Genetic Characterization of a Neurovirulent West Nile Virus Variant Associated with a Fatal Great Grey Owl Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area—Zoo Košice
2.2. Flavivirus RNA Detection in Brain Tissue
2.3. Virus Isolation and Virus Quantification
2.4. Sequencing of the WNV Isolate
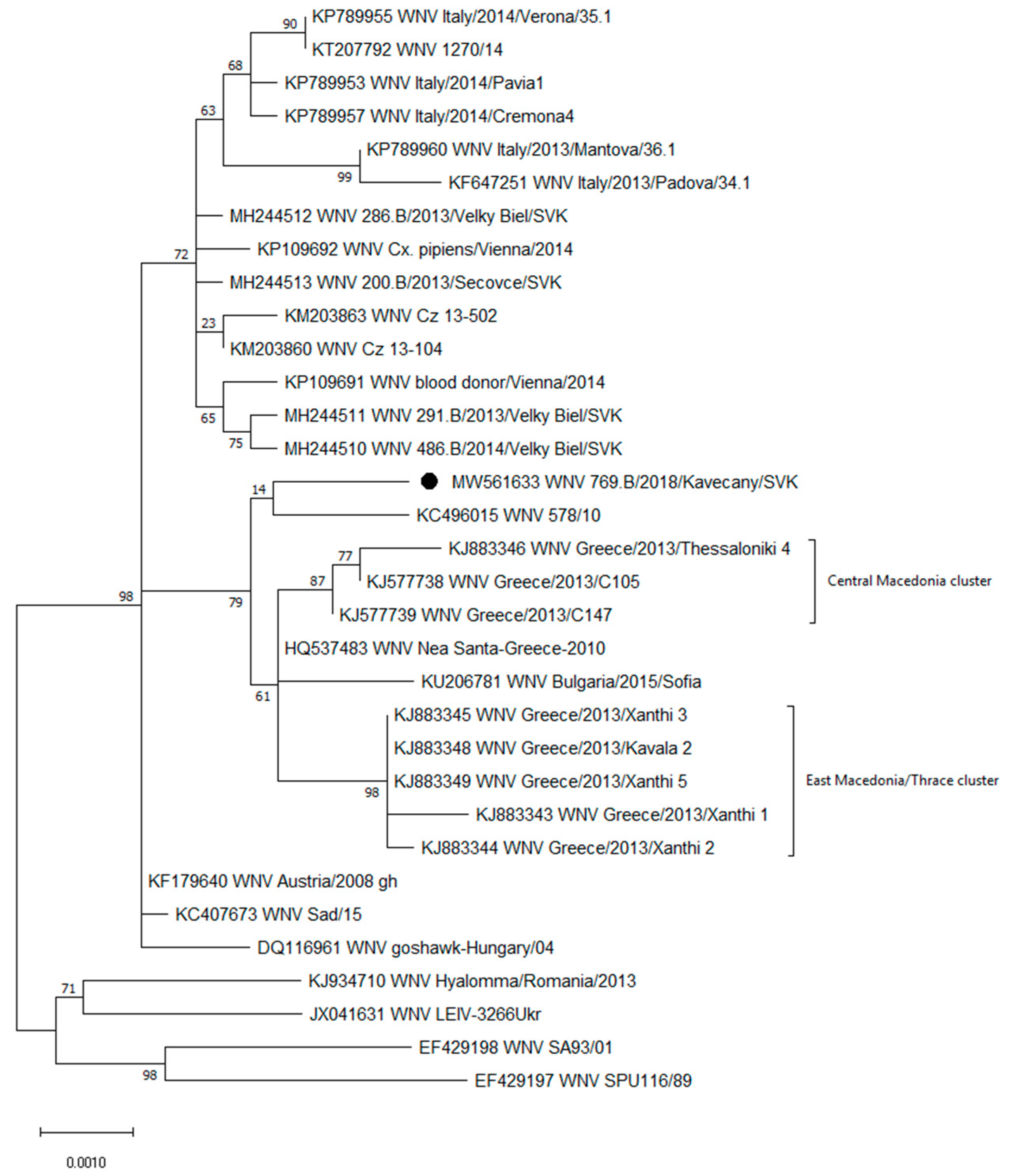
2.5. Phylogenetic Analyses
2.6. Serological Surveillance of Captive Owls in Zoo Košice
2.7. Mosquito Screening in Zoo Košice
2.8. Processing of Mosquitoes for WNV Detection
3. Results
3.1. Detection and Quantification of WNV from the Dead Owl
3.2. Genetic Characterization
3.3. Serological Examination of Owls
3.4. Mosquito Screening in Zoo Košice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef]
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda. Am. J. Trop. Med. Hyg. 1940, 1, 471–492. [Google Scholar] [CrossRef]
- Gray, T.J.; Webb, C.E. A review of the epidemiological and clinical aspects of West Nile virus. Int. J. Gen. Med. 2014, 7, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pare, J.; Moore, A. West Nile virus in horses—What do you need to know to diagnose the disease? Can. Vet. J. 2018, 59, 1119–1120. [Google Scholar] [PubMed]
- Hubalek, Z. Mosquito-borne viruses in Europe. Parasitol. Res. 2008, 103 (Suppl. S1), S29–S43. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Triana, L.M.; Jeffries, C.L.; Mansfield, K.L.; Carnell, G.; Fooks, A.R.; Johnson, N. Emergence of west nile virus lineage 2 in europe: A review on the introduction and spread of a mosquito-borne disease. Front. Public Health 2014, 2, 271. [Google Scholar] [CrossRef] [Green Version]
- Labuda, M.; Kožuch, O.; Grešíková, M. Isolation of West Nile virus from Aedes cantans mosquitoes in western Slovakia. Acta Virol. 1974, 18, 429–433. [Google Scholar]
