Reactivations of Latent Viral Infections Are Associated with an Increased Thr389 p70S6k Phosphorylation in Peripheral Lymphocytes of Renal Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. PBMC Isolation and Flow Cytometry
2.3. Statistical Analysis
3. Results
3.1. BKPyV Viremia Is Associated with Increased p70S6k Phosphorylation
3.2. HCMV DNAemia in Renal Transplant Recipients with a High-Risk Constellation Is Associated with Increased p70S6k Phosphorylation
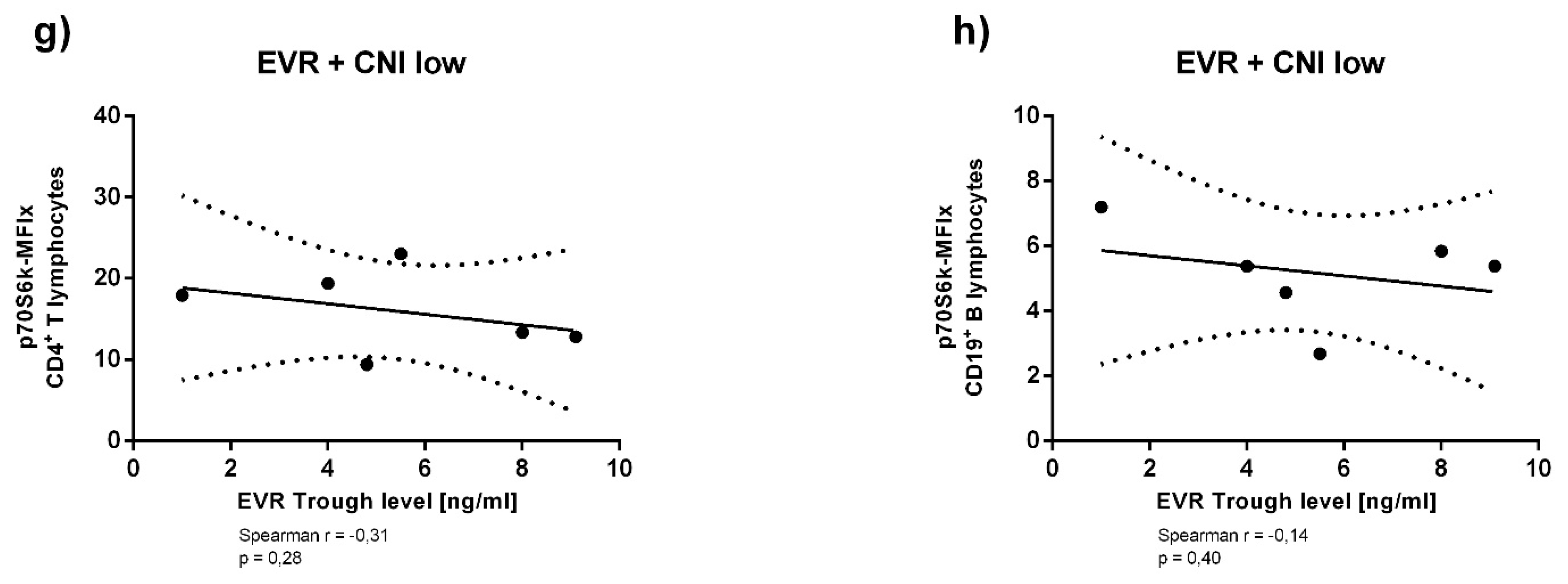
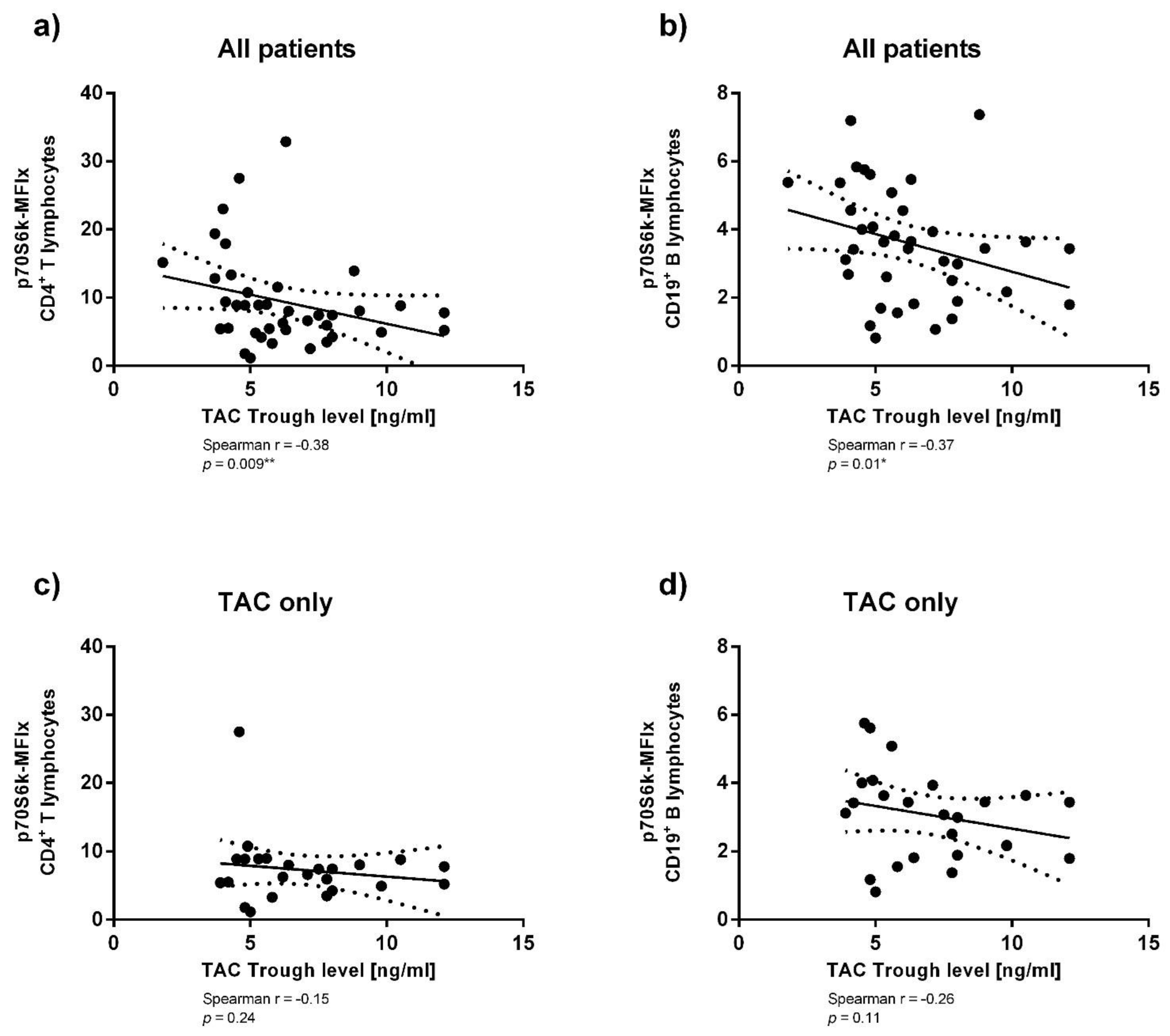
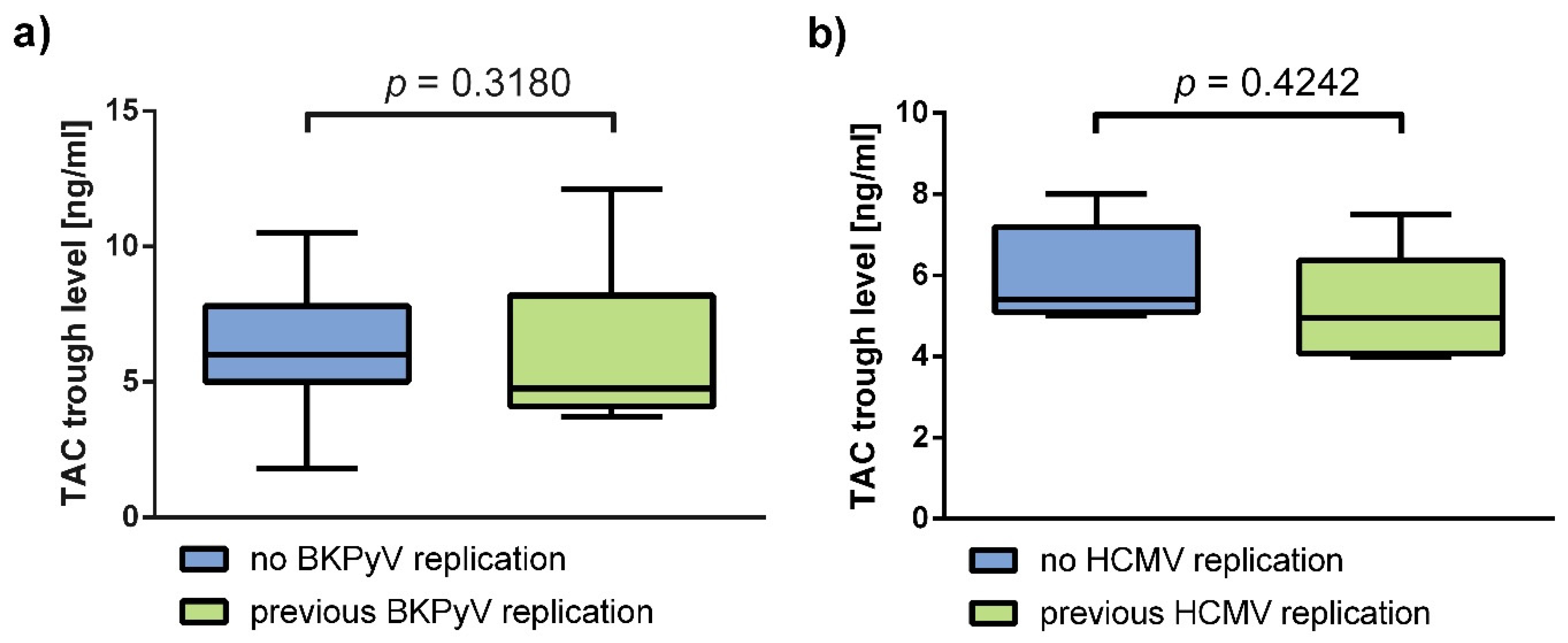
3.3. Immunosuppressant Trough Levels and p70S6k Phosphorylation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Supplementary Analyses of Immunosuppressant Trough Levels and p70S6k Phosphorylation


References
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes: Systematic review of kidney transplantation. Am. J. Transplant. 2011, 11, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. Special issue: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009, 9, S1–S155. [Google Scholar] [CrossRef]
- Albring, A.; Wendt, L.; Harz, N.; Engler, H.; Wilde, B.; Kribben, A.; Lindemann, M.; Schedlowski, M.; Witzke, O. Relationship between pharmacokinetics and pharmaco-dynamics of calcineurin inhibitors in renal transplant patients. Clin. Transplant. 2015, 29, 294–300. [Google Scholar] [CrossRef]
- Fishman, J.A. Infection in organ transplantation. Am. J. Transplant. 2017, 17, 856–879. [Google Scholar] [CrossRef]
- Kaulfuß, M.; Rohn, H.; Witzke, O. Infektionen nach Nierentransplantation. Nephrologe 2018, 13, 161–166. [Google Scholar] [CrossRef]
- Razonable, R.R.; Humar, A.; The AST Infectious Diseases Community of Practice. Cytomegalovirus in solid organ transplantation: CMV in solid organ transplantation. Am. J. Transplant. 2013, 13, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Witzke, O.; Hauser, I.A.; Bartels, M.; Wolf, G.; Wolters, H.; Nitschke, M. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation 2012, 93, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Korth, J.; Widera, M.; Dolff, S.; Guberina, H.; Bienholz, A.; Brinkhoff, A.; Anastasiou, O.E.; Kribben, A.; Dittmer, U.; Verheyen, J.; et al. Impact of low-level BK polyomavirus viremia on intermediate-term renal allograft function. Transpl. Infect. Dis. 2018, 20, e12817. [Google Scholar] [CrossRef]
- Blazquez-Navarro, A.; Dang-Heine, C.; Wittenbrink, N.; Bauer, C.; Wolk, K.; Sabat, R.; Westhoff, T.H.; Sawitzki, B.; Reinke, P.; Thomusch, O.; et al. BKV, CMV, and EBV interactions and their effect on graft function one year post-renal transplantation: Results from a large multi-centre study. EBioMedicine 2018, 34, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Schold, J.D.; Rehman, S.; Kayler, L.K.; Magliocca, J.; Srinivas, T.R.; Meier-Kriesche, H.-U. Treatment for BK virus: Incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transpl. Int. 2009, 22, 626–634. [Google Scholar] [CrossRef]
- Kadambi, P.V.; Josephson, M.A.; Williams, J.; Corey, L.; Jerome, K.R.; Meehan, S.M.; Limaye, A.P. Treatment of refractory BK virus-associated nephropathy with cidofovir. Am. J. Transplant. 2003, 3, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H.; Brennan, D.C.; Drachenberg, C.B.; Ginevri, F.; Gordon, J.; Limaye, A.P.; Mihatsch, M.J.; Nickeleit, V.; Ramos, E.; Randhawa, P.; et al. Polyomavirus-associated nephropathy in renal transplantation: Interdisciplinary analyses and recommendations. Transplantation 2005, 79, 1277–1286. [Google Scholar] [CrossRef]
- Dharnidharka, V.R.; Cherikh, W.S.; Abbott, K.C. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation 2009, 87, 1019–1026. [Google Scholar] [CrossRef]
- Moscarelli, L.; Caroti, L.; Antognoli, G.; Zanazzi, M.; Maria, L.D.; Carta, P.; Minetti, E. Everolimus leads to a lower risk of BKV viremia than mycophenolic acid in de novo renal transplantation patients: A single-center experience. Clin. Transpl. 2013, 27, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Polanco, N.; González Monte, E.; Folgueira, M.D.; Morales, E.; Gutiérrez Martínez, E.; Bengoa, I.; Hernández, A.; Morales, J.M.; Praga, M.; Andrés, A. Everolimus-based immuno-suppression therapy for BK virus nephropathy. Transplant. Proc. 2015, 47, 57–61. [Google Scholar] [CrossRef]
