Abstract
The concerning increase in HIV-1 resistance argues for prioritizing the development of host-targeting antiviral drugs because such drugs can offer high genetic barriers to the selection of drug-resistant viral variants. Targeting host proteins could also yield drugs that act on viral life cycle events that have proven elusive to inhibition, such as intracellular events of HIV-1 immature capsid assembly. Here, we review small molecule inhibitors identified primarily through HIV-1 self-assembly screens and describe how all act either narrowly post-entry or broadly on early and late events of the HIV-1 life cycle. We propose that a different screening approach could identify compounds that specifically inhibit HIV-1 Gag assembly, as was observed when a potent rabies virus inhibitor was identified using a host-catalyzed rabies assembly screen. As an example of this possibility, we discuss an antiretroviral small molecule recently identified using a screen that recapitulates the host-catalyzed HIV-1 capsid assembly pathway. This chemotype potently blocks HIV-1 replication in T cells by specifically inhibiting immature HIV-1 capsid assembly but fails to select for resistant viral variants over 37 passages, suggesting a host protein target. Development of such small molecules could yield novel host-targeting antiretroviral drugs and provide insight into chronic diseases resulting from dysregulation of host machinery targeted by these drugs.
Keywords:
HIV-1 assembly; HIV-1 capsid; Gag; antiretroviral; antiviral; drug screen; viral-host interactions; ABCE1; DDX6; RNA granule 2. Spontaneous Assembly or Host-Catalyzed Assembly of HIV-1 Gag? Two Models with Implications for Assembly Inhibitors
The working model one uses to study a stage of the viral life cycle influences the design of drugs screens used to identify inhibitors, which in turn results in some inhibitors being identified while other promising inhibitors are missed—thus the starting model matters. For decades the dominant model for understanding Gag multimerization has been the self-assembly model, which proposes that Gag polypeptides multimerize spontaneously in the presence of nucleic acids due to intrinsic properties that promote Gag-RNA and Gag-Gag interactions (reviewed in [30,31,32]). This model has been supported by in vitro studies in which assembly of recombinant Gag peptides is studied in the presence of nucleic acids but in the absence of the host proteins that are present when Gag assembles in cells. These studies have revealed important properties of Gag domains in promoting Gag-Gag and Gag-RNA interactions. They have also evolved over time to incorporate a role for host phospholipids such as IP6 in self-assembly [31]. However, evidence, described below, that intracellular Gag associates with viral and cellular RNA by trafficking to host ribonucleoprotein (RNP) complexes has not been incorporated into the self-assembly model [31,33,34].
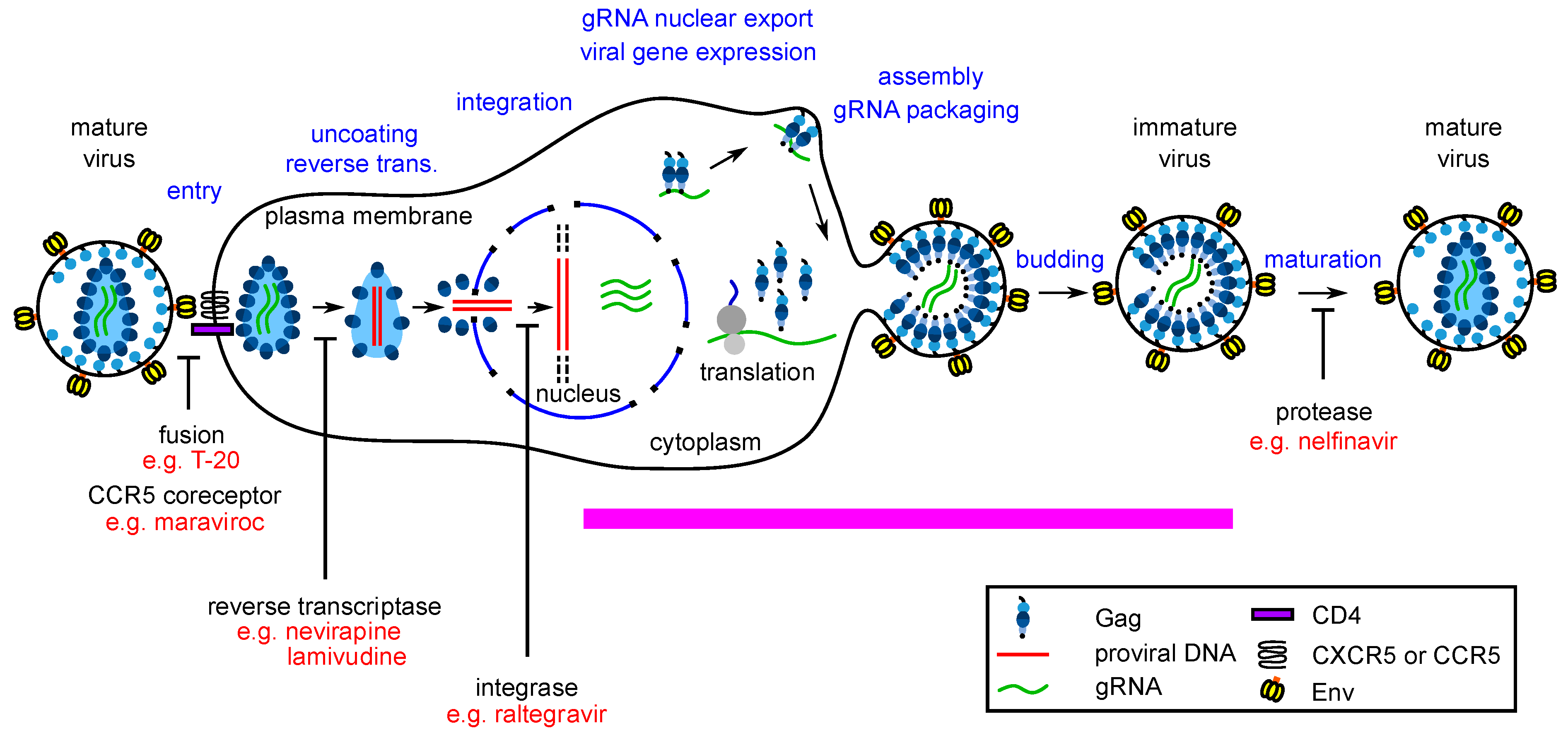
Data in support of host-catalyzed Gag assembly was first generated when, in an attempt to better approximate HIV-1 infection in vivo, assembly of nascent Gag polypeptides was studied in the context of host proteins, using cell extracts (a cell-free system). Unlike self-assembly studies, which examine recombinant Gag at high concentrations in the complete absence of host proteins [35,36], the cell-free system allowed a direct test of the hypothesis that Gag assembles spontaneously in a cellular environment. In the cell-free system, where newly synthesized Gag is present at concentrations more typical of what is observed in infected cells, Gag was found to assemble into particles that closely resembled immature capsids in an energy-dependent manner—not spontaneously; moreover, the energy dependence was post-translational and was therefore independent of the energy requirement expected during Gag synthesis [37,38]. Pulse-chase studies revealed that during assembly in the cell-free system as well as in cells, Gag progresses through a pathway of assembly intermediates that are transient, sequential multiprotein complexes of different sizes, defined by their approximate sedimentation (S) values (~10S, ~80S/150S, and ~500S) [37,39]. Progression of Gag along this pathway culminates in formation of the ~750S completely assembled immature capsid. The order of assembly intermediates revealed by pulse-chase studies [37,39] was confirmed by analysis of assembly-defective Gag mutants, each of which was found to be arrested at a characteristic step in the pathway ([37,39,40,41,42]; Figure 2). ATP hydrolysis is also required at a discrete point in the assembly pathway ([37]; Figure 2). Since HIV-1 Gag does not bind and hydrolyze ATP, these findings indicated that in a cellular context, Gag assembly is energy-dependent and catalyzed by host enzymes. Consistent with this observation, two host ATPases were subsequently identified as facilitators of HIV-1 Gag assembly. The first was ATP-Binding Cassette protein E1 (ABCE1), an essential and highly conserved enzyme found in eukaryotes and archaebacteria. Knockout of ABCE1 leads to rapid cell death [43] most likely because it is required for recycling of ribosomes (reviewed in [44]); thus, the role of ABCE1 in promoting post-translational steps in immature capsid assembly was demonstrated using depletion and reconstitution experiments in cell extracts and dominant negative experiments in cells [45]. Subsequently, a second ATPase, the Dead-box RNA helicase DDX6, was also shown to facilitate HIV-1 immature capsid assembly in cells, with loss of assembly upon knockdown, and rescue of assembly by wild-type DDX6 but not by an ATPase deficient DDX6 mutant [46].