- Csank, T.; Drzewniokova, P.; Korytar, L.; Major, P.; Gyuranecz, M.; Pistl, J.; Bakonyi, T. A Serosurvey of Flavivirus Infection in Horses and Birds in Slovakia. Vector Borne Zoonotic Dis. 2018, 18, 206–213. [Google Scholar] [CrossRef]
- Csank, T.; Korytar, L.; Posivakova, T.; Bakonyi, T.; Pistl, J.; Csanady, A. Surveillance on antibodies against West Nile virus, Usutu virus, tick-borne encephalitis virus and Tribe virus in wild birds in Drienovska wetland, Slovakia. Biologia 2019, 74, 813–820. [Google Scholar] [CrossRef]
- Hubalek, Z.; Ludvikova, E.; Jahn, P.; Treml, F.; Rudolf, I.; Svobodova, P.; Sikutova, S.; Betasova, L.; Bires, J.; Mojzis, M.; et al. West Nile Virus Equine Serosurvey in the Czech and Slovakias. Vector Borne Zoonotic Dis. 2013, 13, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Korytar, L.; Penazziova, K.; Pistl, J.; Ticha, E.; Cabanova, V.; Csank, T. Retrospective review and current knowledge on the occurrence of West Nile virus in mosquito vectors, reservoirs and hosts in Slovakia (Central Europe). Acta Virol. 2020, 64, 187–200. [Google Scholar] [CrossRef]
- Drzewniokova, P.; Barzon, L.; Franchin, E.; Lavezzo, E.; Bakonyi, T.; Pistl, J.; Csank, T. The complete genome sequence analysis of West Nile virus strains isolated in Slovakia (central Europe). Arch. Virol. 2019, 164, 273–277. [Google Scholar] [CrossRef]
- Čabanová, V.; Šikutová, S.; Straková, P.; Šebesta, O.; Víchová, B.; Zubríková, D.; Miterpáková, M.; Mendel, J.; Hurníková, Z.; Hubálek, Z.; et al. Co-Circulation of West Nile and Usutu Flaviviruses in Mosquitoes in Slovakia, 2018. Viruses 2019, 11, 639. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control. Epidemiological Update: West Nile Virus Transmission Season in Europe. 2019. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2019 (accessed on 9 February 2021).
