Small Molecules—Prospective Novel HCMV Inhibitors
Abstract
1. Introduction
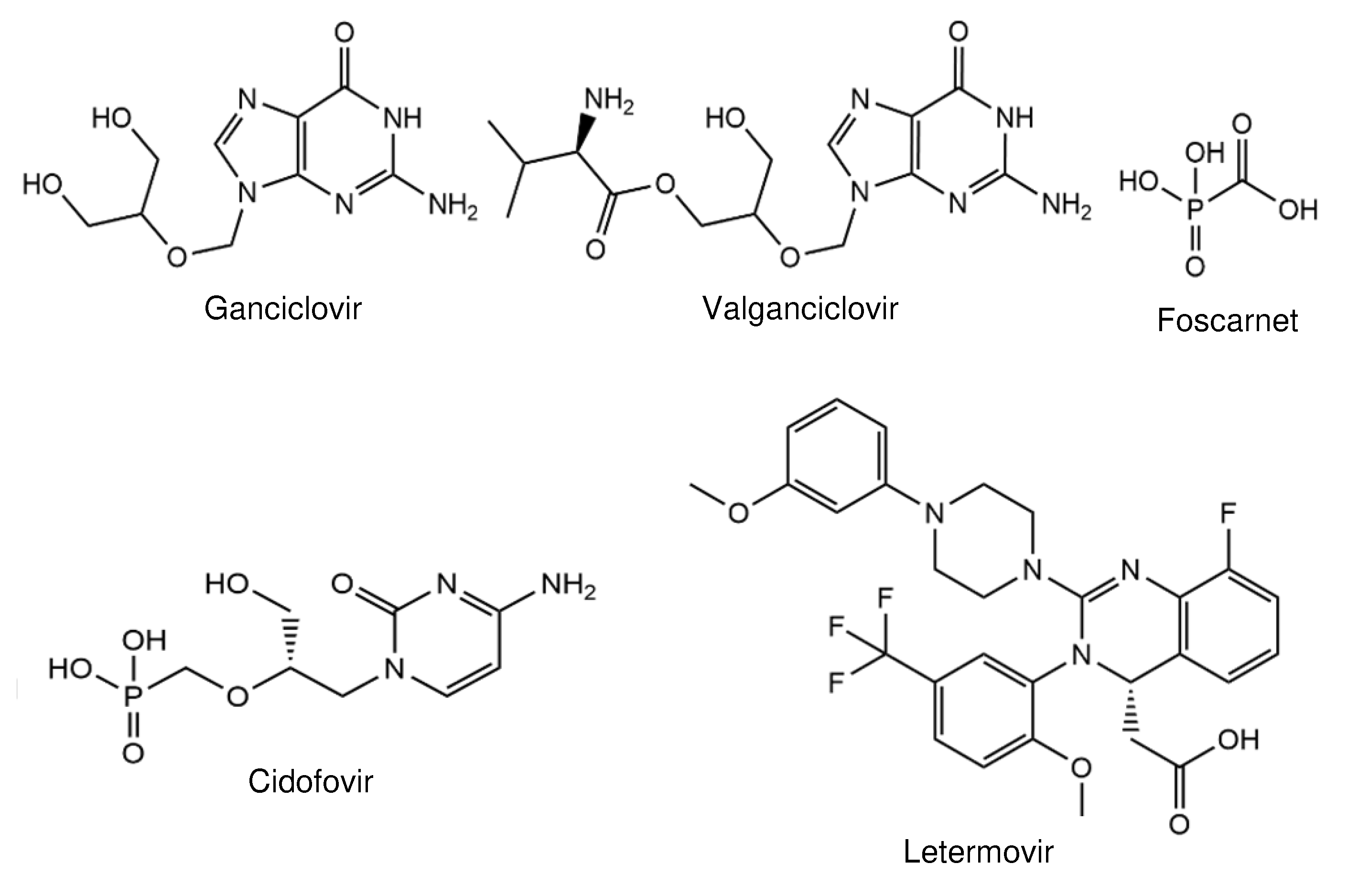
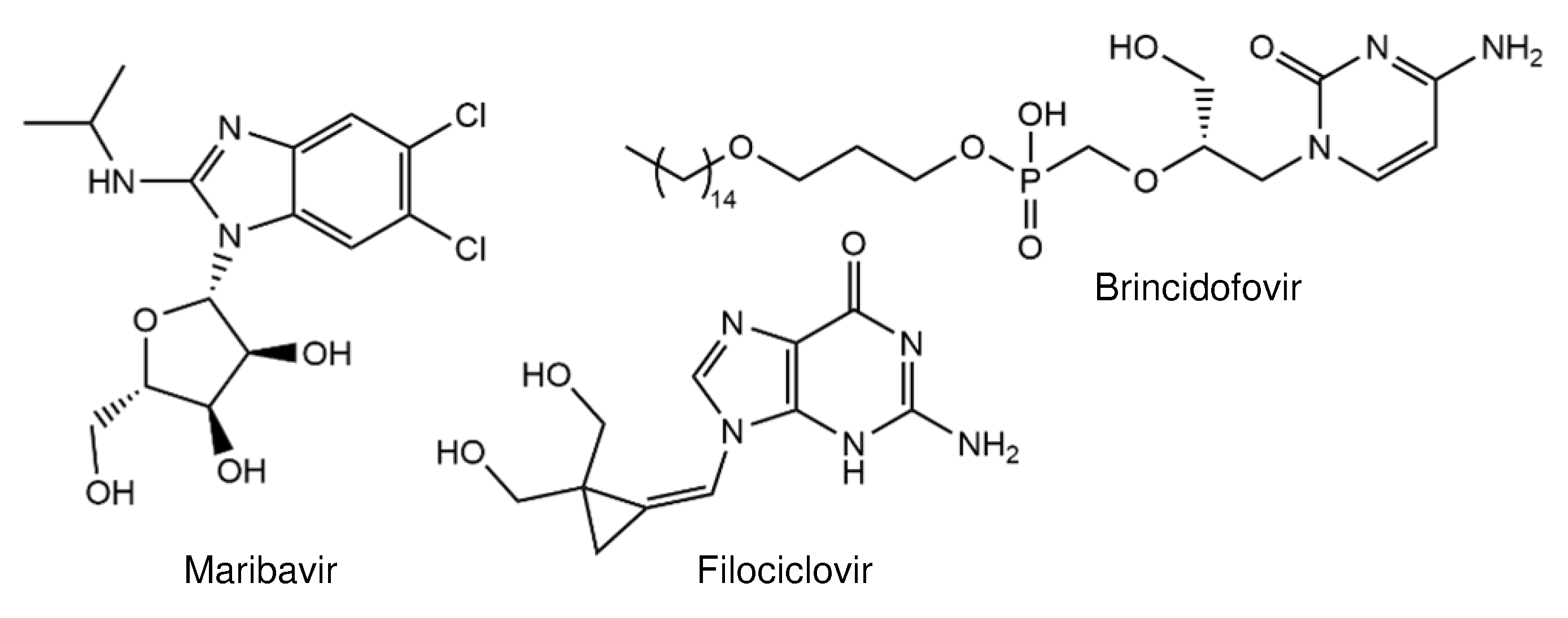
2. Compounds in Clinical Trials
3. Novel Inhibitors Targeting Host-Cell Factors
3.1. Dispirotripiperazines—Blocking Entry
3.2. Artemisinins Bind Vimentin
4. Novel Inhibitors Targeting Viral Proteins
4.1. Nucleoside Inhibitors
4.2. Quinazoline Targeting HCMV Kinase
4.3. Inhibitor of the Small Terminase Subunit pUL89
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramanan, P.; Razonable, R.R. Cytomegalovirus Infections in Solid Organ Transplantation: A Review. Infect. Chemother. 2013, 45, 260–271. [Google Scholar] [CrossRef]
- Sinzger, C.; Digel, M.; Jahn, G. Cytomegalovirus Cell Tropism. Curr. Top. Microbiol. Immunol. 2008, 325, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Biron, K.K. Antiviral drugs for cytomegalovirus diseases. Antivir. Res. 2006, 71, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Chou, S. Antiviral drug resistance in human cytomegalovirus. Transpl. Infect. Dis. 1999, 1, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Crumpacker, C.S. Ganciclovir. N. Engl. J. Med. 1996, 335, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Razonable, R.R. Drug-resistant cytomegalovirus: Clinical implications of specific mutations. Curr. Opin. Organ Transplant. 2018, 23, 388–394. [Google Scholar] [CrossRef]
- Erice, A.; Chou, S.; Biron, K.K.; Stanat, S.C.; Balfour, H.H., Jr.; Jordan, M.C. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised patients. N. Engl. J. Med. 1989, 320, 289–293. [Google Scholar] [CrossRef]
- Lurain, N.S.; Chou, S. Antiviral Drug Resistance of Human Cytomegalovirus. Clin. Microbiol. Rev. 2010, 23, 689–712. [Google Scholar] [CrossRef]
- Chemaly, R.F.; Ullmann, A.J.; Stoelben, S.; Richard, M.P.; Bornhäuser, M.; Groth, C.; Einsele, H.; Silverman, M.; Mullane, K.M.; Brown, J.; et al. Letermovir for Cytomegalovirus Prophylaxis in Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2014, 370, 1781–1789. [Google Scholar] [CrossRef]