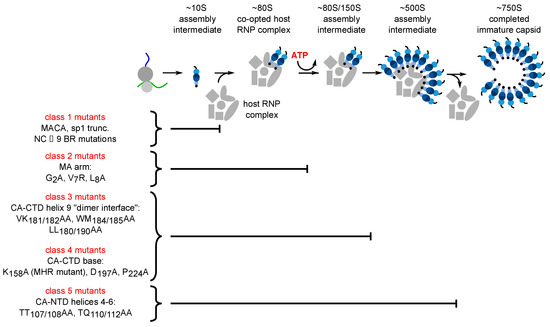
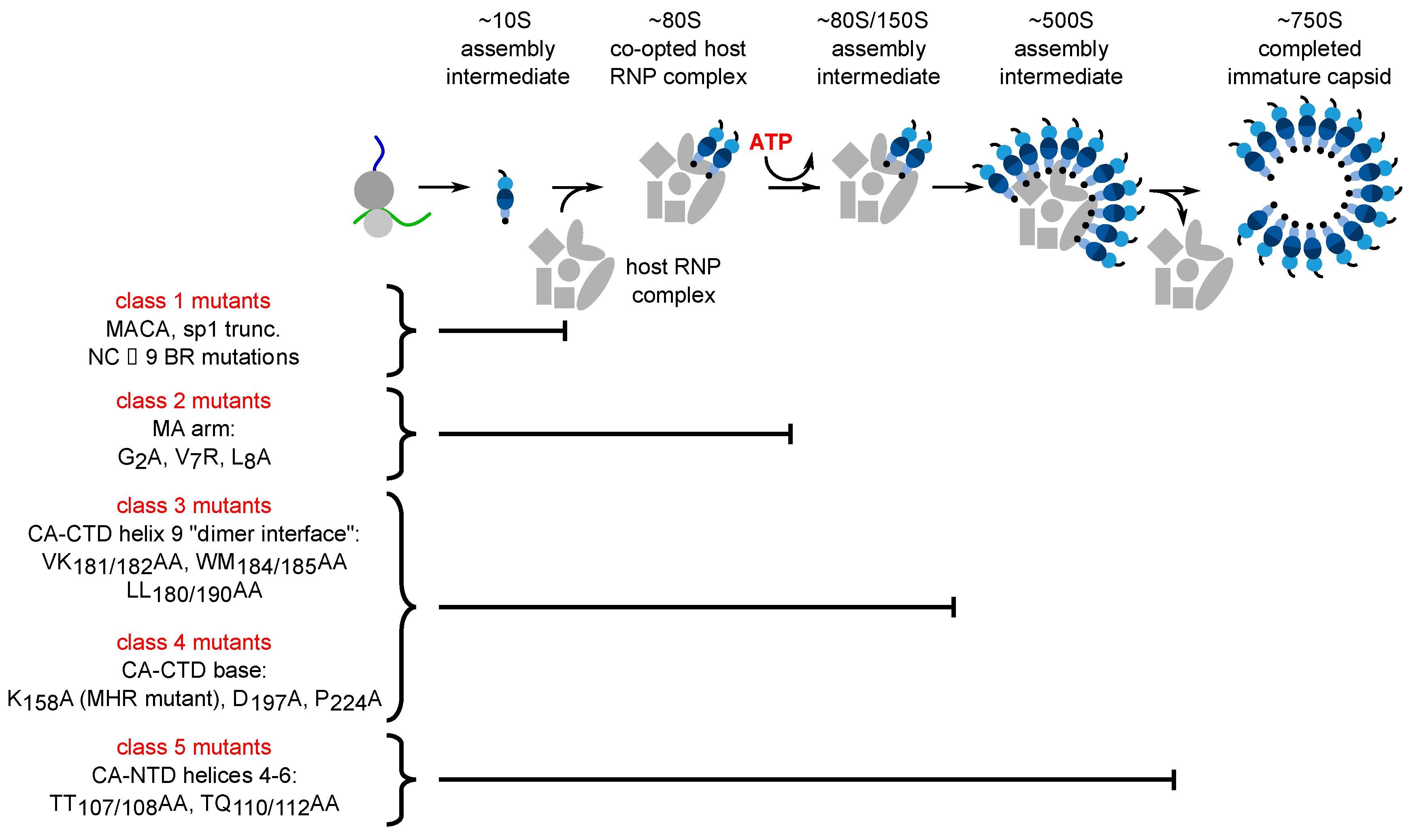
Figure 2.
Each assembly-defective Gag mutant is arrested at a characteristic point in the HIV-1 capsid assembly pathway. Initial studies in the cell-free system and in HIV-1-expressing cells revealed that Gag progresses through a stepwise pathway of multiprotein complexes defined as intermediates in a pathway of assembly based on pulse-chase experiments (reviewed in [47])). Subsequent experiments confirmed the order of intermediates in this pathway by showing that each assembly-defective Gag mutant is arrested at a characteristic point in this pathway. Altogether five different categories of mutants have been identified (Class 1–5), one for each point in the pathway. The difference between Class 3 and Class 4 being that Class 3 mutants are arrested as cytosolic ~80S complexes while Class 4 mutants are arrested as PM-associated ~80S complexes, suggesting that the ~80S assembly intermediate is the complex in which assembling Gag traffics from the cytosol to the PM. Later studies identified some of the host proteins in the assembly intermediates. Relevant references are in the text.
Figure 2.
Each assembly-defective Gag mutant is arrested at a characteristic point in the HIV-1 capsid assembly pathway. Initial studies in the cell-free system and in HIV-1-expressing cells revealed that Gag progresses through a stepwise pathway of multiprotein complexes defined as intermediates in a pathway of assembly based on pulse-chase experiments (reviewed in [47])). Subsequent experiments confirmed the order of intermediates in this pathway by showing that each assembly-defective Gag mutant is arrested at a characteristic point in this pathway. Altogether five different categories of mutants have been identified (Class 1–5), one for each point in the pathway. The difference between Class 3 and Class 4 being that Class 3 mutants are arrested as cytosolic ~80S complexes while Class 4 mutants are arrested as PM-associated ~80S complexes, suggesting that the ~80S assembly intermediate is the complex in which assembling Gag traffics from the cytosol to the PM. Later studies identified some of the host proteins in the assembly intermediates. Relevant references are in the text.
The mechanisms by which ABCE1 and DDX6 promote Gag assembly have not been fully elucidated. However, in the case of DDX6, clues come from the known role of RNA helicases in RNP remodeling, a process by which proteins that are associated with RNAs and dictate RNA fates are replaced by other proteins [48]—thus, DDX6 could facilitate the association of Gag with viral RNA during assembly. In keeping with this possibility, in HIV-1 expressing cells DDX6 is known to colocalize with HIV-1 genomic RNA at PM sites of virus assembly and budding [49]. HIV-1 Gag is also colocalized with DDX6 and ABCE1 in situ, including at the PM at sites of budding [29,39,41,46,49,50]. Together the data support a model in which, in cells, newly synthesized Gag traffics to—and assembles in—specific host RNP complexes that contain both the HIV-1 genomic RNA that is packaged into the assembling virus and host enzymes that facilitate the assembly and packaging process (Figure 3A). Additionally, by sequestering the assembly process within host RNP complexes where non-translating cellular RNAs are stored, the virus is likely able to shield assembling progeny virus from host innate immune effectors.
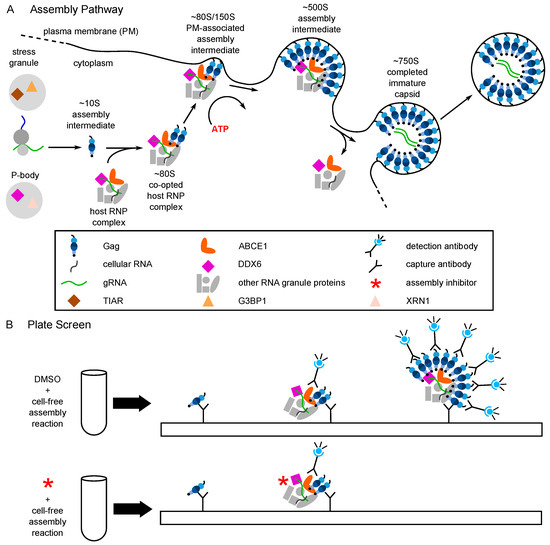
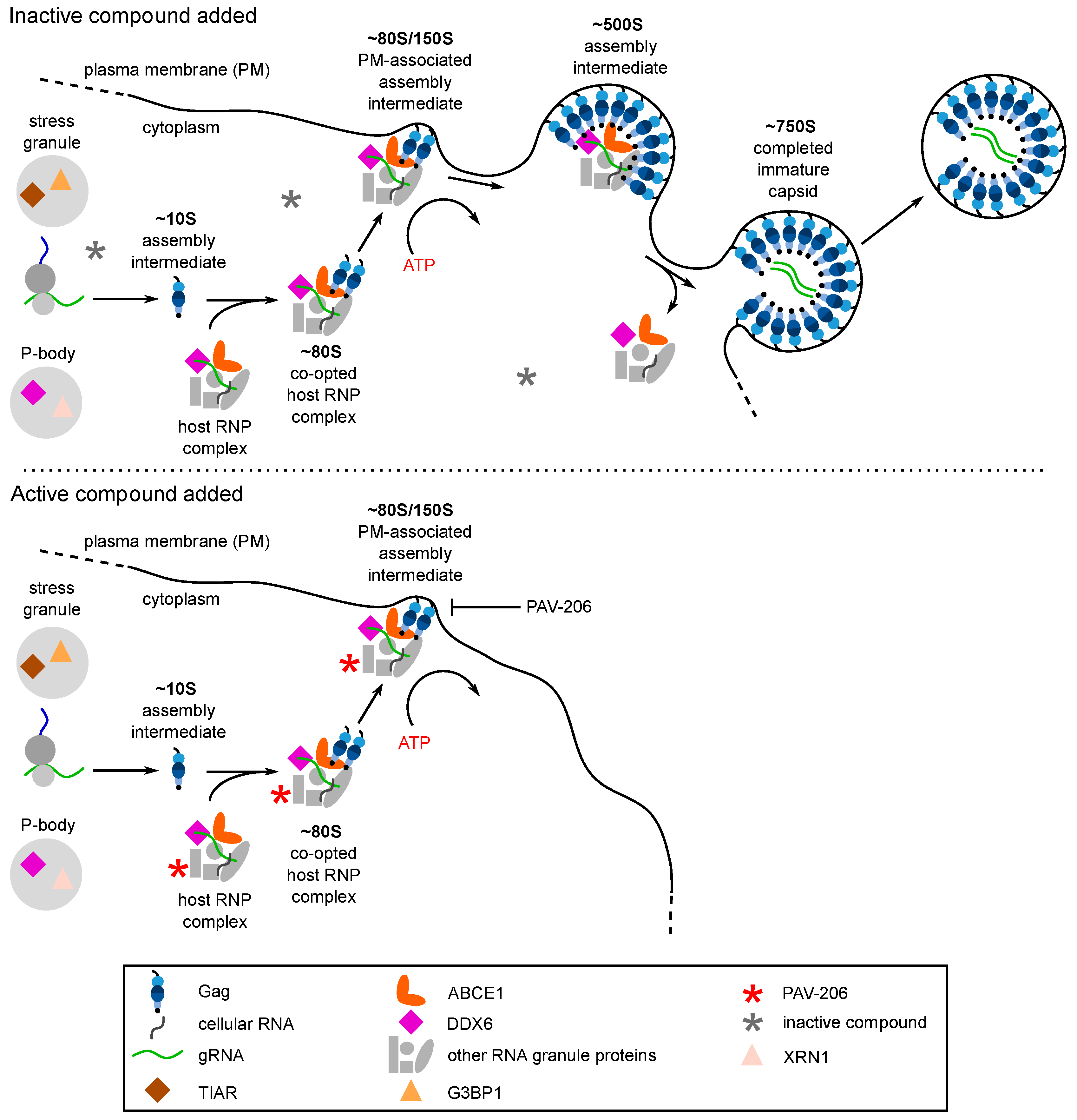
Figure 3.
