; Van Geelen, A.; Izumiya, Y.; Ellison, T.J.; Wang, D.-H.; Ann, D.K.; Luciw, P.A.; Kung, H.-J. Kruppel-Associated Box Domain-Associated Protein-1 as a Latency Regulator for Kaposi’s Sarcoma-Associated Herpesvirus and Its Modulation by the Viral Protein Kinase. Cancer Res. 2009, 69, 5681–5689. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kozlov, S.V.; El-Guindy, A.; Bhaduri-McIntosh, S. Retrograde Regulation by the Viral Protein Kinase Epigenetically Sustains the Epstein-Barr Virus Latency-to-Lytic Switch to Augment Virus Production. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Alekseev, O.; Donegan, W.E.; Donovan, K.R.; Limonnik, V.; Azizkhan-Clifford, J. HSV-1 Hijacks the Host DNA Damage Response in Corneal Epithelial Cells through ICP4-Mediated Activation of ATM. Investig. Opthalmology Vis. Sci. 2020, 61, 39. [Google Scholar] [CrossRef]
- Alekseev, O.; Donovan, K.; Azizkhan-Clifford, J. Inhibition of Ataxia Telangiectasia Mutated (ATM) Kinase Suppresses Herpes Simplex Virus Type 1 (HSV-1) Keratitis. Investig. Opthalmology Vis. Sci. 2014, 55, 706–715. [Google Scholar] [CrossRef]
- Heston, L.; El-Guindy, A.; Countryman, J.; Cruz, C.D.; Delecluse, H.J.; Miller, G. Amino acids in the basic domain of Epstein-Barr virus ZEBRA protein play distinct roles in DNA binding, activation of early lytic gene expression, and promotion of viral DNA replication. J. Virol. 2006, 80, 9115–9133. [Google Scholar] [CrossRef] [PubMed]
- Wang’ondu, R.; Teal, S.; Park, R.; Heston, L.; Delecluse, H.; Miller, G. DNA Damage Signaling Is Induced in the Absence of Epstein-Barr Virus (EBV) Lytic DNA Replication and in Response to Expression of ZEBRA. PLoS ONE 2015, 10, e0126088. [Google Scholar] [CrossRef]
- Hagemeier, S.R.; Barlow, E.A.; Meng, Q.; Kenney, S.C. The Cellular Ataxia Telangiectasia-Mutated Kinase Promotes Epstein-Barr Virus Lytic Reactivation in Response to Multiple Different Types of Lytic Reactivation-Inducing Stimuli. J. Virol. 2012, 86, 13360–13370. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Deshpande, R.; Paull, T.T. ATM activation in the presence of oxidative stress. Cell Cycle 2010, 9, 4805–4811. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Kozlov, S.; Lavin, M.F.; Person, M.D.; Paull, T.T. ATM Activation by Oxidative Stress. Science 2010, 330, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, S.A.; Klein, G. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int. J. Cancer 1981, 28, 131–135. [Google Scholar] [CrossRef]
- Fahmi, H.; Cochet, C.; Hmama, Z.; Opolon, P.; Joab, I. Transforming growth factor beta 1 stimulates expression of the Epstein-Barr virus BZLF1 immediate-early gene product ZEBRA by an indirect mechanism which requires the MAPK kinase pathway. J. Virol. 2000, 74, 5810–5818. [Google Scholar] [CrossRef] [PubMed]
- Mellinghoff, I.; Daibata, M.; Humphreys, R.E.; Mulder, C.; Takada, K.; Sairenji, T. Early events in Epstein-Barr virus genome expression after activation: Regulation by second messengers of B cell activation. Virology 1991, 185, 922–928. [Google Scholar] [CrossRef]
- Shirley, C.M.; Chen, J.; Shamay, M.; Li, H.; Zahnow, C.A.; Hayward, S.D.; Ambinder, R.F. Bortezomib induction of C/EBPbeta mediates Epstein-Barr virus lytic activation in Burkitt lymphoma. Blood 2011, 117, 6297–6303. [Google Scholar] [CrossRef]
- Westphal, E.M.; Blackstock, W.; Feng, W.; Israel, B.; Kenney, S.C. Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: A potential method for treating EBV-positive malignancies. Cancer Res. 2000, 60, 5781–5788. [Google Scholar] [PubMed]
- Feng, W.H.; Israel, B.; Raab-Traub, N.; Busson, P.; Kenney, S.C. Chemotherapy induces lytic EBV replication and confers ganciclovir susceptibility to EBV-positive epithelial cell tumors. Cancer Res. 2002, 62, 1920–1926. [Google Scholar] [PubMed]
- Shakespear, M.R.; Halili, M.A.; Irvine, K.M.; Fairlie, D.P.; Sweet, M.J. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011, 32, 335–343. [Google Scholar] [CrossRef]