- Bogner, E.; Radsak, K.; Stinski, M.F. The Gene Product of Human Cytomegalovirus Open Reading Frame UL56 Binds the pac Motif and Has Specific Nuclease Activity. J. Virol. 1998, 72, 2259–2264. [Google Scholar] [CrossRef]
- Scheffczik, H.; Savva, C.G.W.; Holzenburg, A.; Kolesnikova, L.; Bogner, E. The terminase subunits pUL56 and pUL89 of human cytomegalovirus are DNA metabolizing proteins with toroidal structure. Nucleic Acids Res. 2002, 30, 1695–1703. [Google Scholar] [CrossRef]
- Thoma, C.; Borst, E.; Messerle, M.; Rieger, M.; Hwang, J.-S.; Bogner, E. Identification of the Interaction Domain of the Small Terminase Subunit pUL89 with the Large Subunit pUL56 of Human Cytomegalovirus. Biochemistry 2006, 45, 8855–8863. [Google Scholar] [CrossRef]
- Theiß, J.; Sung, M.W.; Holzenburg, A.; Bogner, E. Full-length human cytomegalovirus terminase pUL89 adopts a two-domain structure specific for DNA packaging. PLoS Pathog. 2019, 15, e1008175. [Google Scholar] [CrossRef]
- Borst, E.M.; Kleine-Albers, J.; Gabaev, I.; Babić, M.; Wagner, K.; Binz, A.; Degenhardt, I.; Kalesse, M.; Jonjić, S.; Bauerfeind, R.; et al. The Human Cytomegalovirus UL51 Protein Is Essential for Viral Genome Cleavage-Packaging and Interacts with the Terminase Subunits pUL56 and pUL89. J. Virol. 2013, 87, 1720–1732. [Google Scholar] [CrossRef]
- Chou, S. RapidIn VitroEvolution of Human Cytomegalovirus UL56 Mutations That Confer Letermovir Resistance. Antimicrob. Agents Chemother. 2015, 59, 6588–6593. [Google Scholar] [CrossRef]
- James, S.H. Letermovir Resistance in Hematopoietic Stem Cell Transplant Recipients: The Risks Associated with Cytomegalovirus Prophylaxis. J. Infect. Dis. 2020, 221, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.M.; Barnard, R.; Holder, D.; Leavitt, R.; Levitan, D.; Maguire, M.; Nickle, D.; Teal, V.; Wan, H.; Van Alewijk, D.C.J.G.; et al. Letermovir Resistance Analysis in a Clinical Trial of Cytomegalovirus Prophylaxis for Hematopoietic Stem Cell Transplant Recipients. J. Infect. Dis. 2020, 221, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zemlicka, J.; Kern, E.R.; Drach, J.C. Fluoroanalogues of Anti-Cytomegalovirus Agent Cyclopropavir: Synthesis and Antiviral Activity of (E)- and (Z)-9- {[2,2-Bis(Hydroxymethyl)-3-Fluorocyclopropylidene]Methyl}-Adenines