The host-catalyzed HIV-1 assembly pathway was developed into a screen for small molecule inhibitors. (A) Schematic showing the host-catalyzed HIV-1 assembly pathway, starting with Gag synthesis and formation of the early ~10S assembly intermediate. The ~80S assembly intermediate is formed when ~10S Gag co-opts a host RNP complex containing ABCE1 and DDX6, two host enzymes that have been shown to facilitate assembly. The co-opted host RNP complex is distinct from but related to P-bodies and stress granules, which are larger host RNP complexes. The ~80S assembly intermediate targets to the plasma membrane where Gag multimerization continues, resulting in sequential formation of the ~150S and ~500S assembly intermediates and the fully assembled ~750S immature capsid. Upon completion of assembly, the host RNP complex is released. Relevant references are in the text. (B) A cell-free protein synthesis and assembly plate screen that recapitulates the host-catalyzed HIV-1 assembly pathway was developed and utilized to identify small molecule inhibitors of the pathway [29]. In this screen, a capture antibody directed against Gag binds Gag monomers, oligomers, and multimers generated in a cell-free assembly reaction. The same anti-Gag antibody is used as a detection antibody that binds to captured oligomers and multimers, but not monomers whose binding site is occupied. The signal produced by the detection antibody is proportional to the amount of anti-Gag binding with a larger fluorescent signal indicating more extensive multimerization. The upper diagram shows the large signal that is produced when the HIV-1 cell-free assembly reaction is carried out in the presence of DMSO, which does not inhibit Gag assembly. The lower diagram shows inhibition of that signal when an inhibitor of Gag assembly is added at the start of the cell-free reaction. Legend in the middle applies to panels (A) and (B).
Despite fundamental differences between the host-catalyzed and spontaneous models of Gag assembly, there are many ways in which the host-catalyzed model complements the self-assembly model. For example, the self-assembly studies show that RNA plays a key role in promoting Gag-Gag interactions [32]. However, unlike self-assembly reactions that contain recombinant Gag and purified RNA, the cytoplasm lacks soluble RNA, with all host and viral RNAs being instead sequestered within multiprotein complexes containing host RNPs [49]. Indeed, the naked viral RNA molecules shown in many viral life cycle diagrams are problematic given the lack of evidence for naked viral RNA genomes in the cytoplasm of infected cells. Data in support of the host-catalyzed model demonstrate that Gag associates with RNA in cells by trafficking to host RNP complexes, thereby forming assembly intermediates that contain Gag, HIV-1 genomic RNA, ABCE1, DDX6, and other host proteins [41,49]. Thus, assembly intermediates appear to be formed when Gag co-opts host RNP complexes that exist in uninfected host cells [49], setting the stage for Gag to replace host RNPs associated with HIV-1 genomic RNA. The RNP complexes that are co-opted resemble host RNA granules since they can be detected by light microscopy but are distinct from the much larger and better studied DDX6-containing RNA granules called P-bodies [29,49]. Like canonical RNA granules, the co-opted host RNP complexes are likely sites of host mRNA storage and metabolism, repurposed by the virus for the purpose of immature capsid assembly and genomic RNA packaging.
Similarly, data showing that HIV-1 utilizes host ATPases to facilitate virus assembly [45,46], thereby increasing the efficiency of virus progeny production, make sense from a broader virologic perspective given that viruses with larger genomes such as DNA viruses encode their own ATPases that function during packaging and assembly [51]. Thus, the host-catalyzed model of Gag assembly is in keeping with basic principles of virology and RNA cell biology.
In a recent paper Deng et al. argue against the existence of the stepwise pathway for HIV-1 assembly [52], but for multiple reasons this study is problematic. First, Deng et al. conclude that all Gag-containing complexes that comigrate with ribosomes likely consist of Gag-binding to ribosomes based solely on their finding that recombinant Gag-GFP purified from bacteria can bind to purified ribosomes, presumably nonspecifically, and without showing that Gag binds to ribosomes in cells by a similar mechanism [52]. They did not address the possibility that complexes containing Gag bound to ribosomes, if they exist in cells, could co-exist with similar sized Gag-containing complexes of an entirely different composition such as assembly intermediates. Notably, well established techniques allow assembly intermediates to be isolated away from other Gag-containing complexes of similar sizes, including ribosomes, by immunoprecipitation using antibodies directed against ABCE1 and RNA granule proteins, the host proteins that are components of these assembly intermediates [39,41,45,46,49,50,53,54]. As Deng et al. did not use such techniques to isolate assembly intermediates, it is likely that they were studying other complexes in addition to assembly intermediates, such as translating Gag associated with ribosomes, newly synthesized Gag, Gag undergoing degradation, and Gag in complexes that have partially dissociated. Indeed, the Gag-containing ribosomal complexes described by Deng et al. [52] are almost certainly not assembly intermediates, since assembly intermediates contain proteins that are not found in ribosomes, such as the RNA granule proteins DDX6, AGO2, and DCP2, and lack the abundant small ribosomal protein S6 [29,41,46,49,50,54]. Notably, complexes containing Gag and RNA granule proteins are not biochemical artifacts since they have also been found in situ using two different imaging techniques in multiple cell types [29,41,46,49,50,54].
A second reason why Deng et al. may have failed to detect assembly intermediates relates to their use of antibody-mediated detection of Gag epitopes to assess the multimerization state of Gag-containing complexes. This technique is problematic since it is well known that Gag epitopes become inaccessible as Gag multimerizes [55]. Given that completed immature capsids contain thousands of Gag proteins [56], the detection of mainly Gag monomers and dimers by Deng et al. (Figure 3 in [52]) raises the possibility that their approach simply excluded late assembly intermediates. Adding to that concern, no positive control was provided to demonstrate that their method is capable of capturing higher order Gag multimers. In a third problematic approach, Deng et al. argue that the smaller Gag-containing complexes found in cells do not behave like assembly intermediates since they are labeled equivalently during SILAC pulse labeling [52]. However, given that assembly happens very rapidly (in <15 min based on imaging in [23,57]), a pulse label of 2 h, the shortest pulse used by Deng et al. [52], would be expected to label many cohorts of assembling Gag and would actually approximate steady-state labeling which lacks the resolution needed to dissect the kinetics of a highly transient process. In contrast, the pulse-chase conditions that originally identified the HIV-1 assembly intermediates involved a much shorter pulse of radiolabel (4–15 min) followed by unlabeled chase periods of varying lengths, allowing small cohorts of assembling Gag to be followed as the size of these Gag-containing complexes changed over time and thereby establishing the progression of a small population of labeled Gag through complexes of increasing size [37,39]. Additionally, for pulse chase experiments performed in HIV-1-expressing cells, anti-ABCE1 immunoprecipitation was used to isolate Gag-containing assembly intermediates from other Gag-containing complexes [37,39]. At later times, much of the radiolabeled Gag that was observed initially in ~80S and later in ~500S ABCE1-containing complexes was no longer in those complexes, appearing instead in released virus like particles (VLPs) in the medium, suggesting that the ~80S and later the ~500S ABCE1-containing complexes are precursors to released VLPs (Figures 2 and 5 in [37,39]).
Finally, because Deng et al. find that the assembly-defective G2A Gag mutant forms a complex that is similar in size to our ~500S assembly intermediate, they argue that the ~500S complex formed by WT Gag must not be a late assembly intermediate. However, multiple groups have shown that non-myristoylated G2A Gag can overcome the assembly block, multimerize, and produce virus like particles in the cytoplasm [58,59,60], most likely due to overexpression. In the absence of controls for overexpression [52], Deng et al. cannot rule out that in their hands G2A multimerized to form late assembly intermediates and VLPs, with the VLPs being cytoplasmic as observed by others [58,59,60] and therefore not forming the puncta characteristic of membrane-associated assembly.
Thus, while many aspects of the assembly pathway we have proposed remain to be understood, the study by Deng et al. did not employ approaches that would have allowed them to make conclusions about HIV-1 assembly intermediates and therefore sheds little light on the stepwise pathway of HIV-1 capsid assembly. Those concerns having been noted, probably the best approach to validating any viral life cycle model is to use that model to identify novel classes of inhibitors that are predicted by the model and can also be used to further understand the model.
3. Drugs Screens Over Two Decades Have Identified Small Molecule Inhibitors That Bind to CA
Until now, two screening approaches have been used to identify small molecules that inhibit viral late events and/or bind to CA: (1) hypothesis-neutral cell-based replication assays; and (2) in vitro reactions that assay self-assembly of recombinant CA peptides or CA-NC peptides (the latter encompasses CA and the adjacent NC domain of Gag). As HIV-1 capsid inhibitors have been reviewed relatively recently [22], here we will describe some of the best studied small molecule inhibitors that have emerged from these two types of assembly screens.
A full viral replication screen yielded the CA-binding small molecule PF74 (PF-3450074; [61]). PF74 displays sub-micromolar potency and inhibits early post-entry events in the viral life cycle as well as integration and viral late events [62,63,64,65]. PF74 binds a conserved pocket in CA-NTD at the interface with CA-CTD that also serves as the binding site for the host factors cleavage and polyadenylation specific factor 6 (CPSF6) and nucleoporin 153 (NUP153); thus, PF74 may act as a competitive inhibitor of CPSF6 and NUP153 binding to CA [62,66,67]. Consistent with crystal structure data, resistance mutations that arise in CA upon selection with PF74 are located in the region of the PF74 binding site [68].
Most other screens for assembly inhibitors have involved self-assembly assays, which largely focus on self-assembly of recombinant CA, in part because of the critical role played by CA during immature and mature capsid assembly as well as during capsid disassembly. The CA domain of the Gag polyprotein contains residues critical for Gag-Gag interactions that mediate multimerization during assembly of the immature capsid. Subsequently, after Gag cleavage, CA is a key component in assembly of the mature capsid. After target cell entry, CA disassembly is required for reverse transcription, trafficking of the pre-integration complex to the nucleus, and integration (reviewed in [69]). CA consists of two subdomains, the N- and C-terminal domains of CA (CA-NTD and CA-CTD), whose crystal structures have been solved [70], allowing the self-assembly and replication assay screens described above to be complemented by in silico modeling.
A CA self-assembly assay was used to validate the first CA-targeting small molecule, CAP-1, which was initially identified in silico as a potential CA-binding compound [71]. CAP-1 results in production of non-infectious virions with abnormal cores [71] and binds to an induced hydrophobic pocket at the base of CA-NTD that is distinct from the PF74 binding pocket [71,72]. After identification of CAP-1, a high throughput CA-NC peptide self-assembly screen of a compound library identified two additional groups of small molecule inhibitors: benzimidazole (BM) compounds, which act in a manner similar to CAP-1 to produce non-infectious virus; and benzodiazepine (BD) compounds, which allow Gag processing but not release suggesting a budding defect [73]. BM and BD compounds bind to the same pocket in CA-NTD as CAP-1 but with expanded contacts, as shown in crystal structures and confirmed by selection in cell culture yielding resistance mutations in the region of the binding site in CA ([73]. While CAP-1 inhibits HIV-1 replication with a half maximal effective concentration (EC50) in the micromolar range [71], the best BD and BM compounds are more potent, with EC50s of 60–70 nM and a large difference between the 50% cytotoxic concentration (CC50) and the EC50 (CC50/EC50 > 300; [73]).