- Čabanová, V.; Tichá, E.; Stewart Bradbury, R.; Zubríková, D.; Valentová, D.; Chovancová, G.; Grešaková, L.; Víchová, B.; šikutová, S.; Csank, T.; et al. Current status of West Nile and Usutu viruses in four distinct territorial units of Slovakia: Description of the first confirmed human West Nile Fever case and a vector surveillance study. Eurosurveillance 2021. Under Review. [Google Scholar]
- Csank, T.; Bhide, K.; Bencurova, E.; Dolinska, S.; Drzewniokova, P.; Major, P.; Korytar, L.; Bockova, E.; Bhide, M.; Pistl, J. Detection of West Nile virus and tick-borne encephalitis virus in birds in Slovakia, using a universal primer set. Arch. Virol. 2016, 161, 1679–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaramozzino, N.; Crance, J.-M.; Jouan, A.; De Briel, D.A.; Stoll, F.O.; Garin, D. Comparison of Flavivirus Universal Primer Pairs and Development of a Rapid, Highly Sensitive Heminested Reverse Transcription-PCR Assay for Detection of Flaviviruses Targeted to a Conserved Region of the NS5 Gene Sequences. J. Clin. Microbiol. 2001, 39, 1922–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios, G.; Quan, P.-L.; Jabado, O.J.; Conlan, S.; Hirschberg, D.L.; Liu, Y.; Zhai, J.; Renwick, N.; Hui, J.; Hegyi, H.; et al. Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerg. Infect. Dis. 2007, 13, 73–81. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ivanics, E.; Erdelyi, K.; Ursu, K.; Ferenczi, E.; Weissenbock, H.; Nowotny, N. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. CABIOS 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Hoysak, D.J.; Weatherhead, P.J. Sampling Blood from Birds: A Technique and an Assessment of Its Effect. Condor 1991, 93, 746–752. [Google Scholar] [CrossRef] [Green Version]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.B.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; p. 577. [Google Scholar]
- Kolodziejek, J.; Marinov, M.; Kiss, B.J.; Alexe, V.; Nowotny, N. The complete sequence of a West Nile virus lineage 2 strain detected in a Hyalomma marginatum marginatum tick collected from a song thrush (Turdus philomelos) in eastern Romania in 2013 revealed closest genetic relationship to strain Volgograd 2007. PLoS ONE 2014, 9, e109905. [Google Scholar] [CrossRef] [Green Version]
- Barzon, L.; Papa, A.; Lavezzo, E.; Franchin, E.; Pacenti, M.; Sinigaglia, A.; Masi, G.; Trevisan, M.; Squarzon, L.; Toppo, S.; et al. Phylogenetic characterization of Central/Southern European lineage 2 West Nile virus: Analysis of human outbreaks in Italy and Greece, 2013–2014. Clin. Microbiol. Infect. 2015, 21, e1–e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, G.V.; Calle, P.P.; Mangiafico, J.A.; Raphael, B.L.; Danner, D.K.; Hile, J.A.; Clippinger, T.L.; Smith, J.F.; Cook, R.A.; McNamara, T. An outbreak of West Nile virus in a New York City captive wildlife population. Am. J. Trop. Med. Hyg. 2002, 67, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, U.; Santos, P.D.; Groschup, M.H.; Hattendorf, C.; Eiden, M.; Hoper, D.; Eisermann, P.; Keller, M.; Michel, F.; Klopfleisch, R.; et al. West Nile Virus Epidemic in Germany Triggered by Epizootic Emergence, 2019. Viruses 2020, 12, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijks, J.; Kik, M.; Slaterus, R.; Foppen, R.; Stroo, A.; IJzer, J.; Stahl, J.; Gröne, A.; Koopmans, M.; van der Jeugd, H.; et al. Widespread Usutu virus outbreak in birds in The Netherlands, 2016. Eurosurveillance 2016, 21, 30391. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.D.; Patterson, J.S.; Kiupel, M.; Simmons, H.A.; Grimes, S.D.; Sarver, C.F.; Fulton, R.M.; Steficek, B.A.; Cooley, T.M.; Massey, J.P.; et al. Clinical and pathologic features of West Nile virus infection in native North American owls (Family strigidae). Avian Dis. 2003, 47, 602–610. [Google Scholar] [CrossRef]