and Guanines. Nucleosides Nucleotides Nucleic Acids 2007, 26, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Kern, E.R.; Bidanset, D.J.; Hartline, C.B.; Yan, Z.; Zemlicka, J.; Quenelle, D.C. Oral Activity of a Methylenecyclopropane Analog, Cyclopropavir, in Animal Models for Cytomegalovirus Infections. Antimicrob. Agents Chemother. 2004, 48, 4745–4753. [Google Scholar] [CrossRef]
- Rouphael, N.G.; Hurwitz, S.J.; Hart, M.; Beck, A.; Anderson, E.J.; Deye, G.; Osborn, B.; Cai, S.Y.; Focht, C.; Amegashie, C.; et al. Phase Ib Trial to Evaluate the Safety and Pharmacokinetics of Multiple Ascending Doses of Filociclovir (MBX-400, Cyclopropavir) in Healthy Volunteers. Antimicrob. Agents Chemother. 2019, 63, e00717-19. [Google Scholar] [CrossRef] [PubMed]
- Florescu, D.F.; Keck, M.A. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev. Anti-Infect. Ther. 2014, 12, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Winston, D.J.; Rowley, S.D.; Vance, E.; Papanicolaou, G.A.; Mullane, K.M.; Brundage, T.M.; Robertson, A.T.; Godkin, S.; Momméja-Marin, H.; et al. CMX001 to Prevent Cytomegalovirus Disease in Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2013, 369, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Winston, D.J.; Chemaly, R.F.; Mullane, K.M.; Shore, T.B.; Papanicolaou, G.A.; Chittick, G.; Brundage, T.M.; Wilson, C.; Morrison, M.E.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial of Oral Brincidofovir for Cytomegalovirus Prophylaxis in Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 369–381. [Google Scholar] [CrossRef]
- Krosky, P.M.; Baek, M.-C.; Coen, D.M. The Human Cytomegalovirus UL97 Protein Kinase, an Antiviral Drug Target, Is Required at the Stage of Nuclear Egress. J. Virol. 2003, 77, 905–914. [Google Scholar] [CrossRef]
- Prichard, M.N. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev. Med. Virol. 2009, 19, 215–229. [Google Scholar] [CrossRef]
- Biron, K.K.; Harvey, R.J.; Chamberlain, S.C.; Good, S.S.; Smith, A.A., III; Davis, M.G.; Talarico, C.L.; Miller, W.H.; Ferris, R.; Dornsife, R.E.; et al. Potent and selective inhibition of human cytomeg-alovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob. Agents Chemother. 2002, 46, 2365–2372. [Google Scholar] [CrossRef]