Other CA-binding small molecules have also been identified using variations of the self-assembly assay. For example, ebselen, an organoselenium compound, was identified using a high throughput screen that monitored recombinant CA-CTD peptide dimerization (the first step of self-assembly) using time-resolved fluorescence resonance energy transfer [74]. Ebselen binds to CA-CTD and inhibits viral replication at an early, post-entry stage with an EC50 of 3.2 µM and a CC50 of >30 µM in peripheral blood mononuclear cells (PBMCs) [74].
More recently, self-assembly screens yielded the most promising CA-binding small molecule identified to date, GS-6207 [75]. Both GS-6207 (GS-CA2) and the related small molecule GS-CA1 bind to the pocket at the interface of CA-NTD and CA-CTD that also binds PF74, CPSF6, and NUP153 [76,77]. In keeping with this, selection results in resistance mutations that map to this binding site. GS-6207 and GS-CA1 inhibit HIV-1 replication with EC50s of 60 pM and 32 pM, respectively, in human CD4+ primary T cells, and CC50 values of >50 µM [76,77]. Both GS-6207 and GS-CA1inhibit at multiple points in the early and late parts of the viral life cycle, as is the case for PF74, but with greater potency against early events. Thus, because of this broad targeting of early and late events, GS-6207 and GS-CA1 lack specificity. However, due in part to their very high potency, both compounds are amenable to long-acting injectable therapy [22,75], and GS-6207 has advanced to Phase 1 clinical trials, where it was found to be safe and well tolerated [76].
5. Identification of PAV-206, a Potent Small Molecule Inhibitor of HIV-1 Immature Capsid Assembly
The cell-free system in which the host-catalyzed HIV-1 assembly pathway was first identified was also used to develop an HIV-1 capsid assembly plate screen [29], analogous to that developed for RABV [78]. In this HIV-1 capsid assembly screen (Figure 3B), a cell extract is programmed with Gag mRNA, leading to synthesis of Gag polypeptides. These Gag polypeptides co-opt host RNP complexes that are present in the extract and contain the previously identified host facilitators of assembly, resulting in formation of sequential assembly intermediates. Plates containing wells coated with anti-Gag antibody result in capture of these Gag-containing assembly intermediates. A soluble anti-Gag antibody conjugated to a detection agent is added, resulting in a larger signal upon detection of Gag multimers (expected in late assembly intermediates) than upon detection of Gag monomers or dimers (expected in early assembly intermediates). Thus, small molecules that inhibit the assembly pathway are detected because they reduce the signal generated by the detection antibody. Under the theory that similar cellular machinery could be used by different viruses, analogous cell-free assembly screens were set up for seven other viruses that are human pathogens, and a master hit collection was generated consisting of 249 small molecules that inhibited assembly in one or more of these eight virus assembly screens [29].
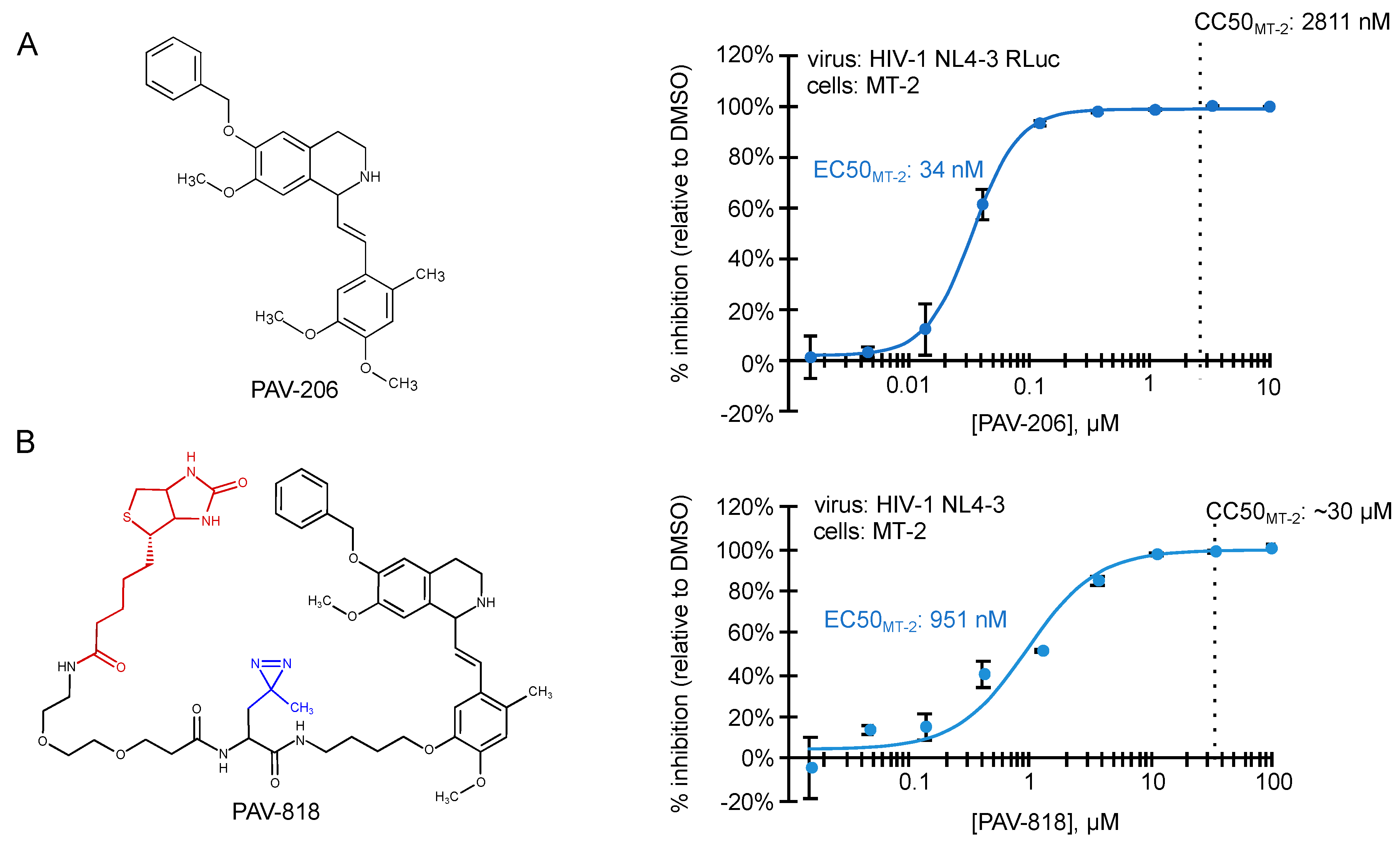
Analysis of compounds from the master hit collection in cell culture assays identified a chemotype that was used for analog development, resulting in PAV-206, a small molecule that inhibits replication in an HIV-1 infected MT-2 T cell line and in HIV-1 infected human peripheral blood PBMCs with EC50s of 34 nM and 75 nM, respectively (Figure 4; [29]). Notably, studies of a PAV-206 analog in a HIV-1-infected human MT-2 T cells showed that this chemotype likely acts by inhibiting formation of the last assembly intermediate in the HIV-1 capsid assembly pathway [29].
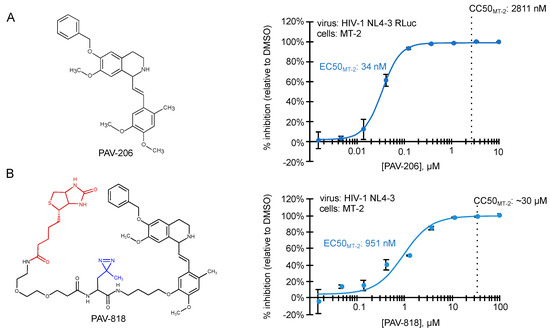
Figure 4.
Small molecules that potently inhibit HIV-1 immature capsid assembly were recently described [29]. Structures of two ARV compounds are shown to the left. Dose–response curves for their inhibition of HIV-1 replication in the MT-2 T cell line are shown to the right, with EC50 and CC50 indicated. (A) PAV-206, is a tetrahydroisoquinolone derivative with excellent drug-like properties and a selectivity index (CC50/EC50) of ~82 in MT-2 cells. Other studies show that PAV-206 has a selectivity index of 48 in PBMCs and inhibits virus production most likely by reducing formation of the late ~500S assembly intermediate [29]. (B) PAV-818 is an analog of PAV-206 that retains ARV activity and contains the following modifications—a biotin tag for colocalization studies (shown in red) and a diazirine group for crosslinking (shown in blue). PAV-818 has a selectivity index (CC50/EC50) of ~32 in MT-2 cells.
