NbTMP14 Is Involved in Tomato Spotted Wilt Virus Infection and Symptom Development by Interaction with the Viral NSm Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Virus Inoculation
2.2. Plasmid Construction and N. benthamiana Transformation
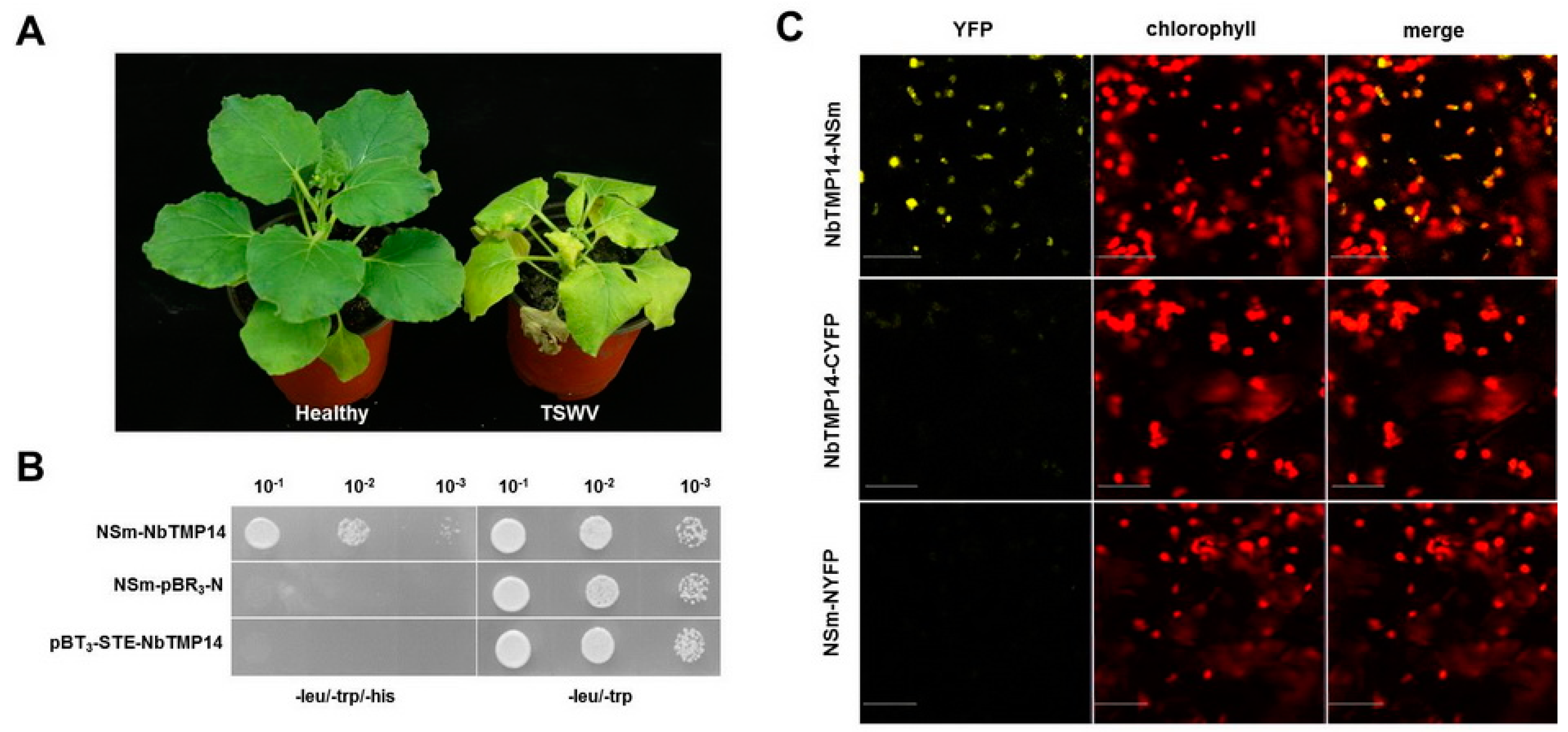
2.3. Y2H and BiFC Assays
2.4. Confocal and Electron Microscopy
2.5. TRV-VIGS in N. benthamiana
2.6. RT-qPCR and Western Blot Assay
3. Results
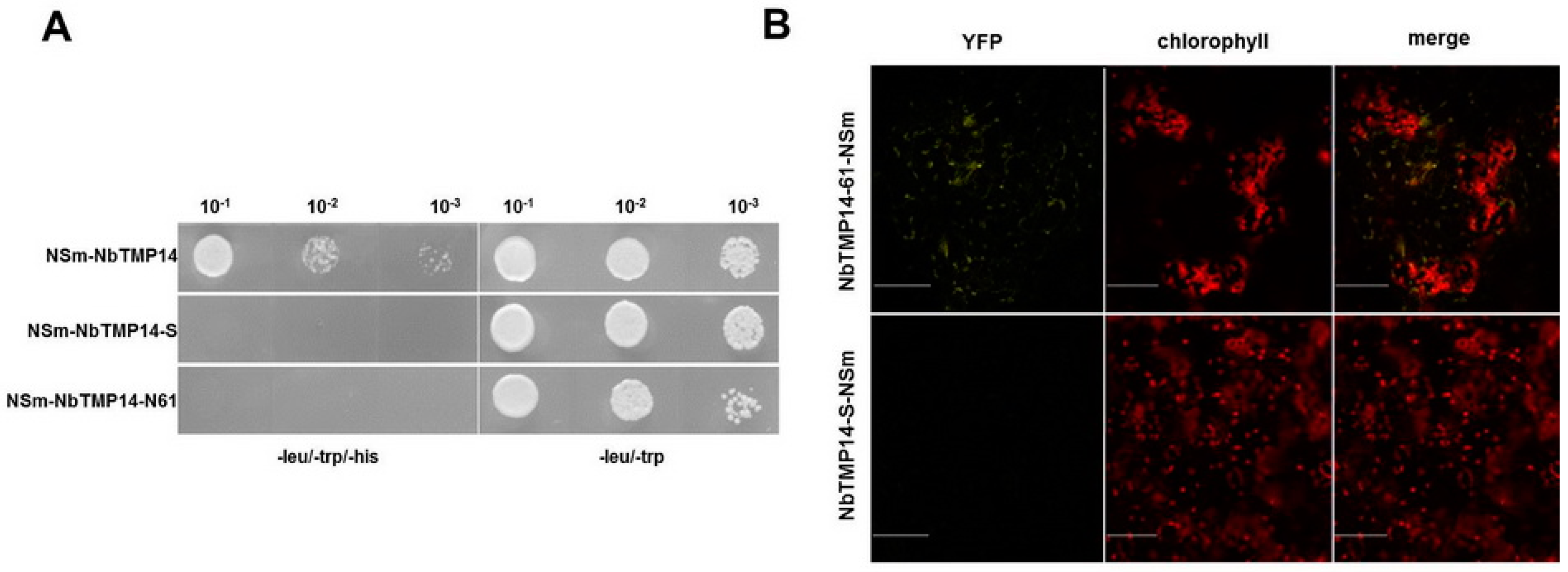
3.1. TSWV NSm Interacted with the N. benthamiana TMP14 Protein
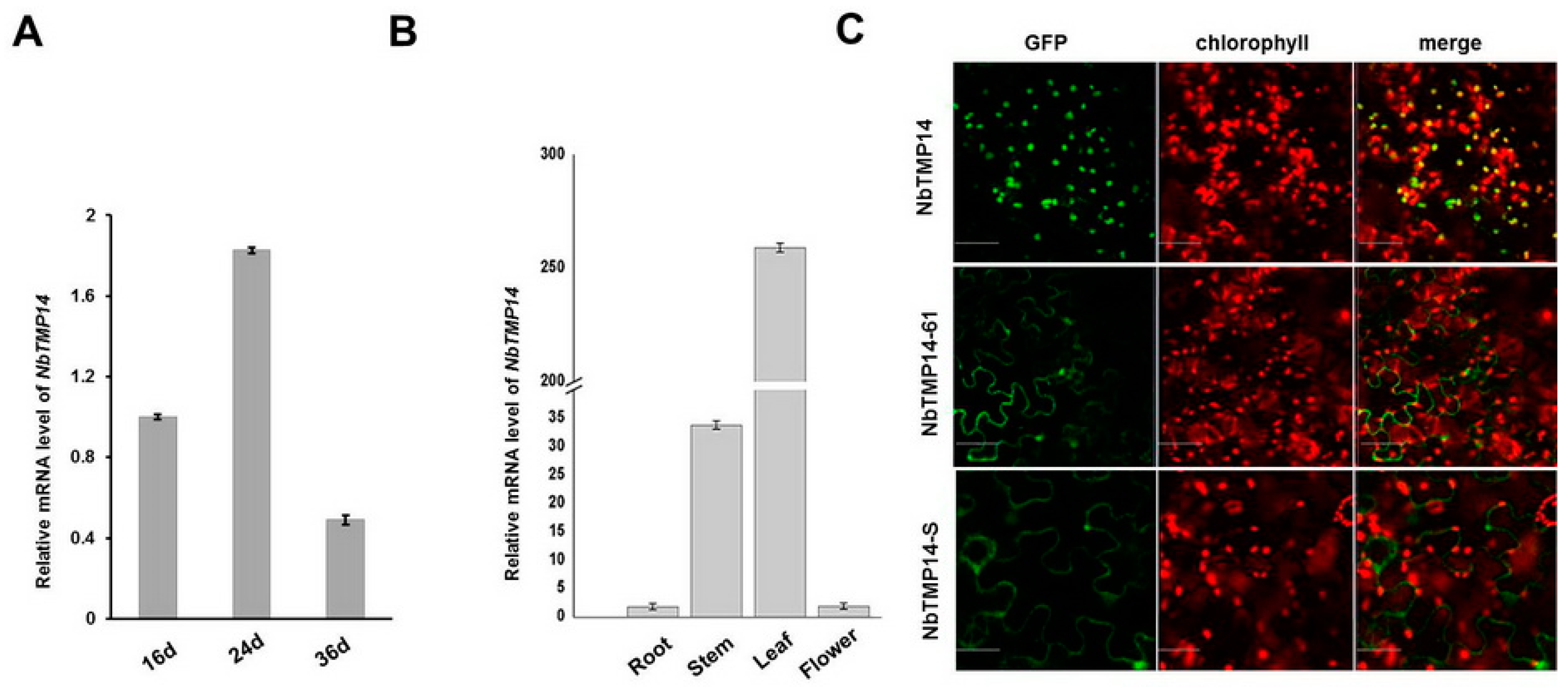
3.2. Sequence Analysis, Expression Pattern, and Subcellular Localization of NbTMP14
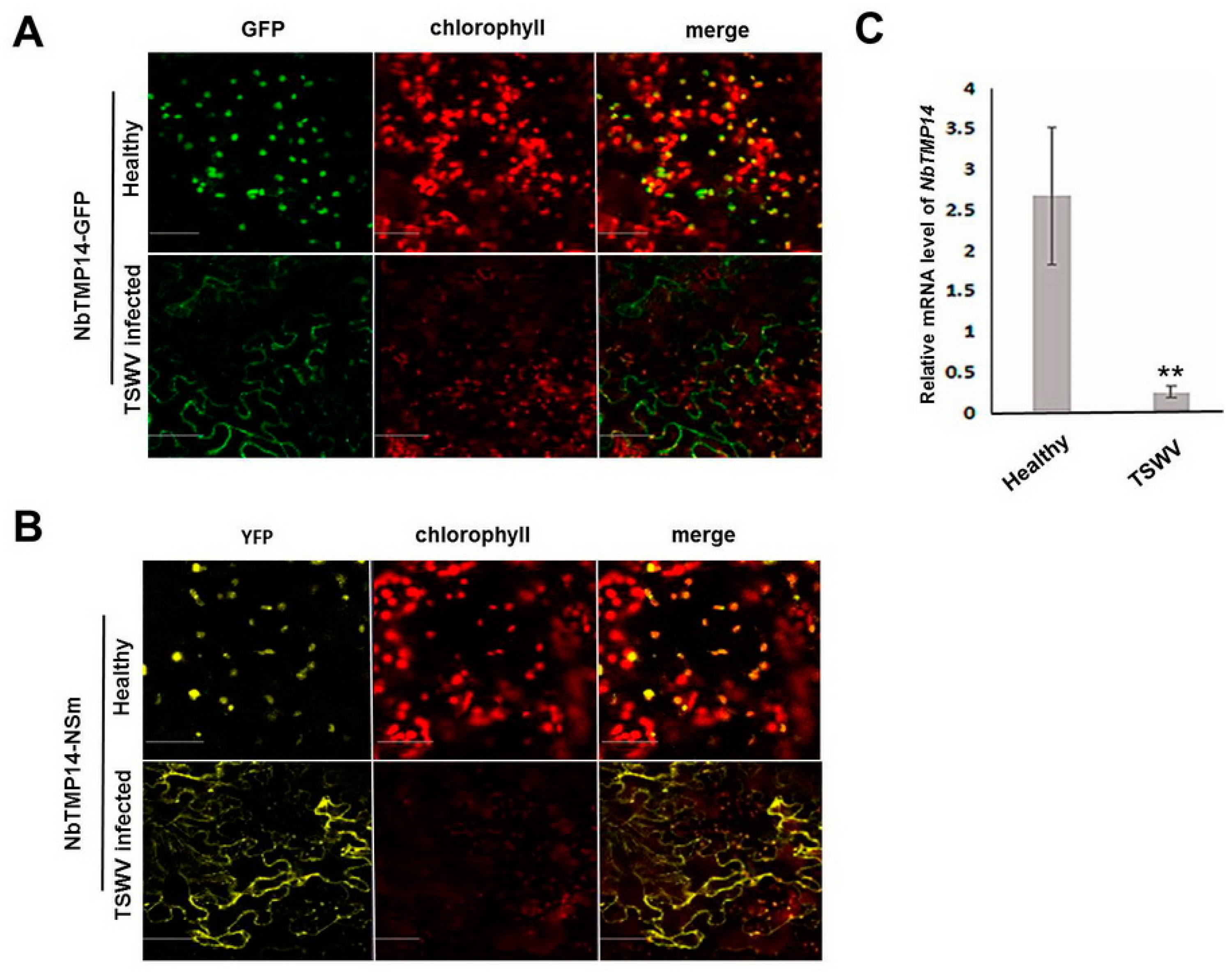
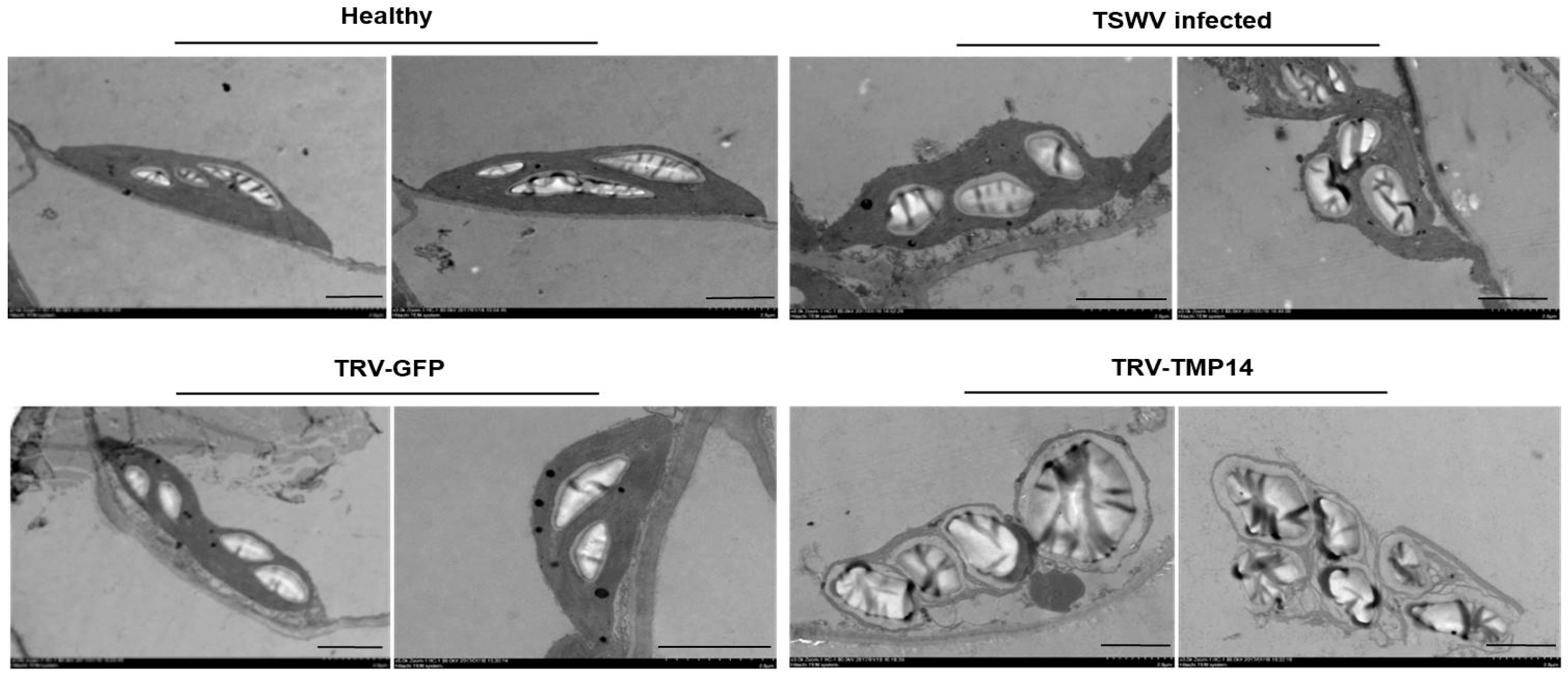
3.3. TSWV Infection Caused Structural Alteration of Chloroplasts by Disturbing NbTMP14 Expression and Subcellular Localization
3.4. NSm Interacted with Mature Proteins and Disturbed the Expression of NbTMP14
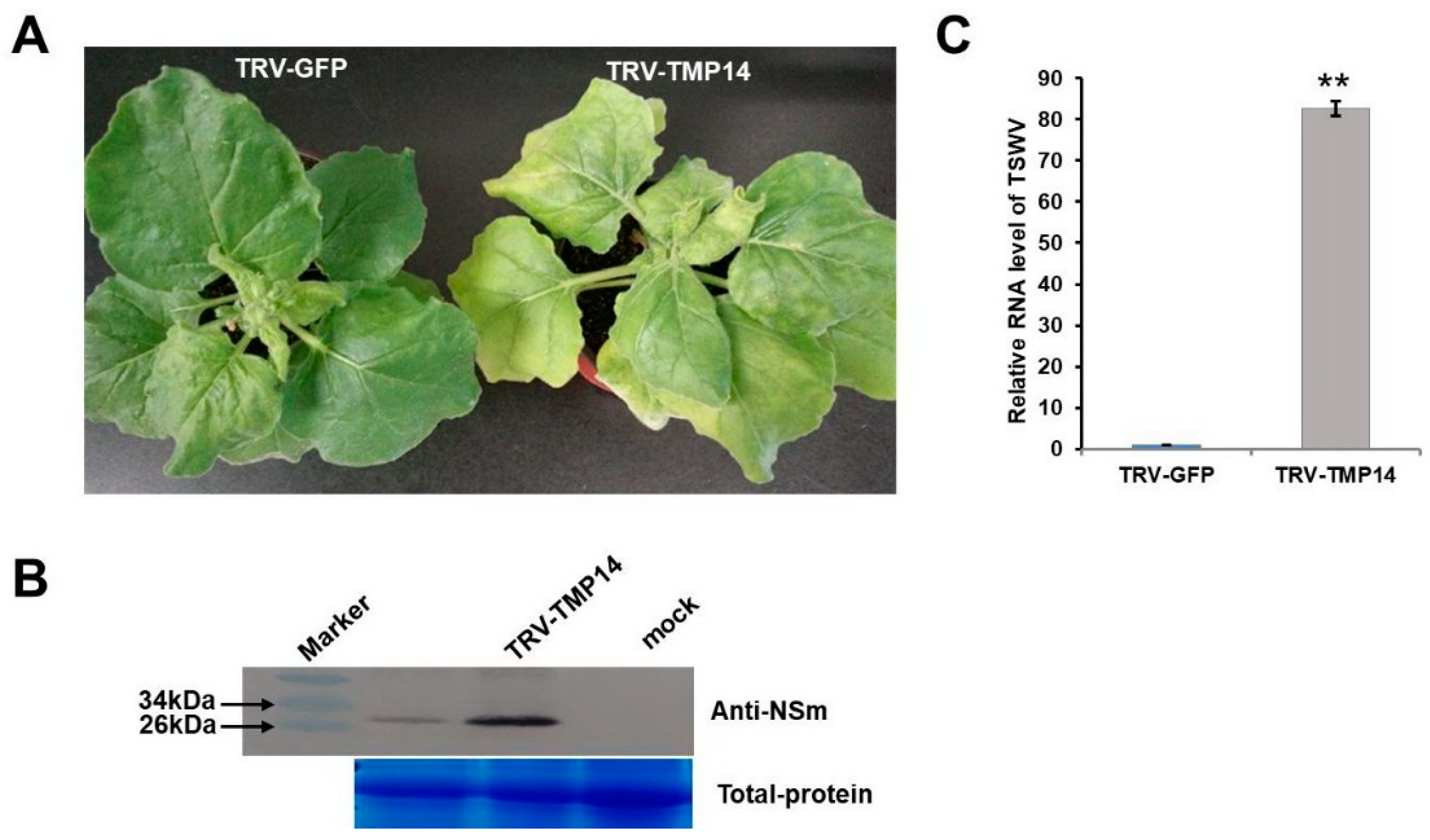
3.5. NbTMP14 Played a Positive Role in Resistance to TSWV
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kormelink, R.; Garcia, M.L.; Goodin, M.; Sasaya, T.; Haenni, A.L. Negative-strand RNA Viruses: The Plant-Infecting Counterparts. Virus Res. 2011, 162, 184–202. [Google Scholar] [CrossRef]
- Scholthof, K.B.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 Plant Viruses in Molecular Plant Pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.E.; Whitfield, A.E. The genus Tospovirus: Emerging Bunyaviruses that Threaten Food Security. Annu. Rev. Virol. 2016, 3, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Turina, M.; Kormelink, R.; Resende, R.O. Resistance to Tospoviruses in Vegetable Crops: Epidemiological and Molecular Aspects. Annu. Rev. Phytopathol. 2016, 54, 347–371. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Changes to Taxonomy and the International Code of Virus Classification and Nomenclature Ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 2017, 162, 2505–2538. [Google Scholar] [CrossRef]