- Williams, S.L.; Hartline, C.B.; Kushner, N.L.; Harden, E.A.; Bidanset, D.J.; Drach, J.C.; Townsend, L.B.; Underwood, M.R.; Biron, K.K.; Kern, E.R. In Vitro Activities of Benzimidazole d- and l-Ribonucleosides against Herpesviruses. Antimicrob. Agents Chemother. 2003, 47, 2186–2192. [Google Scholar] [CrossRef]
- Winston, D.J.; Young, J.-A.H.; Pullarkat, V.; Papanicolaou, G.A.; Vij, R.; Vance, E.; Alangaden, G.J.; Chemaly, R.F.; Petersen, F.; Chao, N.; et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood 2008, 111, 5403–5410. [Google Scholar] [CrossRef]
- Chou, S.; Song, K.; Wu, J.; Bo, T.; Crumpacker, C. Drug resistance mutations and associated phenotypes detected in clinical trials of maribavir for treatment of cytomegalovirus infection. J. Infect. Dis. 2020, jiaa462. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Bogner, E.; Novoselova, E.; Zorn, K.M.; Ekins, S.; Makarov, V. Dispirotripiperazine-core compounds, their biological activity with a focus on broad antiviral property, and perspectives in drug design (mini-review). Eur. J. Med. Chem. 2021, 211, 113014. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, M.; Karger, A.; Meerbach, A.; Egerer, R.; Stelzner, A.; Makarov, V. Binding of a N,N′-bisheteryl derivative of dispirotripiperazine to heparan sulfate residues on the cell surface specifically prevents infection of viruses from different families. Virology 2003, 311, 134–143. [Google Scholar] [CrossRef]
- Paeschke, R.; Woskobojnik, I.; Makarov, V.; Schmidtke, M.; Bogner, E. DSTP-27 Prevents Entry of Human Cytomegalovirus. Antimicrob. Agents Chemother. 2014, 58, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Kaminka, M.E.; Kalinkina, M.A.; Pushkina, T.V.; Tupikina, S.M.; Riabova, O.B.; Makarov, V.A.; Granik, V.G. Antiulcer ac-tivity of furoxanopyrimidine derivatives. Eksp. Klin. Farm. 2004, 67, 30–33. [Google Scholar]
- Adfeldt, R.; Schmitz, J.; Kropff, B.; Thomas, M.; Monakhova, N.; Hölper, J.E.; Klupp, B.G.; Mettenleiter, T.C.; Makarov, V.; Bogner, E. Diazadispiroalkane derivatives—new viral entry inhibitors. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef]