6. Evidence That PAV-206 Acts by Targeting Host Complexes Critical for HIV-1 Assembly
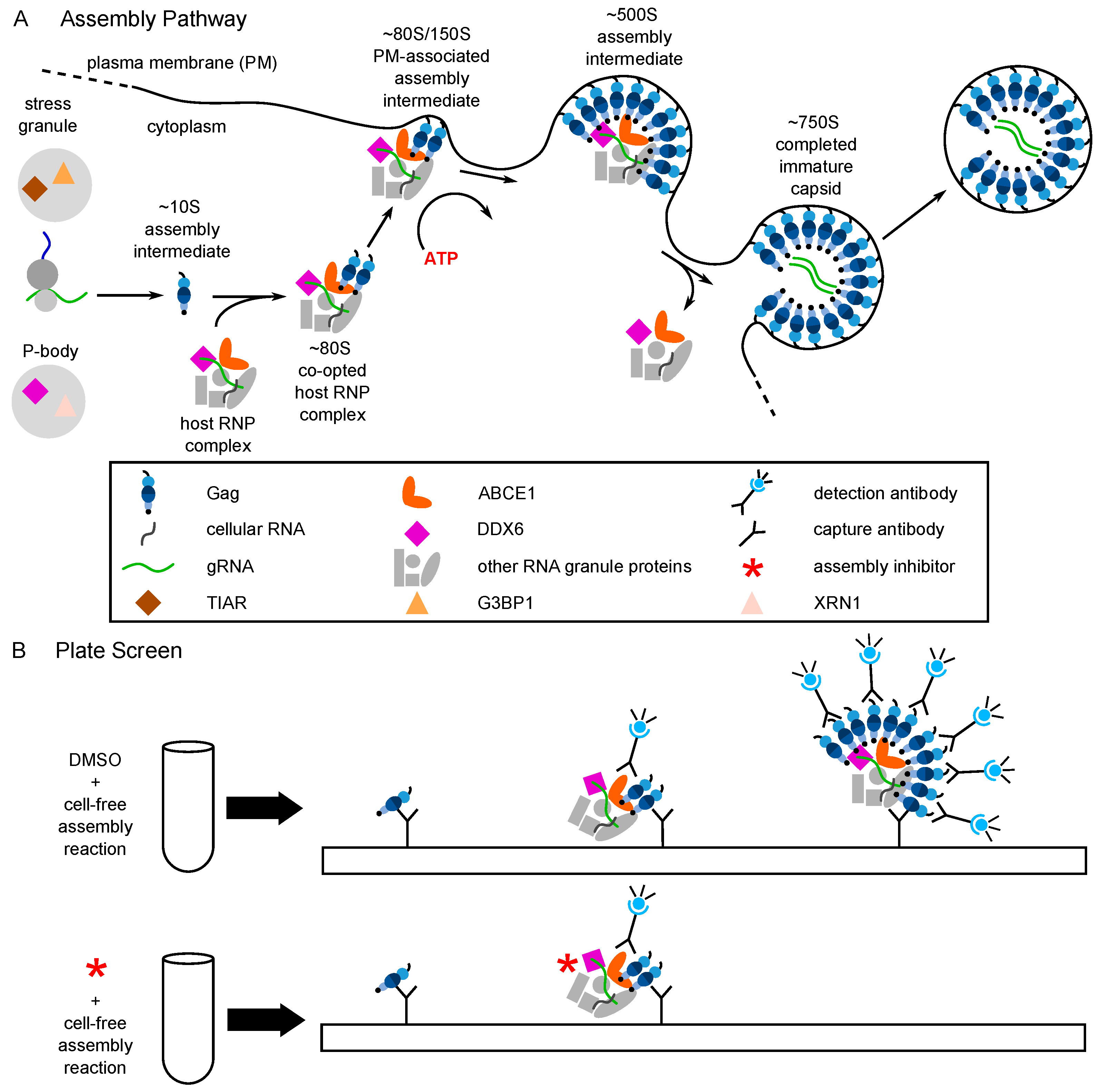
The finding that PAV-206 analogs likely inhibit assembly of the immature HIV-1 capsid raised the possibility that these analogs bind directly to assembling Gag or to a host protein associated with assembling Gag, such as a host component of the Gag-containing assembly intermediates. Analysis of a biotinylated analog of PAV-206 using the proximity ligation assay (PLA) found that the PAV-206 analog displays a dose-dependent colocalization with Gag in HIV-1-expressing cells, while a biotinylated control compound that lacks antiretroviral activity does not colocalize with Gag (Figure 5A; [29]). While PLA is a highly sensitive method for demonstrating colocalization [79], finding the compound colocalized with Gag does not distinguish between the compound binding directly to Gag vs. binding to a host protein in a Gag-containing multiprotein complex.
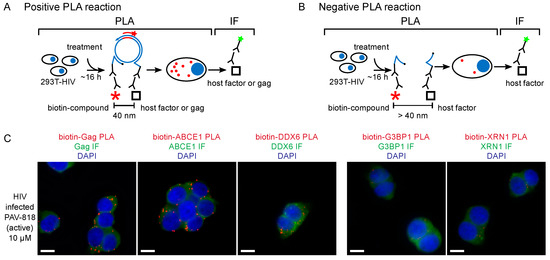
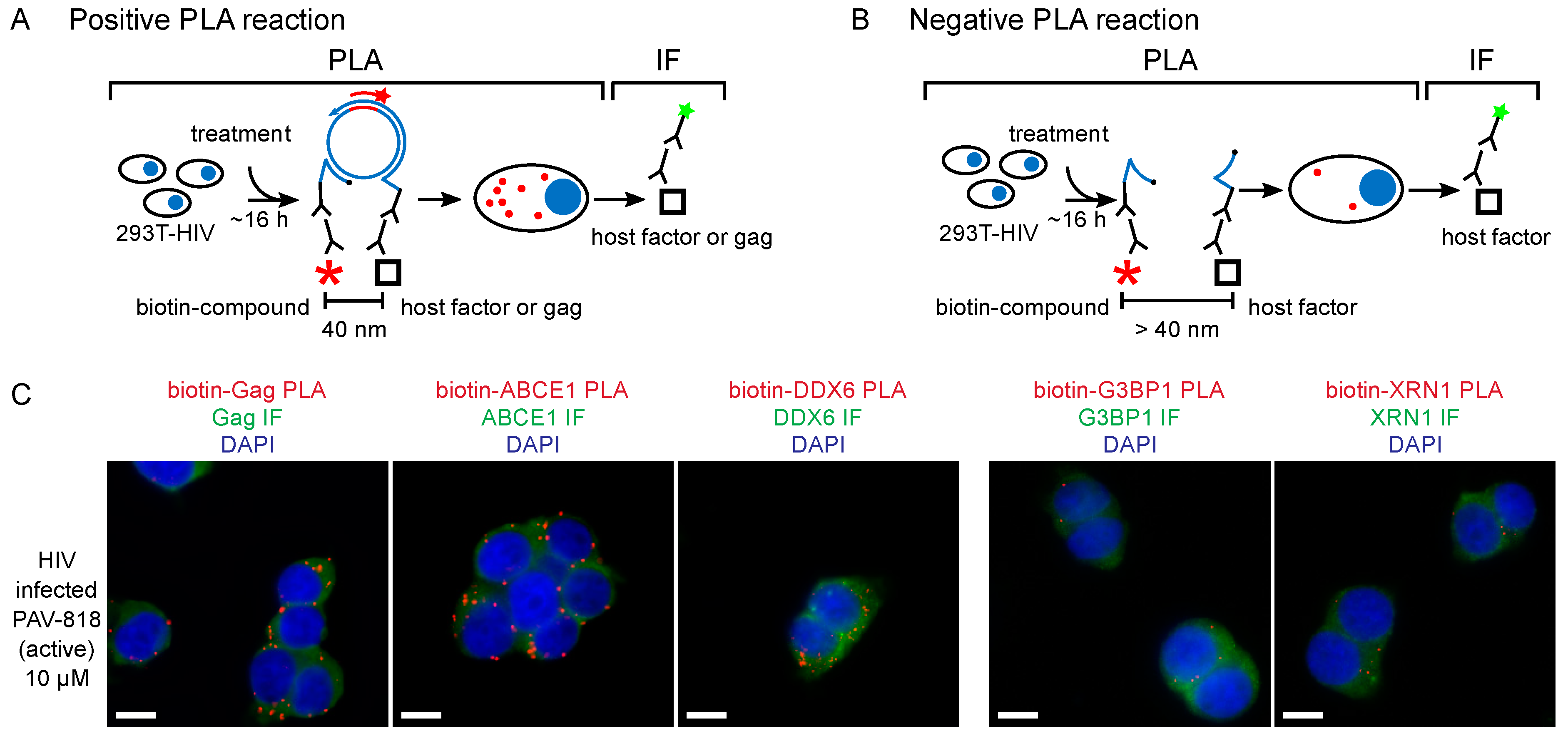
Figure 5.
The biotinylated antiretroviral analog of PAV-206 colocalizes with three components of assembly intermediates but does not colocalize with two host proteins that are not associated with Gag or assembly intermediates [29]. (A) Schematic of the PLA approach for detecting colocalization of PAV-206 with either Gag or the host proteins ABCE1 and DDX6. 293T cells chronically infected with HIV-1 (293T-HIV) were treated with 10 µM PAV-818 (the biotinylated active compound). PLA was performed on treated cells by incubating with primary antibody pairs (either rabbit anti-biotin with mouse anti-Gag; mouse anti-biotin with rabbit anti-ABCE1; or mouse anti-biotin with rabbit anti-ABCE1) followed by incubation with PLA secondary antibodies (anti-rabbit IgG coupled to [+] PLA oligonucleotide and anti-mouse IgG coupled to [−] PLA oligonucleotide). Addition of other PLA reagents results in connector oligonucleotides annealing to the “+” and “–” oligonucleotides only if the primary antibodies are colocalized (within 40 nm); this in turn leads to the PLA amplification reaction. The addition of an oligonucleotide that recognizes a sequence in the amplified regions and is coupled to a red fluorophore (red star) results in intense spots only at sites where the two antibody targets (biotinylated compound and Gag, or biotinylated compound and host protein) are colocalized in situ. After PLA, IF was performed by adding secondary antibody conjugated to a green fluorophore (green star) to detect any unoccupied Gag or host protein antibody, thus marking Gag- or host-protein expressing cells with low-level green fluorescence. (B) This schematic illustrates a scenario in which the biotinylated compound and host protein are more than 40 nm apart. The connector oligonucleotide will not anneal to the antibody-conjugated [+] and [−] PLA oligonucleotides; thus, little to no amplification will occur and few or no red spots will be observed. (C) Data in the three panels on the left show colocalization by PLA of Gag, ABCE1, and DDX6 with PAV-818, respectively. Data in the two panels on the right show lack of colocalization with two host proteins that are found in P-bodies and stress granules, respectively, G3BP1 and XRN1, but are not known to be associated with Gag or found in assembly intermediates. Additional PLA negative controls, PLA dose–response curves, and quantitation are found in [29].
A sensitive approach to determining whether a drug binds to a viral protein involves selecting for HIV-1 variants that are resistant to the drug. Every CA-binding inhibitor that has been studied extensively to date results in rapid development of resistant variants upon selection in vitro [22], including the two highly potent but broadly acting small molecules GS-CA1 and GS-6027 [77,80]. Similarly, the first and second generation maturation inhibitors, a different class of small molecules that inhibit Gag cleavage, also select for resistance mutations in vitro (reviewed in [81]).
Interestingly, upon selection for resistance to the immature capsid assembly inhibitor PAV-206 in MT-2 cells infected with HIV-1, no PAV-206-specific resistance mutations were identified during the 37 weeks of selection nor did high level resistance (at least 16-fold above the EC50) develop during that time [29]. This contrasted markedly with a selection experiment that was carried out in parallel using the well-studied protease inhibitor nelfinavir [82]; during the 37-week selection period, this control selection yielded three well known nelfinavir resistance mutations as well as resistance 16-fold above the EC50 [29]. The failure to develop rapid PAV-206-specific resistance upon selection suggested that PAV-206 does not bind directly to Gag or another viral protein. The lack of drug-specific resistance mutations, along with the finding that PAV-206 colocalizes with Gag, suggests that PAV-206 binds to a host factor associated with assembling Gag. Indeed, consistent with that possibility, experiments demonstrated that in addition to colocalizing with Gag, PAV-206 also colocalizes with two host factors that are associated with Gag in assembly intermediates—the two host enzymes that are known to facilitate assembly and reside in RNA-granule-like assembly intermediates, ABCE1 and DDX6 [29] (Figure 5A). Notably, controls showed that two other host proteins, G3BP1 found in stress granules and XRN1 found in P-bodies, are not colocalized with PAV-206. Since stress granules and P-bodies are prominent RNA granules that are not thought to play a role in HIV-1 assembly [29] (Figure 5B), these findings argue that the colocalization of PAV-206 with ABCE1 and DDX6 is not simply due to non-specific sticking of PAV-206 to host RNA granule proteins. Together the data support a model (Figure 6) in which PAV-206 likely binds to a host factor in the HIV-1 capsid assembly intermediates that are formed when Gag co-opts a host multiprotein complex that contains ABCE1, DDX6, and other host proteins. Association of PAV-206 with these RNA-granule-like intermediates appears to inhibit assembly of Gag, thereby reducing the production of viral particles.