- German, T.L.; Ullman, D.E.; Moyer, J.W. Tospoviruses: Diagnosis, molecular biology, phylogeny, and vector relationships. Ann. Rev. Phytopathol. 1992, 30, 315–348. [Google Scholar] [CrossRef] [PubMed]
- Mumford, R.A.; Barker, I.; Wood, K.R. The biology of the tospoviruses. Ann. Appl. Biol. 1996, 128, 159–183. [Google Scholar] [CrossRef]
- Parrella, G.; Gognalons, P.; Gebre-Selassiè, K.; Vovlas, C.; Marchoux, G. An Update of the Host Range of Tomato Spotted Wilt Virus. J. Plant Pathol. 2003, 85, 227–264. [Google Scholar]
- Rotenberg, D.; Jacobson, A.L.; Schneweis, D.J.; Whitfield, A.E. Thrips Transmission of Tospoviruses. Curr. Opin. Virol. 2015, 15, 80–89. [Google Scholar] [CrossRef]
- Adkins, S. Tomato Spotted Wilt Virus-positive Steps towards Negative Success. Mol. Plant Pathol. 2000, 1, 151–157. [Google Scholar] [CrossRef]
- Soellick, T.; Uhrig, J.F.; Bucher, G.L.; Kellmann, J.W.; Schreier, P.H. The Movement Protein NSm of Tomato Spotted Wilt Tospovirus (TSWV): RNA Binding, Interaction with the TSWV N Protein, and Identification of Interacting Plant Proteins. Proc. Natl. Acad. Sci. USA 2000, 97, 2373–2378. [Google Scholar] [CrossRef]
- Kormelink, R.; Storms, M.; van Lent, J.; Peters, D.; Goldbach, R. Expression and Subcellular Location of the NSm Protein of Tomato Spotted Wilt Virus (TSWV), a Putative Viral Movement Protein. Virology 1994, 200, 56–65. [Google Scholar] [CrossRef]
- Feng, Z.; Xue, F.; Xu, M.; Chen, X.; Zhao, W.; Garcia-Murria, M.J.; Mingarro, I.; Liu, Y.; Huang, Y.; Jiang, L.; et al. The ER-membrane Transport System is Critical for Intercellular Trafficking of the NSm Movement Protein and Tomato Spotted Wilt Tospovirus. PLoS Pathog. 2016, 12, e1005443. [Google Scholar] [CrossRef]