- Ana, A.; Perez Andrés, M.; Julia, P.; Pedro, P.; Arno, W.; Kimberly, V.W.; Julio, A.; Michelle, W. Syndromic surveillance for West Nile virus using raptors in rehabilitation. BMC Vet. Res. 2017, 13, 368. [Google Scholar] [CrossRef] [Green Version]
- Gamino, V.; Hofle, U. Pathology and tissue tropism of natural West Nile virus infection in birds: A review. Vet. Res. 2013, 44, 39. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, U.; Luhken, R.; Keller, M.; Cadar, D.; van der Grinten, E.; Michel, F.; Albrecht, K.; Eiden, M.; Rinder, M.; Lachmann, L.; et al. West Nile virus epizootic in Germany, 2018. Antivir. Res. 2019, 162, 39–43. [Google Scholar] [CrossRef]
- Gancz, A.Y.; Campbell, D.G.; Barker, I.K.; Lindsay, R.; Hunter, B. Detecting West Nile virus in owls and raptors by an antigen-capture assay. Emerg. Infect. Dis. 2004, 10, 2204–2206. [Google Scholar] [CrossRef]
- Wheeler, S.S.; Barker, C.M.; Fang, Y.; Veronica Armijos, M.; Carroll, B.D.; Husted, S.; Johnson, W.O.; Reisen, W.K. Differential Impact of West Nile Virus on California Birds. Condor 2009, 111, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lühken, R.; Jöst, H.; Cadar, D.; Thomas, S.M.; Bosch, S.; Tannich, E.; Becker, N.; Ziegler, U.; Lachmann, L.; Schmidt-Chanasit, J. Distribution of Usutu Virus in Germany and Its Effect on Breeding Bird Populations. Emerg. Infect. Dis. 2017, 23, 1994–2001. [Google Scholar] [CrossRef] [Green Version]
- Szentpali-Gavaller, K.; Lim, S.M.; Dencso, L.; Banyai, K.; Koraka, P.; Osterhaus, A.D.; Martina, B.E.; Bakonyi, T.; Balint, A. In Vitro and in Vivo Evaluation of Mutations in the NS Region of Lineage 2 West Nile Virus Associated with Neuroinvasiveness in a Mammalian Model. Viruses 2016, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Papa, A.; Danis, K.; Baka, A.; Bakas, A.; Dougas, G.; Lytras, T.; Theocharopoulos, G.; Chrysagis, D.; Vassiliadou, E.; Kamaria, F.; et al. Ongoing outbreak of West Nile virus infections in humans in Greece, July–August 2010. Eurosurveillance 2010, 15. [Google Scholar] [CrossRef]
- Baymakova, M.; Trifonova, I.; Panayotova, E.; Dakova, S.; Pacenti, M.; Barzon, L.; Lavezzo, E.; Hristov, Y.; Ramshev, K.; Plochev, K.; et al. Fatal Case of West Nile Neuroinvasive Disease in Bulgaria. Emerg. Infect. Dis. 2016, 22, 2203–2204. [Google Scholar] [CrossRef] [Green Version]
- Brault, A.C.; Huang, C.Y.; Langevin, S.A.; Kinney, R.M.; Bowen, R.A.; Ramey, W.N.; Panella, N.A.; Holmes, E.C.; Powers, A.M.; Miller, B.R. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat. Genet. 2007, 39, 1162–1166. [Google Scholar] [CrossRef]
- Chappell, K.J.; Stoermer, M.J.; Fairlie, D.P.; Young, P.R. West Nile Virus NS2B/NS3 protease as an antiviral target. Curr. Med. Chem. 2008, 15, 2771–2784. [Google Scholar] [CrossRef]
| Accession Number | E | NS1 | NS2B | NS3 | NS4B | NS5 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 159T (449) | 399R (689) | 44K (835) | 119I (1493) | 249P (1754) | 486L (1991) | 14G (2287) | 49A (2322) | 113V (2386) | 298T (2827) | |