- Andrassy, J.; Hoffmann, V.S.; Rentsch, M.; Stangl, M.; Habicht, A.; Meiser, B.; Fischereder, M.; Jauch, K.-W.; Guba, M. Is cytomegalovirus prophylaxis dispensable in patients receiving an mTOR inhibitor–based immunosuppression? A systematic review and meta-analysis. Transplant. J. 2012, 94, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Nashan, B.; Gaston, R.; Emery, V.; Säemann, M.D.; Mueller, N.J.; Couzi, L.; Dantal, J.; Shihab, F.; Mulgaonkar, S.; Kim, Y.S.; et al. Review of cytomegalovirus infection findings with mammalian target of rapamycin inhibitor-based immunosuppressive therapy in de novo renal transplant recipients. Transplantation 2012, 93, 1075–1085. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Fantus, D.; Thomson, A.W. Evolving perspectives of mTOR complexes in immunity and transplantation: mTOR in immunity and transplantation. Am. J. Transplant. 2015, 15, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S. Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant. Proc. 2003, 35, S7–S14. [Google Scholar] [CrossRef]
- Buchkovich, N.J.; Yu, Y.; Zampieri, C.A.; Alwine, J.C. The TORrid affairs of viruses: Effects of mammalian DNA viruses on the PI3K–Akt–mTOR signalling pathway. Nat. Rev. Microbiol. 2008, 6, 266–275. [Google Scholar] [CrossRef]
- Roy, S.; Arav-Boger, R. New cell-signaling pathways for controlling cytomegalovirus replication: Cellular control of CMV replication. Am. J. Transplant. 2014, 14, 1249–1258. [Google Scholar] [CrossRef]
- Liacini, A.; Seamone, M.E.; Muruve, D.A.; Tibbles, L.A. Anti-BK virus mechanisms of sirolimus and leflunomide alone and in combination: Toward a new therapy for BK virus infection. Transplantation 2010, 90, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H.; Yakhontova, K.; Lu, M.; Manzetti, J. BK polyomavirus replication in renal tubular epithelial cells is inhibited by Sirolimus, but activated by Tacrolimus through a pathway involving FKBP-12: BKPyV inhibition by Sirolimus requires FKBP-12. Am. J. Transplant. 2016, 16, 821–832. [Google Scholar] [CrossRef]