- Zeng, Q.Z.; Yang, F.; Li, C.G.; Xu, L.H.; He, X.H.; Mai, F.Y.; Zeng, C.Y.; Zhang, C.C.; Zha, Q.B.; Ouyang, D.Y. Paclitaxel Enhances the Innate Immunity by Promoting NLRP3 Inflammasome Activation in Macrophages. Front. Immunol. 2019, 10, 72. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.D. Inflammasome activation and assembly at a glance. J. Cell Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kanneganti, T.D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Burton, E.M.; Goldbach-Mansky, R.; Bhaduri-McIntosh, S. A promiscuous inflammasome sparks replication of a common tumor virus. Proc. Natl. Acad. Sci. USA 2020, 117, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Gjyshi, O.; Roy, A.; Dutta, S.; Veettil, M.V.; Dutta, D.; Chandran, B. Activated Nrf2 Interacts with Kaposi’s Sarcoma-Associated Herpesvirus Latency Protein LANA-1 and Host Protein KAP1 To Mediate Global Lytic Gene Repression. J. Virol. 2015, 89, 7874–7892. [Google Scholar] [CrossRef]
- Sun, R.; Liang, D.; Gao, Y.; Lan, K. Kaposi’s Sarcoma-Associated Herpesvirus-Encoded LANA Interacts with Host KAP1 To Facilitate Establishment of Viral Latency. J. Virol. 2014, 88, 7331–7344. [Google Scholar] [CrossRef]
- Siegel, A.M.; Heimall, J.; Freeman, A.F.; Hsu, A.P.; Brittain, E.; Brenchley, J.M.; Douek, D.C.; Fahle, G.H.; Cohen, J.I.; Holland, S.M.; et al. A Critical Role for STAT3 Transcription Factor Signaling in the Development and Maintenance of Human T Cell Memory. Immunity 2011, 35, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.W.; Dixit, V.D. Mechanisms of disease: Inflammasome activation and the development of type 2 diabetes. Front. Immunol. 2013, 4, 50. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, M.; Zhao, S.; Lu, Q.; Ni, L.; Zou, C.; Lu, L.; Xu, X.; Guan, H.; Zheng, Z.; et al. Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: A novel inhibitory effect of minocycline. Inflamm. Res. 2017, 66, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Johnson, D.W.; Teixeira-Pinto, A.; Craig, J.C.; Wong, G. Incidence and predictors of post-transplant lymphoproliferative disease after kidney transplantation during adulthood and childhood: A registry study. Nephrol. Dial. Transpl. 2018, 33, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M.; Metallidis, S.; Tziomalos, K. Herpes Zoster and Diabetes Mellitus: A Review. Diabetes Ther. 2018, 9, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Quiles, C.; Lopez-Lacort, M.; Ampudia-Blasco, F.J.; Diez-Domingo, J. Risk and impact of herpes zoster on patients with diabetes: A population-based study, 2009–2014. Hum. Vaccines Immunother. 2017, 13, 2606–2611. [Google Scholar] [CrossRef]
- Kennedy, P.G.; Rovnak, J.; Badani, H.; Cohrs, R.J. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J. Gen. Virol. 2015, 96, 1581–1602. [Google Scholar] [CrossRef]
- Thorley-Lawson, D.; Deitsch, K.W.; Duca, K.A.; Torgbor, C. The Link between Plasmodium falciparum Malaria and Endemic Burkitt’s Lymphoma—New Insight into a 50-Year-Old Enigma. PLoS Pathog. 2016, 12, e1005331. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhaduri-McIntosh, S.; McIntosh, M.T. Inflammasome, the Constitutive Heterochromatin Machinery, and Replication of an Oncogenic Herpesvirus. Viruses 2021, 13, 846. https://doi.org/10.3390/v13050846
Bhaduri-McIntosh S, McIntosh MT. Inflammasome, the Constitutive Heterochromatin Machinery, and Replication of an Oncogenic Herpesvirus. Viruses. 2021; 13(5):846. https://doi.org/10.3390/v13050846
Chicago/Turabian StyleBhaduri-McIntosh, Sumita, and Michael T. McIntosh. 2021. "Inflammasome, the Constitutive Heterochromatin Machinery, and Replication of an Oncogenic Herpesvirus" Viruses 13, no. 5: 846. https://doi.org/10.3390/v13050846
APA StyleBhaduri-McIntosh, S., & McIntosh, M. T. (2021). Inflammasome, the Constitutive Heterochromatin Machinery, and Replication of an Oncogenic Herpesvirus. Viruses, 13(5), 846. https://doi.org/10.3390/v13050846