| MW561633■/SVK | T | R | K | I | P | L | G | A | V | T |
| KC496015□/HUN | I | K | R | . | . | F | . | . | . | A |
| KU206781▼/BGR | M | K | R | . | . | F | . | . | M | A |
| HQ537483●/GRC | I | K | R | . | . | F | . | . | M | A |
| KJ883349▼/GRC | I | K | R | . | . | F | . | . | M | A |
| KJ883345▼/GRC | I | K | R | . | . | F | . | . | M | A |
| KJ883348▼/GRC | I | K | R | . | . | F | . | . | M | A |
| KJ883343▼/GRC | I | K | R | . | . | F | . | . | M | A |
| KJ883344▼/GRC | I | K | R | . | . | F | . | . | M | A |
| KJ883346▼/GRC | I | K | R | . | . | F | . | . | M | A |
| KJ577738▼/GRC | I | K | R | . | . | F | . | . | M | A |
| KJ577739▼/GRC | I | K | R | . | . | F | . | . | M | A |
| MH244513■/SVK | . | K | R | V | H | F | S | T | . | . |
| MH244512■/SVK | . | K | R | V | H | F | S | T | . | . |
| MH244511■/SVK | A | K | R | V | H | F | S | T | . | . |
| MH244510■/SVK | A | K | R | V | H | F | S | T | . | . |
| KM203863●/CZE | . | K | R | V | H | F | S | T | . | . |
| KM203860●/CZE | . | K | R | V | H | F | S | T | . | . |
| KP789955▼/ITA | . | K | R | V | H | F | S | T | . | . |
| KP789957▼/ITA | . | K | R | V | H | F | S | T | . | . |
| KP789953▼/ITA | . | K | R | V | H | F | S | T | . | . |
| KP789960▼/ITA | . | K | R | V | H | F | S | T | . | . |
| KF647251▼/ITA | . | K | R | V | H | F | S | T | . | . |
| KT207792●/ITA | . | K | R | V | H | F | S | T | . | . |
| DQ116961■/HUN | I | K | R | V | H | F | S | T | . | A |
| KP109692●/AUT | . | K | R | V | H | F | S | T | . | . |
| KP109691▼/AUT | A | K | R | V | H | F | S | T | . | . |
| KF179640■/AUT | I | K | R | V | H | F | S | T | . | A |
| KC407673■/SRB | I | K | R | V | H | F | S | T | . | A |
| KJ934710○/ROU | M | K | . | V | H | F | S | T | . | A |
| JX041631■/UKR | I | K | . | V | H | F | . | T | . | A |
| EF429198▼/SAF | I | K | . | V | H | F | S | T | . | A |
| EF429197▼/SAF | I | K | . | V | H | F | S | T | . | A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peňazziová, K.; Korytár, Ľ.; Pastorek, P.; Pistl, J.; Rusňáková, D.; Szemes, T.; Čabanová, V.; Ličková, M.; Boršová, K.; Klempa, B.; et al. Genetic Characterization of a Neurovirulent West Nile Virus Variant Associated with a Fatal Great Grey Owl Infection. Viruses 2021, 13, 699. https://doi.org/10.3390/v13040699
Peňazziová K, Korytár Ľ, Pastorek P, Pistl J, Rusňáková D, Szemes T, Čabanová V, Ličková M, Boršová K, Klempa B, et al. Genetic Characterization of a Neurovirulent West Nile Virus Variant Associated with a Fatal Great Grey Owl Infection. Viruses. 2021; 13(4):699. https://doi.org/10.3390/v13040699
Chicago/Turabian StylePeňazziová, Katarína, Ľuboš Korytár, Patrik Pastorek, Juraj Pistl, Diana Rusňáková, Tomáš Szemes, Viktória Čabanová, Martina Ličková, Kristína Boršová, Boris Klempa, and et al. 2021. "Genetic Characterization of a Neurovirulent West Nile Virus Variant Associated with a Fatal Great Grey Owl Infection" Viruses 13, no. 4: 699. https://doi.org/10.3390/v13040699
APA StylePeňazziová, K., Korytár, Ľ., Pastorek, P., Pistl, J., Rusňáková, D., Szemes, T., Čabanová, V., Ličková, M., Boršová, K., Klempa, B., & Csank, T. (2021). Genetic Characterization of a Neurovirulent West Nile Virus Variant Associated with a Fatal Great Grey Owl Infection. Viruses, 13(4), 699. https://doi.org/10.3390/v13040699