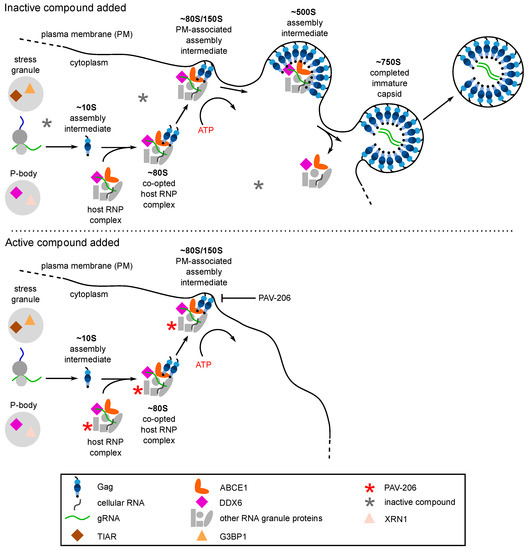
Figure 6.
PAV-206 appears to inhibit HIV-1 replication by reducing formation of the ~500S assembly intermediate, most likely by binding to a host protein in assembly intermediates. The top panel shows that the assembly pathway is unaffected by an inactive control compound. For details of events in this pathway, see the Figure 3A legend. The bottom panel is based on recent findings [29] and shows that addition of PAV-206 or one of its active analogs results in association of the small molecule (red asterisk) with an as yet unidentified host protein in the assembly intermediates, leading to a reduction in the formation of the final ~500S assembly intermediate and little to no virus production.
The direct-binding target of PAV-206 and its analogs has not yet been identified, and much remains to be done before an analog of PAV-206 can be advanced to a preclinical stage. Nevertheless, there are important lessons in the discovery of this potent and specific small molecule inhibitor of Gag assembly. First, the findings show that a screen recapitulating the host-catalyzed HIV-1 assembly pathway led to the first potent and selective inhibitor of Gag assembly, after 15 years of screens based on CA and Gag self-assembly, indicating that screens that more closely recapitulate the cell biology of HIV-1 assembly can identify molecules missed by other screens. Second, the conclusion that the PAV-206 appears to target a host protein argues that host-targeting inhibitors of HIV-1 assembly could be highly successful. Third, identification of PAV-206 suggests that development of ARV drugs that will not select for resistant viral variants is an achievable goal—although it may require a shift in the paradigm that is used for target identification and antiviral drug development as discussed below. Finally, the success of this screen in discovering a potent assembly inhibitor is an important validation of the host-catalyzed model of HIV-1 capsid assembly model, since an inhibitor of this pathway would only be expected to block virus production if the pathway is indeed critical for virus assembly as has been proposed [41,45,46,49]. Thus, the finding that the first potent and specific inhibitor of assembly appears to be directed against a component of assembly intermediates is strong evidence that these complexes play a critical role in HIV-1 immature capsid assembly.
7. Host-Targeted Assembly Inhibitors—Challenges and Future Directions
Antiviral drugs that bind to viral proteins constitute the low-hanging fruit of antiviral drug development, attractive on many fronts. However, the best viral targets have been the subject of numerous screens over the past three decades and could be nearly fully tapped from a small molecule perspective. This leaves host-targeted therapies as the approach that is most likely to yield big gains in the future. While more complex and requiring novel approaches, host-targeting drugs are likely to yield the exciting reward of high genetic barriers that will greatly extend drug utility [83]. The identification of RABV and HIV assembly inhibitors through the use of screens that recapitulate host-catalyzed assembly pathways [29,78] makes a strong case that the main reason we lack host-targeting inhibitors of virus assembly is because we have not sufficiently understood host-catalyzed assembly pathways and explored these novel screening approaches.
Given the increasing dependence on ARV drugs not just for treatment but also for prevention [84,85], the importance of developing drugs that are less prone to development of resistance cannot be overstated. However, to achieve such success will require entirely new approaches. Screens aimed at identifying drugs that bind to host dependency factors, proteins known to facilitate virus events in the viral life cycle, may not be successful if such proteins are studied in isolation. Many viral–host interactions involve transient, poorly understood multiprotein complexes that likely contain host proteins that are “moonlighting”, i.e., performing a second non-canonical and often very different function, when present in a different context [86]. Screens that remove such cellular proteins from their multiprotein complex environment may be much less successful in identifying inhibitors of host-dependent steps of the viral life cycle than more holistic pathway screens. Such pathway screens, exemplified by the screens used to identify the HIV-1 and RABV assembly inhibitors described above, recapitulate a spectrum of key steps in virus assembly including translation of the capsid protein and the stepwise formation of assembly intermediates catalyzed by host enzymes [29,78].
Assembly pathway screens have two added advantages. First, they might identify small molecules that inhibit replication of unrelated viruses if those viruses use common host machinery during assembly [15]. Secondly, the multiprotein complexes involved in virus assembly could play important roles in non-viral diseases that result from disordered protein homeostasis [87,88], thereby leading to small molecule therapeutics effective against chronic degenerative diseases. For example, studies suggest that the neuronal protein ARC, which is found in synapses and is critical for learning and memory, evolved from an ancient retroelement Gag protein [89,90,91]; thus, the host machinery involved in Gag assembly could easily be critical for assembly of multiprotein complexes that are critical for neuronal function. Hence the importance of further understanding the host-targeting assembly inhibitor PAV-206. Diseases that result from disordered homeostasis and aggregation of neuronal multiprotein complexes include degenerative neurologic diseases for which few drug treatments exist [88]. Thus, from many perspectives, a better understanding of host-catalyzed assembly pathways and screens that result from them offers new and exciting avenues for combatting disease.
Author Contributions
Conceptualization, J.R.L., J.C.R., and V.R.L.; writing—original draft preparation, J.R.L.; writing—review and editing, J.R.L. and J.C.R.; project administration, J.R.L.; funding acquisition, J.R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Institutes of Health grants R01 AI150457, R56 AI145498, and R21AI154963 to J.R.L. and by Prosetta Biosciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. J.R.L. is a cofounder of Prosetta Biosciences, and J.C.R. is a consultant for Prosetta Biosciences. V.R.L. is a cofounder, CEO, and CTO of Prosetta Biosciences.
References
- UNAIDS. Global HIV & AIDS Statistics—2020 Fact Sheet. 2020. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 19 January 2021).
- Collier, D.A.; Monit, C.; Gupta, R.K. The Impact of HIV-1 Drug Escape on the Global Treatment Landscape. Cell Host Microbe 2019, 26, 48–60. [Google Scholar] [CrossRef]
- Boender, T.S.; Sigaloff, K.C.; McMahon, J.H.; Kiertiburanakul, S.; Jordan, M.R.; Barcarolo, J.; Ford, N.; Rinke de Wit, T.F.; Bertagnolio, S. Long-term Virological Outcomes of First-Line Antiretroviral Therapy for HIV-1 in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2015, 61, 1453–1461. [Google Scholar] [CrossRef]
- Gregson, J.; Kaleebu, P.; Marconi, V.C.; van Vuuren, C.; Ndembi, N.; Hamers, R.L.; Kanki, P.; Hoffmann, C.J.; Lockman, S.; Pillay, D.; et al. Occult HIV-1 drug resistance to thymidine analogues following failure of first-line tenofovir combined with a cytosine analogue and nevirapine or efavirenz in sub Saharan Africa: A retrospective multi-centre cohort study. Lancet Infect. Dis. 2017, 17, 296–304. [Google Scholar] [CrossRef]
- TenoRes Study, G. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: A multicentre retrospective cohort study. Lancet Infect. Dis. 2016, 16, 565–575. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gregson, J.; Parkin, N.; Haile-Selassie, H.; Tanuri, A.; Andrade Forero, L.; Kaleebu, P.; Watera, C.; Aghokeng, A.; Mutenda, N.; et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: A systematic review and meta-regression analysis. Lancet Infect. Dis. 2018, 18, 346–355. [Google Scholar] [CrossRef]
- Laborde-Balen, G.; Taverne, B.; Ndour, C.T.; Kouanfack, C.; Peeters, M.; Ndoye, I.; Delaporte, E. The fourth HIV epidemic. Lancet Infect. Dis. 2018, 18, 379–380. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Kassaye, S.G.; Barrow, G.; Sundaramurthi, J.C.; Jordan, M.R.; Shafer, R.W. HIV-1 transmitted drug resistance surveillance: Shifting trends in study design and prevalence estimates. J. Int. AIDS Soc. 2020, 23, e25611. [Google Scholar] [CrossRef]
- HIVinfo.NIH.gov. FDA-Approved HIV Medicine. 2020. Available online: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/fda-approved-hiv-medicines (accessed on 19 January 2021).