- Hartmann, B. p70S6 kinase phosphorylation for pharmacodynamic monitoring. Clin. Chim. Acta 2012, 413, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, B.; Schmid, G.; Graeb, C.; Bruns, C.J.; Fischereder, M.; Jauch, K.-W.; Heeschen, C.; Guba, M.; Hartmann, G.S.B. Biochemical monitoring of mTOR inhibitor-based immunosuppression following kidney transplantation: A novel approach for tailored immunosuppressive therapy. Kidney Int. 2005, 68, 2593–2598. [Google Scholar] [CrossRef]
- Hasan, M.R.; Tan, R.; Al-Rawahi, G.; Thomas, E.; Tilley, P. Comparative evaluation of laboratory developed real-time PCR assays and RealStar® BKV PCR Kit for quantitative detection of BK polyomavirus. J. Virol. Methods 2016, 234, 80–86. [Google Scholar] [CrossRef]
- Drachenberg, C.B.; Papadimitriou, J.C.; Hirsch, H.H.; Wali, R.; Crowder, C.; Nogueira, J.; Cangro, C.B.; Mendley, S.; Mian, A.; Ramos, E. Histological patterns of polyomavirus nephropathy: Correlation with graft outcome and viral load. Am. J. Transplant. 2004, 4, 2082–2092. [Google Scholar] [CrossRef]
- Schnepf, N.; Scieux, C.; Resche-Riggon, M.; Feghoul, L.; Xhaard, A.; Gallien, S.; Molina, J.M.; Socié, G.; Viglietti, D.; Simon, F.; et al. Fully automated quantification of Cytomeg-alovirus (CMV) in whole blood with the New Sensitive Abbott RealTime CMV assay in the era of the CMV International Standard. J. Clin. Microbiol. 2013, 51, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Hoerning, A.; Wilde, B.; Wang, J.; Tebbe, B.; Jing, L.; Wang, X.; Jian, F.; Zhu, J.; Dolff, S.; Kribben, A.; et al. Pharmacodynamic monitoring of mammalian target of Ra-pamycin inhibition by phosphoflow cytometric determination of p70S6 kinase activity. Transplantation 2015, 99, 210–219. [Google Scholar] [CrossRef]