- Storms, M.M.; Kormelink, R.; Peters, D.; van Vent, J.W.; Goldbach, R.W. The Nonstructural NSm Protein of Tomato Spotted Wilt Virus Induces Tubular Structures in Plant and Insect Cells. Virology 1995, 214, 485–493. [Google Scholar] [CrossRef]
- Storms, M.M.; van der Schoot, C.; Prins, M.; Kormelink, R.; van Lent, J.W.M.; Goldbach, R.W. A Comparison of Two Methods of Microinjection for Assessing Altered Plasmodesmal Gating in Tissues Expressing Viral Movement Proteins. Plant J. 1998, 13, 131–140. [Google Scholar] [CrossRef]
- Prins, M.; Storms, M.M.H.; Kormelink, R.; DeHaan, P.; Goldbach, R. Transgenic Tobacco Plants Expressing the Putative Movement Protein of Tomato Spotted Wilt Tospovirus Exhibit Aberrations in Growth and Appearance. Transgenic Res. 1997, 6, 245–251. [Google Scholar] [CrossRef]
- Hallwass, M.; de Oliveira, A.S.; de Campos Dianese, E.; Lohuis, D.; Boiteux, L.S.; Inoue-Nagata, A.K.; Resende, R.O.; Kormelink, R. The Tomato Spotted Wilt Virus Cell-to-Cell Movement Protein (NSm) Triggers a Hypersensitive Response in Sw-5-containing Resistant Tomato Lines and in Nicotiana benthamiana Transformed with the Functional Sw-5b Resistance Gene Copy. Mol. Plant Pathol. 2014, 15, 871–880. [Google Scholar] [CrossRef]
- Peiró, A.; Cañizares, M.C.; Rubio, L.; López, C.; Moriones, E.; Aramburu, J.; Sánchez-Navarro, J. The Movement Protein (NSm) of Tomato Spotted Wilt Virus Is the Avirulence Determinant in the Tomato Sw-5 Gene-Based Resistance. Mol. Plant Pathol. 2014, 15, 802–813. [Google Scholar] [CrossRef]
- Huang, C.; Liu, Y.; Yu, H.; Yuan, C.; Zeng, J.; Zhao, L.; Tong, Z.; Tao, X. Non-Structural Protein NSm of Tomato Spotted Wilt Virus Is an Avirulence Factor Recognized by Resistance Genes of Tobacco and Tomato via Different Elicitor Active Sites. Viruses 2018, 10, 660. [Google Scholar] [CrossRef]