- Preston, B.D.; Poiesz, B.J.; Loeb, L.A. Fidelity of HIV-1 reverse transcriptase. Science 1988, 242, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.W.; Shafer, R.W. HIV-1 antiretroviral resistance: Scientific principles and clinical applications. Drugs 2012, 72, e1–e25. [Google Scholar] [CrossRef]
- Miao, M.; De Clercq, E.; Li, G. Clinical significance of chemokine receptor antagonists. Expert Opin. Drug Metab. Toxicol. 2020, 16, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, B.M.; Dziuba, N.; Li, G.; Endsley, M.A.; Murray, J.L.; Ferguson, M.R. Host factors mediating HIV-1 replication. Virus Res. 2011, 161, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Gulick, R.M.; Lalezari, J.; Goodrich, J.; Clumeck, N.; DeJesus, E.; Horban, A.; Nadler, J.; Clotet, B.; Karlsson, A.; Wohlfeiler, M.; et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N. Engl. J. Med. 2008, 359, 1429–1441. [Google Scholar] [CrossRef]
- Selvarajah, S.; Lingappa, A.F.; Michon, M.; Yu, S.F.; Macieik, A.; Mallesh, S.; Appaiah, U.; Crabtree, J.; Copeland, K.; Lin, J.; et al. From COVID-19 to the Common Cold: Novel Host-Targeted, Pan-Respiratory Antiviral Small Molecule Therapeutics. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hendrix, C.W.; Collier, A.C.; Lederman, M.M.; Schols, D.; Pollard, R.B.; Brown, S.; Jackson, J.B.; Coombs, R.W.; Glesby, M.J.; Flexner, C.W.; et al. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J. Acquir. Immune Defic. Syndr. 2004, 37, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Hardy, W.D.; Gulick, R.M.; Mayer, H.; Fatkenheuer, G.; Nelson, M.; Heera, J.; Rajicic, N.; Goodrich, J. Two-year safety and virologic efficacy of maraviroc in treatment-experienced patients with CCR5-tropic HIV-1 infection: 96-week combined analysis of MOTIVATE 1 and 2. J. Acquir. Immune Defic. Syndr. 2010, 55, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Gulick, R.M.; Fatkenheuer, G.; Burnside, R.; Hardy, W.D.; Nelson, M.R.; Goodrich, J.; Mukwaya, G.; Portsmouth, S.; Heera, J.R. Five-year safety evaluation of maraviroc in HIV-1-infected treatment-experienced patients. J. Acquir. Immune Defic. Syndr. 2014, 65, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Weehuizen, J.M.; Wensing, A.M.J.; Mudrikova, T.; Wit, F.; Hoepelman, A.I.M. Efficacy and safety of long-term maraviroc use in a heterogeneous group of HIV-infected patients: A retrospective cohort study. Int. J. Antimicrob. Agents 2019, 54, 215–222. [Google Scholar] [CrossRef]
- Francisci, D.; Pirro, M.; Schiaroli, E.; Mannarino, M.R.; Cipriani, S.; Bianconi, V.; Alunno, A.; Bagaglia, F.; Bistoni, O.; Falcinelli, E.; et al. Maraviroc Intensification Modulates Atherosclerotic Progression in HIV-Suppressed Patients at High Cardiovascular Risk. A Randomized, Crossover Pilot Study. Open Forum Infect. Dis. 2019, 6, ofz112. [Google Scholar] [CrossRef] [PubMed]
- Piconi, S.; Pocaterra, D.; Rainone, V.; Cossu, M.; Masetti, M.; Rizzardini, G.; Clerici, M.; Trabattoni, D. Maraviroc Reduces Arterial Stiffness in PI-Treated HIV-infected Patients. Sci. Rep. 2016, 6, 28853. [Google Scholar] [CrossRef] [PubMed]
- Carnes, S.K.; Sheehan, J.H.; Aiken, C. Inhibitors of the HIV-1 capsid, a target of opportunity. Curr. Opin. HIV AIDS 2018, 13, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Jouvenet, N.; Simon, S.M.; Bieniasz, P.D. Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles. Proc. Natl. Acad. Sci. USA 2009, 106, 19114–19119. [Google Scholar] [CrossRef] [PubMed]
- Kutluay, S.B.; Bieniasz, P.D. Analysis of the initiating events in HIV-1 particle assembly and genome packaging. PLoS Pathog. 2010, 6, e1001200. [Google Scholar] [CrossRef] [PubMed]
- Sundquist, W.I.; Krausslich, H.G. HIV-1 Assembly, Budding, and Maturation. Cold Spring Harb. Perspect Med. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Pornillos, O.; Ganser-Pornillos, B.K. Maturation of retroviruses. Curr. Opin. Virol. 2019, 36, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, Z.; Aiken, C. HIV-1 uncoating: Connection to nuclear entry and regulation by host proteins. Virology 2014, 454–455, 371–379. [Google Scholar] [CrossRef]
- Schlicksup, C.J.; Zlotnick, A. Viral structural proteins as targets for antivirals. Curr. Opin. Virol. 2020, 45, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Solas, D.; Kitaygorodskyy, A.; Freeman, B.; Ressler, D.T.B.; Phuong, D.J.; Swain, J.V.; Matlack, K.; Hurt, C.R.; Lingappa, V.R.; et al. Identification of an Antiretroviral Small Molecule That Appears To Be a Host-Targeting Inhibitor of HIV-1 Assembly. J. Virol. 2021, 95. [Google Scholar] [CrossRef]
- Bush, D.L.; Vogt, V.M. In Vitro Assembly of Retroviruses. Annu. Rev. Virol. 2014, 1, 561–580. [Google Scholar] [CrossRef]
- Dick, R.A.; Mallery, D.L.; Vogt, V.M.; James, L.C. IP6 Regulation of HIV Capsid Assembly, Stability, and Uncoating. Viruses 2018, 10, 640. [Google Scholar] [CrossRef]
- Rein, A.; Datta, S.A.; Jones, C.P.; Musier-Forsyth, K. Diverse interactions of retroviral Gag proteins with RNAs. Trends Biochem. Sci. 2011, 36, 373–380. [Google Scholar] [CrossRef]
- Bieniasz, P.; Telesnitsky, A. Multiple, Switchable Protein:RNA Interactions Regulate Human Immunodeficiency Virus Type 1 Assembly. Annu. Rev. Virol. 2018, 5, 165–183. [Google Scholar] [CrossRef]
- Rein, A. RNA Packaging in HIV. Trends Microbiol. 2019, 27, 715–723. [Google Scholar] [CrossRef]
- Campbell, S.; Fisher, R.J.; Towler, E.M.; Fox, S.; Issaq, H.J.; Wolfe, T.; Phillips, L.R.; Rein, A. Modulation of HIV-like particle assembly in vitro by inositol phosphates. Proc. Natl. Acad. Sci. USA 2001, 98, 10875–10879. [Google Scholar] [CrossRef]
- Campbell, S.; Rein, A. In vitro assembly properties of human immunodeficiency virus type 1 Gag protein lacking the p6 domain. J. Virol. 1999, 73, 2270–2279. [Google Scholar] [CrossRef]
- Lingappa, J.R.; Hill, R.L.; Wong, M.L.; Hegde, R.S. A multistep, ATP-dependent pathway for assembly of human immunodeficiency virus capsids in a cell-free system. J. Cell Biol. 1997, 136, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Lingappa, J.R.; Newman, M.A.; Klein, K.C.; Dooher, J.E. Comparing capsid assembly of primate lentiviruses and hepatitis B virus using cell-free systems. Virology 2005, 333, 114–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dooher, J.E.; Schneider, B.L.; Reed, J.C.; Lingappa, J.R. Host ABCE1 is at Plasma Membrane HIV Assembly Sites and Its Dissociation from Gag is Linked to Subsequent Events of Virus Production. Traffic 2007, 8, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Lingappa, J.R.; Dooher, J.E.; Newman, M.A.; Kiser, P.K.; Klein, K.C. Basic residues in the nucleocapsid domain of Gag are required for interaction of HIV-1 gag with ABCE1 (HP68), a cellular protein important for HIV-1 capsid assembly. J. Biol. Chem. 2006, 281, 3773–3784. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.A.; Reed, J.C.; Geary, C.D.; Swain, J.V.; Lingappa, J.R. A temporospatial map that defines specific steps at which critical surfaces in the Gag MA and CA domains act during immature HIV-1 capsid assembly in cells. J. Virol. 2014, 88, 5718–5741. [Google Scholar] [CrossRef]
- Singh, A.R.; Hill, R.L.; Lingappa, J.R. Effect of mutations in Gag on assembly of immature human immunodeficiency virus type 1 capsids in a cell-free system. Virology 2001, 279, 257–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Z.Q.; Dong, J.; Ishimura, A.; Daar, I.; Hinnebusch, A.G.; Dean, M. The Essential Vertebrate ABCE1 Protein Interacts with Eukaryotic Initiation Factors. J. Biol. Chem. 2006, 281, 7452–7457. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Quiles, C.; Mateo-Bonmati, E.; Micol, J.L. ABCE Proteins: From Molecules to Development. Front. Plant. Sci. 2018, 9, 1125. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, C.; Klein, K.C.; Kiser, P.K.; Singh, A.R.S.; Firestein, B.L.; Riba, S.C.; Lingappa, J.R. Identification of a host protein essential for assembly of immature HIV-1 capsids. Nature 2002, 415, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Molter, B.; Geary, C.D.; McNevin, J.; McElrath, J.; Giri, S.; Klein, K.C.; Lingappa, J.R. HIV-1 Gag co-opts a cellular complex containing DDX6, a helicase that facilitates capsid assembly. J. Cell Biol. 2012, 198, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Lingappa, J.R.; Reed, J.C.; Tanaka, M.; Chutiraka, K.; Robinson, B.A. How HIV-1 Gag assembles in cells: Putting together pieces of the puzzle. Virus Res. 2014. [Google Scholar] [CrossRef]
- Jarmoskaite, I.; Russell, R. RNA helicase proteins as chaperones and remodelers. Annu. Rev. Biochem. 2014, 83, 697–725. [Google Scholar] [CrossRef] [PubMed]
- Barajas, B.C.; Tanaka, M.; Robinson, B.A.; Phuong, D.J.; Chutiraka, K.; Reed, J.C.; Lingappa, J.R. Identifying the assembly intermediate in which Gag first associates with unspliced HIV-1 RNA suggests a novel model for HIV-1 RNA packaging. PLoS Pathog. 2018, 14, e1006977. [Google Scholar] [CrossRef]
- Tanaka, M.; Robinson, B.A.; Chutiraka, K.; Geary, C.D.; Reed, J.C.; Lingappa, J.R. Mutations of Conserved Residues in the Major Homology Region Arrest Assembling HIV-1 Gag as a Membrane-Targeted Intermediate Containing Genomic RNA and Cellular Proteins. J. Virol. 2015, 90, 1944–1963. [Google Scholar] [CrossRef]
- Jardine, P.J. Slow and steady wins the race: Physical limits on the rate of viral DNA packaging. Curr. Opin. Virol. 2019, 36, 32–37. [Google Scholar] [CrossRef]