- Dupont, L.; Reeves, M.B. Cytomegalovirus latency and reactivation: Recent insights into an age old problem. Rev. Med. Virol. 2016, 26, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Wills, M.R.; Poole, E.; Lau, B.; Krishna, B.; Sinclair, J.H. The immunology of human cytomegalovirus latency: Could latent infection be cleared by novel immunotherapeutic strategies? Cell Mol. Immunol. 2015, 12, 128–138. [Google Scholar] [CrossRef]
- Gu, Z.-Y.; Li, Q.; Si, Y.-L.; Li, X.; Hao, H.-J.; Song, H.-J. Prevalence of BK virus and JC virus in peripheral blood leukocytes and normal arterial walls in healthy individuals in China. J. Med. Virol. 2003, 70, 600–605. [Google Scholar] [CrossRef]
- Haghighi, M.F.; Seyyedi, N.; Farhadi, A.; Zare, F.; Kasraian, L.; Dehbidi, G.R.R.; Ranjbaran, R.; Behzad-Behbahani, A. Polyomaviruses BK and JC DNA infection in peripheral blood cells from blood donors. Braz. J. Infect. Dis. 2019, 23, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Jouve, T.; Rostaing, L.; Malvezzi, P. Place of mTOR inhibitors in management of BKV infection after kidney transplantation. J. Nephropathol. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Korth, J.; Anastasiou, O.E.; Verheyen, J.; Dickow, J.; Sertznig, H.; Frericks, N.; Bleekmann, B.; Kribben, A.; Brinkhoff, A.; Wilde, B.; et al. Impact of immune suppressive agents on the BK-Polyomavirus non coding control region. Antivir. Res. 2018, 159, 68–76. [Google Scholar] [CrossRef]
- Kudchodkar, S.B.; Del Prete, G.Q.; Maguire, T.G.; Alwine, J.C. AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. J. Virol. 2007, 81, 3649–3651. [Google Scholar] [CrossRef] [PubMed]
- Kudchodkar, S.B.; Yu, Y.; Maguire, T.G.; Alwine, J.C. Human cytomegalovirus infection alters the substrate specificities and ra-pamycin sensitivities of raptor- and rictor-containing complexes. Proc. Natl. Acad. Sci. USA 2006, 103, 14182–14187. [Google Scholar] [CrossRef]
- Moorman, N.J.; Cristea, I.M.; Terhune, S.S.; Rout, M.P.; Chait, B.T.; Shenk, T. Human cytomegalovirus protein ul38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell Host Microbe 2008, 3, 253–262. [Google Scholar] [CrossRef]