- Lewandowski, D.J.; Adkins, S. The Tubule-Forming NSm Protein from Tomato Spotted Wilt Virus Complements Cell-to-Cell and Long-Distance Movement of Tobacco Mosaic Virus Hybrids. Virology 2005, 342, 26–37. [Google Scholar] [CrossRef]
- Li, W.; Lewandowski, D.J.; Hilf, M.E.; Adkins, S. Identification of Domains of the Tomato Spotted Wilt Virus NSm Protein Involved in Tubule Formation, Movement and Symptomatology. Virology 2009, 390, 110–121. [Google Scholar] [CrossRef]
- Rinne, P.L.H.; van den Boogaard, R.; Mensink, M.G.J.; Kopperud, C.; Kormelink, R.; Goldbach, R.; van der Schoot, C. Tobacco Plants Respond to the Constitutive Expression of the Tospovirus Movement Protein NSm with a Heat-Reversible Sealing of Plasmodesmata that Impairs Development. Plant J. 2005, 43, 688–707. [Google Scholar] [CrossRef]
- Owen, P.C. The Effect of Infection with Tobacco Etch Virus on the Rates of Respiration and Photosynthesis of Tobacco Leaves. Ann. Appl. Biol. 1957, 45, 327–331. [Google Scholar] [CrossRef]
- Owen, P.C. The Effects of Infection with Tobacco Mosaic Virus on the Photosynthesis of Tobacco Leaves. Ann. Appl. Biol. 1957, 45, 456–461. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Hong, Y.; Liu, Y. Chloroplast in Plant-Virus Interaction. Front. Microbiol. 2016, 7, 1565. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Chakraborty, S. Chloroplast: The Trojan Horse in Plant-virus Interaction. Mol. Plant Pathol. 2018, 19, 504–518. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Prabu, G.; Reddy, K.K.; Kushwaha, N.K.; Sharma, V.K.; Yusuf, M.A.; Chakraborty, S. A Geminivirus Betasatellite Damages the Structural and Functional Integrity of Chloroplasts Leading to Symptom Formation and Inhibition of Photosynthesis. J. Exp. Bot. 2015, 66, 5881–5895. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, P.; Kang, L.; Cui, F. Different Pathogenicities of Rice Stripe Virus from the Insect Vector and from Viruliferous Plants. New Phytol. 2016, 210, 196–207. [Google Scholar] [CrossRef]
- Gnanasekaran, P.; Ponnusamy, K.; Chakraborty, S. A Geminivirus Betasatellite Encoded βC1 Protein Interacts with PsbP and Subverts PsbP-mediated Antiviral Defence in Plants. Mol. Plant Pathol. 2019, 20, 943–960. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Y.; Wang, F.; Chen, Y.; Tian, Y.; Jiang, L.; Peng, J.; Zheng, H.; Lin, L.; Yan, C.; et al. Involvement of the Chloroplast Gene Ferredoxin 1 in Multiple Responses of Nicotiana benthamiana to Potato Virus X Infection. J. Exp. Bot. 2020, 71, 2142–2156. [Google Scholar] [CrossRef]
- Khrouchtchova, A.; Hansson, M.; Paakkarinen, V.; Vainonen, J.P.; Zhang, S.; Jensen, P.E.; Scheller, H.V.; Vener, A.V.; Aro, E.M.; Haldrup, A. A Previously Found Thylakoid Membrane Protein of 14kDa (TMP14) is a Novel Subunit of Plant Photosystem I and is Designated PSI-P. FEBS Lett. 2005, 579, 4808–4812. [Google Scholar] [CrossRef]
- Xia, C.; Zheng, Y.; Huang, J.; Zhou, X.; Li, R.; Zha, M.; Wang, X.; Huang, Z.; Lan, H.; Turgeon, R.; et al. Elucidation of the Mechanisms of Long-Distance mRNA Movement in a Nicotiana benthamiana/Tomato Heterograft System. Plant Physiol. 2018, 177, 745–758. [Google Scholar] [CrossRef]
- Thaminy, S.; Auerbach, D.; Arnoldo, A.; Stagljar, I. Identification of novel ErbB3-interacting factors using the split-ubiquitin membrane yeast two-hybrid system. Genome Res. 2003, 13, 1744–1753. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, K.; Sun, Z.; Yan, M.; Chen, C.; Zhang, X.; Tang, Y.; Wu, Y. LNK1 and LNK2 Co-Repressors Interact with the MYB3 Transcription Factor in Phenylpropanoid Biosynthesis. Plant Physiol. 