- Efferth, T.; Marschall, M.; Wang, X.; Huong, S.-M.; Hauber, I.; Olbrich, A.; Kronschnabl, M.; Stamminger, T.; Huang, E.-S. Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J. Mol. Med. 2001, 80, 233–242. [Google Scholar] [CrossRef]
- Kaptein, S.J.F.; Efferth, T.; Leis, M.; Rechter, S.; Auerochs, S.; Kalmer, M.; Bruggeman, C.A.; Vink, C.; Stamminger, T.; Marschall, M. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antivir. Res. 2006, 69, 60–69. [Google Scholar] [CrossRef]
- Roy, S.; Kapoor, A.; Zhu, F.; Mukhopadhyay, R.; Ghosh, A.K.; Lee, H.; Mazzone, J.; Posner, G.H.; Arav-Boger, R. Artemisinins target the intermediate filament protein vimentin for human cytomegalovirus inhibition. J. Biol. Chem. 2020, 295, 15013–15028. [Google Scholar] [CrossRef]
- Shapira, M.Y.; Resnick, I.B.; Chou, S.; Neumann, A.U.; Lurain, N.S.; Stamminger, T.; Caplan, O.; Saleh, N.; Efferth, T.; Marschall, M.; et al. Artesunate as a Potent Antiviral Agent in a Patient with Late Drug-Resistant Cytomegalovirus Infection after Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2008, 46, 1455–1457. [Google Scholar] [CrossRef]
- Germi, R.; Mariette, C.; Alain, S.; Lupo, J.; Thiebaut, A.; Brion, J.; Epaulard, O.; Raymond, C.S.; Malvezzi, P.; Morand, P. Success and failure of artesunate treatment in five transplant recipients with disease caused by drug-resistant cytomegalovirus. Antivir. Res. 2014, 101, 57–61. [Google Scholar] [CrossRef]
- Wolf, D.G.; Shimoni, A.; Resnick, I.B.; Stamminger, T.; Neumann, A.U.; Chou, S.; Efferth, T.; Caplan, O.; Rose, J.; Nagler, A.; et al. Human cytomegalovirus kinetics following institution of artesunate after hematopoietic stem cell transplantation. Antivir. Res. 2011, 90, 183–186. [Google Scholar] [CrossRef][Green Version]
- Oiknine-Djian, E.; Weisblum, Y.; Panet, A.; Wong, H.N.; Haynes, R.K.; Wolf, D.G. The Artemisinin Derivative Artemisone Is a Potent Inhibitor of Human Cytomegalovirus Replication. Antimicrob. Agents Chemother. 2018, 62, e00288-18. [Google Scholar] [CrossRef]