- Clippinger, A.J.; Maguire, T.G.; Alwine, J.C. Human cytomegalovirus infection maintains mTOR activity and its perinuclear localization during amino acid deprivation. J. Virol. 2011, 85, 9369–9376. [Google Scholar] [CrossRef] [PubMed]
- Young, V.P.; Mariano, M.C.; Tu, C.C.; Allaire, K.M.; Avdic, S.; Slobedman, B.; Spencer, J.V. Modulation of the host environment by human cytomegalovirus with viral interleukin 10 in peripheral blood. J. Infect. Dis. 2017, 215, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Lee, S.J.; Kim, S.-H.; Han, J.; Bae, J.; Kim, S.J.; Park, C.-G.; Chun, T. IL-10 inhibits the starvation induced autophagy in macrophages via class I phosphatidylinositol 3-kinase (PI3K) pathway. Mol. Immunol. 2011, 48, 720–727. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Pollizzi, K.N.; Waickman, A.T.; Heikamp, E.; Meyers, D.J.; Horton, M.R.; Xiao, B.; Worley, P.F.; Powell, J.D. The kinase mTOR regulates the differ-entiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC. Nat. Immunol. 2011, 12, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Dauby, N.; Kummert, C.; LeComte, S.; Liesnard, C.; Delforge, M.-L.; Donner, C.; Marchant, A. Primary human cytomegalovirus infection induces the expansion of virus-specific activated and atypical memory B cells. J. Infect. Dis. 2014, 210, 1275–1285. [Google Scholar] [CrossRef] [PubMed]



| Variable | Measure | Overall (n = 48) | Female (n = 18) | Male (n = 30) | |
|---|---|---|---|---|---|
| Age [y] | Mean (SD) | 53.8 (13.6) | 54.5 (10.7) | 53.4 (15.2) | |
| Time since Transplantation [y] | Median (IQR) | 2.1 (1.0; 5.0) | 1.7 (1.1; 4.5) | 2.5 (0.8; 6.1) | |
| Serum-Creatinine at day of measurement [mg/dL] | Mean (SD) | 1.92 ± 1.34 | 1.90 ± 2.07 | 1.93 ± 0.63 | |
| Immunosuppression | |||||
| CNI-based | n | 24 | 12 | 12 | |
| mTORi-based | n | 24 | 6 | 18 | |
| EVR only | n | 5 | 2 | 3 | |
| EVR+CNI | n | 19 | 4 | 15 | |
| Virus Replication | CNI-based | mTORi-Based | |||
| HCMV | |||||
| D−/R− without viremia | n | 6 | 8 | 3 | 11 |
| D−/R− with viremia | n | 0 | 0 | 0 | 0 |
| D+, or −/R+ without viremia | n | 4 | 7 | 5 | 6 |