2017, 174, 1348–1358. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, F.; Li, R.; Meng, F. Observation of Chloroplast Structure under High Concentration CO2. J. Anhui Agric. Sci. 2014, 42, 3780–3781, 3790. [Google Scholar]
- Liu, Y.; Schiff, M.; Marathe, R.; Dinesh-Kumar, S.P. Tobacco Rar1, EDS1 and NPR1/NIM1 like Genes are Required for N-mediated Resistance to Tobacco Mosaic Virus. Plant J. 2002, 30, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Kyseláková, H.; Prokopová, J.; Nauš, J.; Novák, O.; Navrátil, M.; Šafáˇrová, D.; Spundová, M.; Ilík, P. Photosynthetic Alterations of Pea Leaves Infected Systemically by Pea Enation Mosaic Virus: A Coordinated Decrease in Efficiencies of CO2 Assimilation and Photosystem II Photochemistry. Plant Physiol. Biochem. 2011, 49, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Manfre, A.; Glenn, M.; Nunez, A.; Moreau, R.; Dardick, C. Light Quantity and Photosystem Function Mediate Host Susceptibility to Turnip Mosaic Virus via a Salicylic Acid-Independent Mechanism. Mol. Plant Microbe Interact. 2011, 24, 315–327. [Google Scholar] [CrossRef]
- Mochizuki, T.; Yamazaki, R.; Wada, T.; Ohki, S.T. Coat Protein Mutations in an Attenuated Cucumber Mosaic Virus Encoding Mutant 2b Protein that Lacks RNA Silencing Suppressor Activity Induces Chlorosis with Photosynthesis Gene Repression and Chloroplast Abnormalities in Infected Tobacco Plants. Virology 2014, 456–457, 292–299. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, X. Role of Rice Stripe Virus NSvc4 in Cell-to-Cell Movement and Symptom Development in Nicotiana benthamiana. Front. Plant Sci. 2012, 3, 269. [Google Scholar] [CrossRef]