- Oiknine-Djian, E.; Bar-On, S.; Laskov, I.; Lantsberg, D.; Haynes, R.K.; Panet, A.; Wolf, D.G. Artemisone demonstrates synergistic antiviral activity in combination with approved and experimental drugs active against human cytomegalovirus. Antivir. Res. 2019, 172, 104639. [Google Scholar] [CrossRef]
- Haynes, R.K.; Fugmann, B.; Stetter, J.; Rieckmann, K.; Heilmann, H.-D.; Chan, H.-W.; Cheung, M.-K.; Lam, W.-L.; Wong, H.-N.; Croft, S.L.; et al. Artemisone—A Highly Active Antimalarial Drug of the Artemisinin Class. Angew. Chem. Int. Ed. 2006, 45, 2082–2088. [Google Scholar] [CrossRef]
- Kozlovskaya, L.I.; Andrei, G.; Orlov, A.A.; Khvatov, E.V.; Koruchekov, A.A.; Belyaev, E.S.; Nikolaev, E.N.; Korshun, V.A.; Snoeck, R.; Osolodkin, D.I.; et al. Antiviral activity spectrum of phenoxazine nucleoside derivatives. Antivir. Res. 2019, 163, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Paramonova, M.P.; Khandazhinskaya, A.L.; Ozerov, A.A.; Kochetkov, S.N.; Snoeck, R.; Andrei, G.; Novikov, M.S. Synthesis and antiviral properties of 1-substituted 3-[ω-(4-oxoquinazolin-4(3h)-yl) alkyl] uracil derivatives. Acta Naturae 2020, 12, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Hutterer, C.; Hamilton, S.; Steingruber, M.; Zeittraeger, I.; Bahsi, H.; Thuma, N.; Naing, Z.; Oerfi, Z.; Oerfi, L.; Socher, E.; et al. The chemical class of quinazoline compounds provides a core structure for the design of anticytomegaloviral kinase inhibitors. Antivir. Res. 2016, 134, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, T.; Reiter, C.; Ibrahim, M.M.; Beutel, J.; Hutterer, C.; Zeitträger, I.; Bahsi, H.; Leidenberger, M.; Friedrich, O.; Kappes, B.; et al. Synthesis of Novel Hybrids of Quinazoline and Artemisinin with High Activities against Plasmodium falciparum, Human Cytomegalovirus, and Leukemia Cells. ACS Omega 2017, 2, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
- Gentry, B.G.; Bogner, E.; Drach, J.C. Targeting the terminase: An important step forward in the treatment and prophylaxis of human cytomegalovirus infections. Antivir. Res. 2019, 161, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mao, L.; Kankanala, J.; Wang, Z.; Geraghty, R.J. Inhibition of Human Cytomegalovirus pUL89 Terminase Subunit Blocks Virus Replication and Genome Cleavage. J. Virol. 2016, 91, e02152-16. [Google Scholar] [CrossRef] [PubMed]