- Deng, Y.; Hammond, J.A.; Pauszek, R.; Ozog, S.; Chai, I.; Rabuck-Gibbons, J.; Lamichhane, R.; Henderson, S.C.; Millar, D.P.; Torbett, B.E.; et al. Discrimination between Functional and Non-functional Cellular Gag Complexes involved in HIV-1 Assembly. J. Mol. Biol. 2021, 433, 166842. [Google Scholar] [CrossRef]
- Dooher, J.E.; Lingappa, J.R. Conservation of a step-wise, energy-sensitive pathway involving HP68 for assembly of primate lentiviral capsids in cells. J. Virol. 2004, 78, 1645–1656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reed, J.C.; Westergreen, N.; Barajas, B.C.; Ressler, D.T.B.; Phuong, D.J.; Swain, J.V.; Lingappa, V.R.; Lingappa, J.R. The formation of RNA granule-derived capsid assembly intermediates appears to be conserved between HIV-1 and the non-primate lentivirus FIV. J. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Waheed, A.A.; Joshi, A.; Freed, E.O. Association of human immunodeficiency virus type 1 gag with membrane does not require highly basic sequences in the nucleocapsid: Use of a novel Gag multimerization assay. J. Virol. 2005, 79, 14131–14140. [Google Scholar] [CrossRef]
- Briggs, J.A.; Simon, M.N.; Gross, I.; Krausslich, H.G.; Fuller, S.D.; Vogt, V.M.; Johnson, M.C. The stoichiometry of Gag protein in HIV-1. Nat. Struct. Mol. Biol. 2004, 11, 672–675. [Google Scholar] [CrossRef]
- Jouvenet, N.; Bieniasz, P.D.; Simon, S.M. Imaging the biogenesis of individual HIV-1 virions in live cells. Nature 2008, 454, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Chazal, N.; Carriere, C.; Gay, B.; Boulanger, P. Phenotypic characterization of insertion mutants of the human immunodeficiency virus type 1 Gag precursor expressed in recombinant baculovirus-infected cells. J. Virol. 1994, 68, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, D.; Jacobs, E.; de Foresta, F.; Thiriart, C.; Francotte, M.; Thines, D.; De Wilde, M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 1989, 59, 103–112. [Google Scholar] [CrossRef]
- O’Carroll, I.P.; Crist, R.M.; Mirro, J.; Harvin, D.; Soheilian, F.; Kamata, A.; Nagashima, K.; Rein, A. Functional redundancy in HIV-1 viral particle assembly. J. Virol. 2012, 86, 12991–12996. [Google Scholar] [CrossRef]
- Cao, J.; Isaacson, J.; Patick, A.K.; Blair, W.S. High-throughput human immunodeficiency virus type 1 (HIV-1) full replication assay that includes HIV-1 Vif as an antiviral target. Antimicrob. Agents Chemother. 2005, 49, 3833–3841. [Google Scholar] [CrossRef]
- Blair, W.S.; Pickford, C.; Irving, S.L.; Brown, D.G.; Anderson, M.; Bazin, R.; Cao, J.; Ciaramella, G.; Isaacson, J.; Jackson, L.; et al. HIV capsid is a tractable target for small molecule therapeutic intervention. PLoS Pathog. 2010, 6, e1001220. [Google Scholar] [CrossRef]
- Fricke, T.; Buffone, C.; Opp, S.; Valle-Casuso, J.; Diaz-Griffero, F. BI-2 destabilizes HIV-1 cores during infection and Prevents Binding of CPSF6 to the HIV-1 Capsid. Retrovirology 2014, 11, 120. [Google Scholar] [CrossRef]
- Rankovic, S.; Ramalho, R.; Aiken, C.; Rousso, I. PF74 Reinforces the HIV-1 Capsid To Impair Reverse Transcription-Induced Uncoating. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, J.; Shah, V.B.; Aiken, C.; Whitby, K. Small-molecule inhibition of human immunodeficiency virus type 1 infection by virus capsid destabilization. J. Virol. 2011, 85, 542–549. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Alam, S.L.; Fricke, T.; Zadrozny, K.; Sedzicki, J.; Taylor, A.B.; Demeler, B.; Pornillos, O.; Ganser-Pornillos, B.K.; Diaz-Griffero, F.; et al. Structural basis of HIV-1 capsid recognition by PF74 and CPSF6. Proc. Natl. Acad. Sci. USA 2014, 111, 18625–18630. [Google Scholar] [CrossRef]
- Price, A.J.; Jacques, D.A.; McEwan, W.A.; Fletcher, A.J.; Essig, S.; Chin, J.W.; Halambage, U.D.; Aiken, C.; James, L.C. Host cofactors and pharmacologic ligands share an essential interface in HIV-1 capsid that is lost upon disassembly. PLoS Pathog. 2014, 10, e1004459. [Google Scholar] [CrossRef]
- Zhou, J.; Price, A.J.; Halambage, U.D.; James, L.C.; Aiken, C. HIV-1 Resistance to the Capsid-Targeting Inhibitor PF74 Results in Altered Dependence on Host Factors Required for Virus Nuclear Entry. J. Virol. 2015, 89, 9068–9079. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.M.; Hope, T.J. HIV-1 capsid: The multifaceted key player in HIV-1 infection. Nat. Rev. Microbiol. 2015, 13, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Ganser-Pornillos, B.K.; Yeager, M.; Pornillos, O. Assembly and architecture of HIV. Adv. Exp. Med. Biol. 2012, 726, 441–465. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Loeliger, E.; Kinde, I.; Kyere, S.; Mayo, K.; Barklis, E.; Sun, Y.; Huang, M.; Summers, M.F. Antiviral inhibition of the HIV-1 capsid protein. J. Mol. Biol. 2003, 327, 1013–1020. [Google Scholar] [CrossRef]
- Kelly, B.N.; Kyere, S.; Kinde, I.; Tang, C.; Howard, B.R.; Robinson, H.; Sundquist, W.I.; Summers, M.F.; Hill, C.P. Structure of the antiviral assembly inhibitor CAP-1 complex with the HIV-1 CA protein. J. Mol. Biol. 2007, 373, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Lemke, C.T.; Titolo, S.; von Schwedler, U.; Goudreau, N.; Mercier, J.F.; Wardrop, E.; Faucher, A.M.; Coulombe, R.; Banik, S.S.; Fader, L.; et al. Distinct effects of two HIV-1 capsid assembly inhibitor families that bind the same site within the N-terminal domain of the viral CA protein. J. Virol. 2012, 86, 6643–6655. [Google Scholar] [CrossRef] [PubMed]
- Thenin-Houssier, S.; de Vera, I.M.; Pedro-Rosa, L.; Brady, A.; Richard, A.; Konnick, B.; Opp, S.; Buffone, C.; Fuhrmann, J.; Kota, S.; et al. Ebselen, a Small-Molecule Capsid Inhibitor of HIV-1 Replication. Antimicrob. Agents Chemother. 2016, 60, 2195–2208. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Gallazzi, F.; Hill, K.J.; Burke, D.H.; Lange, M.J.; Quinn, T.P.; Neogi, U.; Sonnerborg, A. GS-CA Compounds: First-In-Class HIV-1 Capsid Inhibitors Covering Multiple Grounds. Front. Microbiol. 2019, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Link, J.O.; Rhee, M.S.; Tse, W.C.; Zheng, J.; Somoza, J.R.; Rowe, W.; Begley, R.; Chiu, A.; Mulato, A.; Hansen, D.; et al. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature 2020, 584, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Yant, S.R.; Mulato, A.; Hansen, D.; Tse, W.C.; Niedziela-Majka, A.; Zhang, J.R.; Stepan, G.J.; Jin, D.; Wong, M.H.; Perreira, J.M.; et al. A highly potent long-acting small-molecule HIV-1 capsid inhibitor with efficacy in a humanized mouse model. Nat. Med. 2019, 25, 1377–1384. [Google Scholar] [CrossRef]
- Lingappa, U.F.; Wu, X.; Macieik, A.; Yu, S.F.; Atuegbu, A.; Corpuz, M.; Francis, J.; Nichols, C.; Calayag, A.; Shi, H.; et al. Host-rabies virus protein-protein interactions as druggable antiviral targets. Proc. Natl. Acad. Sci. USA 2013, 110, E861–E868. [Google Scholar] [CrossRef]
- Soderberg, O.; Gullberg, M.; Jarvius, M.; Ridderstrale, K.; Leuchowius, K.J.; Jarvius, J.; Wester, K.; Hydbring, P.; Bahram, F.; Larsson, L.G.; et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 2006, 3, 995–1000. [Google Scholar] [CrossRef]
- Marcelin, A.G.; Charpentier, C.; Jary, A.; Perrier, M.; Margot, N.; Callebaut, C.; Calvez, V.; Descamps, D. Frequency of capsid substitutions associated with GS-6207 in vitro resistance in HIV-1 from antiretroviral-naive and -experienced patients. J. Antimicrob. Chemother. 2020, 75, 1588–1590. [Google Scholar] [CrossRef]
- Kleinpeter, A.B.; Freed, E.O. HIV-1 Maturation: Lessons Learned from Inhibitors. Viruses 2020, 12, 940. [Google Scholar] [CrossRef]
- Perry, C.M.; Frampton, J.E.; McCormack, P.L.; Siddiqui, M.A.; Cvetkovic, R.S. Nelfinavir: A review of its use in the management of HIV infection. Drugs 2005, 65, 2209–2244. [Google Scholar] [CrossRef]
- Garbelli, A.; Riva, V.; Crespan, E.; Maga, G. How to win the HIV-1 drug resistance hurdle race: Running faster or jumping higher? Biochem. J. 2017, 474, 1559–1577. [Google Scholar] [CrossRef]
- Eakle, R.; Venter, F.; Rees, H. Pre-exposure prophylaxis (PrEP) in an era of stalled HIV prevention: Can it change the game? Retrovirology 2018, 15, 29. [Google Scholar] [CrossRef]
- Nugent, D.; Gilson, R. Where next with preexposure prophylaxis? Curr. Opin. Infect. Dis. 2017, 30, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, C.J. Multitalented actors inside and outside the cell: Recent discoveries add to the number of moonlighting proteins. Biochem. Soc. Trans. 2019, 47, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Marreiros, R.; Muller-Schiffmann, A.; Bader, V.; Selvarajah, S.; Dey, D.; Lingappa, V.R.; Korth, C. Viral capsid assembly as a model for protein aggregation diseases: Active processes catalyzed by cellular assembly machines comprising novel drug targets. Virus Res. 2015, 207, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Muller-Schiffmann, A.; Trossbach, S.V.; Lingappa, V.R.; Korth, C. Viruses as ’Truffle Hounds’: Molecular Tools for Untangling Brain Cellular Pathology. Trends Neurosci. 2020. [Google Scholar] [CrossRef]
- Campillos, M.; Doerks, T.; Shah, P.K.; Bork, P. Computational characterization of multiple Gag-like human proteins. Trends Genet. 2006, 22, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Campioni, M.R.; Finkbeiner, S. Going retro: Ancient viral origins of cognition. Neuron 2015, 86, 346–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, W.; Wu, J.; Ward, M.D.; Yang, S.; Chuang, Y.A.; Xiao, M.; Li, R.; Leahy, D.J.; Worley, P.F. Structural basis of arc binding to synaptic proteins: Implications for cognitive disease. Neuron 2015, 86, 490–500. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).