- Reuveni, M.; Debbi, A.; Kutsher, Y.; Gelbart, D.; Zemach, H.; Belausov, E.; Levin, I.; Lapidot, M. Tomato Yellow Leaf Curl Virus Effects on Chloroplast Biogenesis and Cellular Structure. Physiol. Mol. Plant Pathol. 2015, 92, 51–58. [Google Scholar] [CrossRef]
- Lehto, K.; Tikkanen, M.; Hiriart, J.B.; Paakkarinen, V.; Aro, E.M. Depletion of the Photosystem II Core Complex in Mature Tobacco Leaves Infected by the Flavum Strain of Tobacco Mosaic Virus. Mol. Plant Microbe Interact. 2003, 16, 1135–1144. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, T.; Hong, Y.; Fan, Z.; Li, H. Decreased level of ferredoxin I in tobacco mosaic virus-infected tobacco is associated with development of the mosaic symptom. Physiol. Mol. Plant Pathol. 2008, 72, 39–45. [Google Scholar] [CrossRef]
- Mazidah, M.; Lau, W.H.; Yusoff, K.; Habibuddin, H.; Tan, Y.H. Ultrastructural Features of Catharanthus roseus Leaves Infected with Cucumber Mosaic Virus. Pertanika J. Trop. Agric. Sci. 2012, 35, 85–92. [Google Scholar]
- Leastro, M.O.; Pallas, V.; Resende, R.O.; Sanchez-Navarro, J.A. The Movement Proteins (NSm) of Distinct Tospoviruses Peripherally Associate with Cellular Membranes and Interact with Homologous and Heterologous NSm and Nucleocapsid Proteins. Virology 2015, 478, 39–49. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, Perception, Signal Transduction and Action in Plant Stress Response, Growth and Development. An Update to the 2007 Review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, C.; Tsuda, K. Salicylic Acid Signal Transduction: The Initiation of Biosynthesis, Perception and Transcriptional Reprogramming. Front. Plant Sci. 2014, 5, 697. [Google Scholar] [CrossRef]