- Kankanala, J.; Kirby, K.A.; Liu, F.; Miller, L.; Nagy, E.; Wilson, D.J.; Parniak, M.A.; Sarafianos, S.G.; Wang, Z. Design, Synthesis, and Biological Evaluations of Hydroxypyridonecarboxylic Acids as Inhibitors of HIV Reverse Transcriptase Associated RNase H. J. Med. Chem. 2016, 59, 5051–5062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, J.; Wang, Z.; Geraghty, R.J. Metal-chelating 3-hydroxypyrimidine-2,4-diones inhibit human cytomegalovirus pUL89 endonuclease activity and virus replication. Antivir. Res. 2018, 152, 10–17. [Google Scholar] [CrossRef] [PubMed]
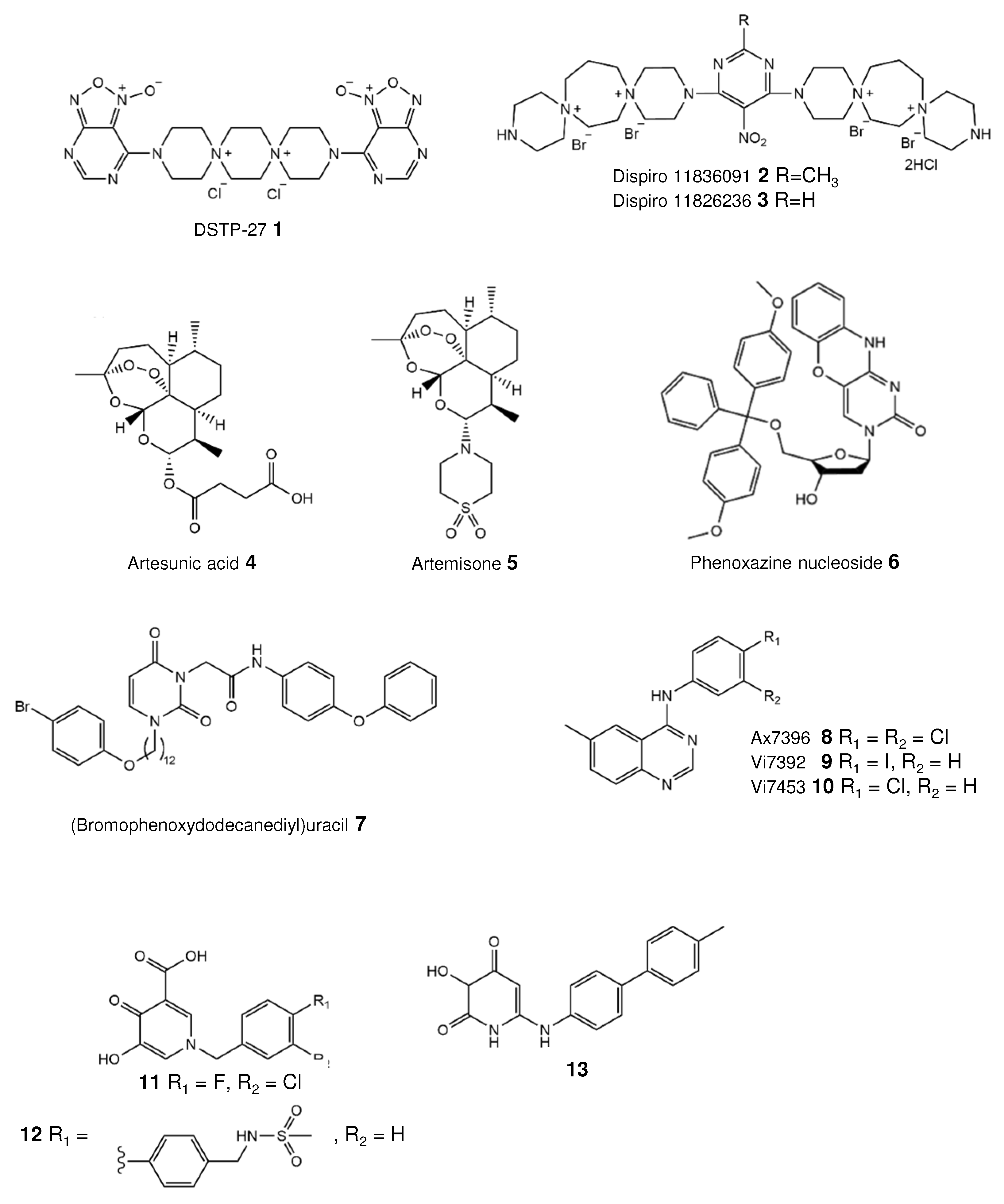
| Compound Name | IC50 (µM) | Mechanism of Action | Ref. |
|---|---|---|---|
| Dispirotripiperazines 11826091 and 11826236 | 1.38–8.95 | Blocking virus attachment by binding cell-surface heparan sulfates | [31] |
| Artesunic acid | 5–15 | Binding of vimentin, a filament protein | [35,37] |
| Artemisone | 0.22–1.46 | Targeting an early phase of the viral replication cycle | [41] |
| Phenoxazine nucleoside 6 | 4 | Nucleoside mimetic | [44] |
| (Bromophenoxydodecanediyl) uracil 7 | 0.8–1.52 | Nucleoside mimetic | [45] |
| Quinazolines Vi7392 and Vi7453 | 0.96–3.31 | Targeting viral protein kinase pUL97 | [46] |
| Hydroxypyridonecarboxylic acid 11 | 4 | Inhibition of the small terminase subunit pUL89 | [49] |
| 3-Hydroxy-pyridinedione 13 | 1.2 | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogner, E.; Egorova, A.; Makarov, V. Small Molecules—Prospective Novel HCMV Inhibitors. Viruses 2021, 13, 474. https://doi.org/10.3390/v13030474
Bogner E, Egorova A, Makarov V. Small Molecules—Prospective Novel HCMV Inhibitors. Viruses. 2021; 13(3):474. https://doi.org/10.3390/v13030474
Chicago/Turabian StyleBogner, Elke, Anna Egorova, and Vadim Makarov. 2021. "Small Molecules—Prospective Novel HCMV Inhibitors" Viruses 13, no. 3: 474. https://doi.org/10.3390/v13030474
APA StyleBogner, E., Egorova, A., & Makarov, V. (2021). Small Molecules—Prospective Novel HCMV Inhibitors. Viruses, 13(3), 474. https://doi.org/10.3390/v13030474