| D+, or −/R+ with viremia | n | 8 | 2 | 5 | 5 |
| D+/R− without viremia | n | 3 | 3 | 2 | 4 |
| D+/R− with viremia | n | 2 | 4 | 2 | 4 |
| Active replication at day of measurement | 0 | 0 | 0 | 0 | |
| Time since last reactivation [d] | Median (IQR) | 167 (24; 411) | - | - | |
| BKPyV | |||||
| No viremia | 16 | 14 | 13 | 17 | |
| Viremia | 8 | 10 | 5 | 13 | |
| PyVAN | 1 | 5 | 1 | 5 | |
| PyVAN with viremia at day of measurement | 0 | 5 | 1 | 4 | |
| Time since last reactivation [d] | Median (IQR) | 263 (5; 462) | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherneha, M.; Korth, J.; Kaulfuß, M.; Trilling, M.; Widera, M.; Rohn, H.; Dolff, S.; Babel, N.; Hoerning, A.; Kribben, A.; et al. Reactivations of Latent Viral Infections Are Associated with an Increased Thr389 p70S6k Phosphorylation in Peripheral Lymphocytes of Renal Transplant Recipients. Viruses 2021, 13, 424. https://doi.org/10.3390/v13030424
Cherneha M, Korth J, Kaulfuß M, Trilling M, Widera M, Rohn H, Dolff S, Babel N, Hoerning A, Kribben A, et al. Reactivations of Latent Viral Infections Are Associated with an Increased Thr389 p70S6k Phosphorylation in Peripheral Lymphocytes of Renal Transplant Recipients. Viruses. 2021; 13(3):424. https://doi.org/10.3390/v13030424
Chicago/Turabian StyleCherneha, Maxim, Johannes Korth, Meike Kaulfuß, Mirko Trilling, Marek Widera, Hana Rohn, Sebastian Dolff, Nina Babel, André Hoerning, Andreas Kribben, and et al. 2021. "Reactivations of Latent Viral Infections Are Associated with an Increased Thr389 p70S6k Phosphorylation in Peripheral Lymphocytes of Renal Transplant Recipients" Viruses 13, no. 3: 424. https://doi.org/10.3390/v13030424
APA StyleCherneha, M., Korth, J., Kaulfuß, M., Trilling, M., Widera, M., Rohn, H., Dolff, S., Babel, N., Hoerning, A., Kribben, A., & Witzke, O. (2021). Reactivations of Latent Viral Infections Are Associated with an Increased Thr389 p70S6k Phosphorylation in Peripheral Lymphocytes of Renal Transplant Recipients. Viruses, 13(3), 424. https://doi.org/10.3390/v13030424