- Lemos, M.; Xiao, Y.; Bjornson, M.; Wang, J.Z.; Hicks, D.; Souza, A.D. The Plastidial Retrograde Signal Methyl Erythritol Cyclopyrophosphate is a Regulator of Salicylic Acid and Jasmonic Acid Crosstalk. J. Exp. Bot. 2016, 67, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Caplan, J.L.; Mamillapalli, P.; Burch-Smith, T.M.; Czymmek, K.; Dinesh Kumar, S.P. Chloroplastic Protein NRIP1 Mediates Innate Immune Receptor Recognition of a Viral Effector. Cell 2008, 132, 449–462. [Google Scholar] [CrossRef]
- Muhlenbock, P.; Szechynska-Hebda, M.; Plaszczyca, M.; Baudo, M.; Mateo, A.; Mullineaux, P.M. Chloroplast Signaling and LESION SIMULATING DISEASE1 Regulate Crosstalk between Light Acclimation and Immunity in Arabidopsis. Plant Cell 2008, 20, 2339–2356. [Google Scholar] [CrossRef]
- Caplan, J.L.; Kumar, A.S.; Park, E.; Padmanabhan, M.S.; Hoban, K.; Modla, S.; Czymmek, K.; Dinesh-Kumar, S.P. Chloroplast Stromules Function during Innate Immunity. Dev. Cell 2015, 34, 45–57. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, J.; Shi, H.; Li, W.; Zhang, C.; Zhang, Y. NbTMP14 Is Involved in Tomato Spotted Wilt Virus Infection and Symptom Development by Interaction with the Viral NSm Protein. Viruses 2021, 13, 427. https://doi.org/10.3390/v13030427
Zhan J, Shi H, Li W, Zhang C, Zhang Y. NbTMP14 Is Involved in Tomato Spotted Wilt Virus Infection and Symptom Development by Interaction with the Viral NSm Protein. Viruses. 2021; 13(3):427. https://doi.org/10.3390/v13030427
Chicago/Turabian StyleZhan, Jin, Huiping Shi, Weimin Li, Chao Zhang, and Yongqiang Zhang. 2021. "NbTMP14 Is Involved in Tomato Spotted Wilt Virus Infection and Symptom Development by Interaction with the Viral NSm Protein" Viruses 13, no. 3: 427. https://doi.org/10.3390/v13030427
APA StyleZhan, J., Shi, H., Li, W., Zhang, C., & Zhang, Y. (2021). NbTMP14 Is Involved in Tomato Spotted Wilt Virus Infection and Symptom Development by Interaction with the Viral NSm Protein. Viruses, 13(3), 427. https://doi.org/10.3390/v13030427




