Structure Unveils Relationships between RNA Virus Polymerases
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Identification of a Common Structural Core for Viral RdRps
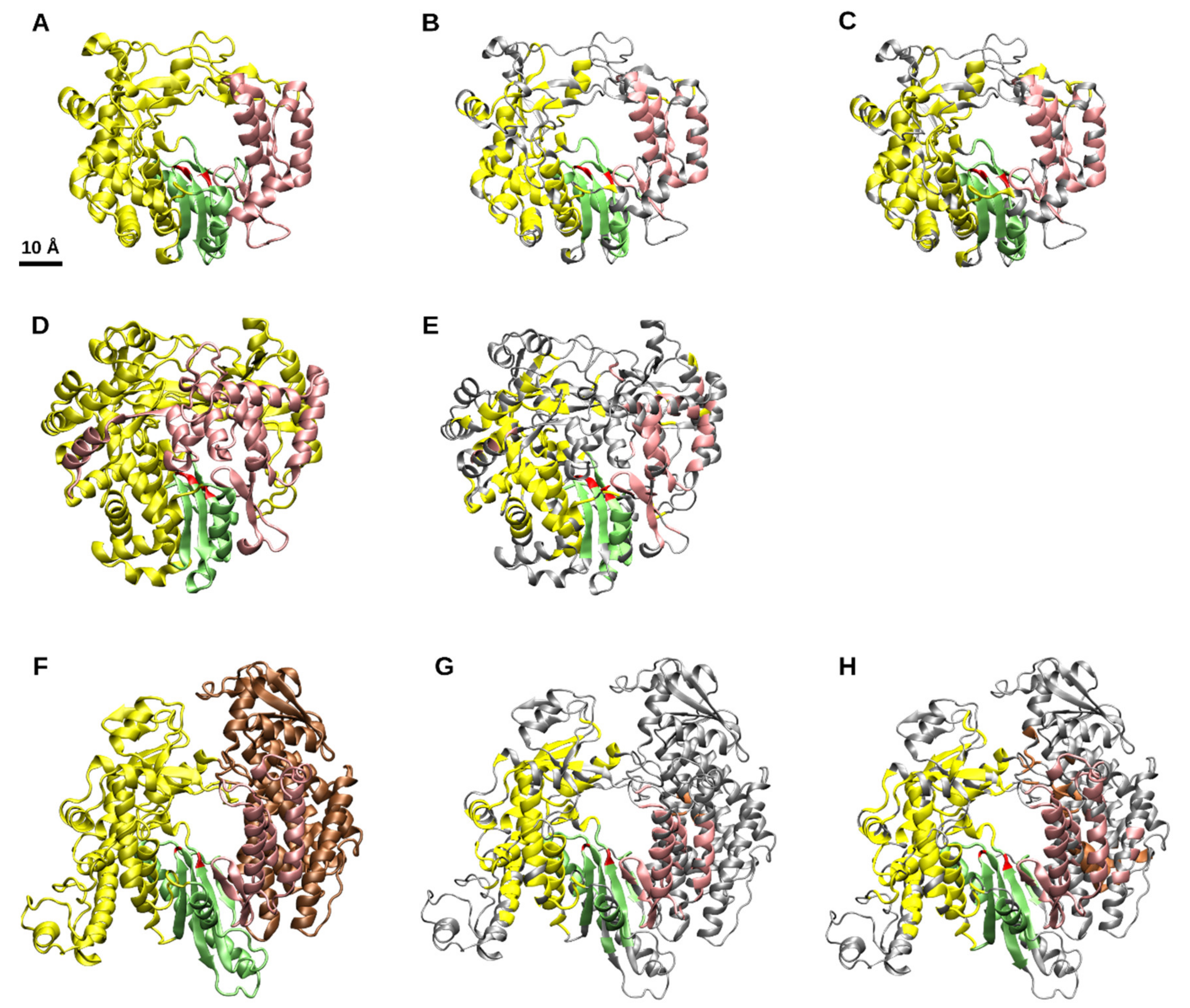

3.2. Structure-Based Distance Tree and Overall Grouping of the RdRp Structures
3.3. Stability of the Structure-Based Distance Tree and the Common Structural Core
3.4. RdRps from (+)RNA and dsRNA Viruses with Permutated Catalytic Site Sequence Group Together
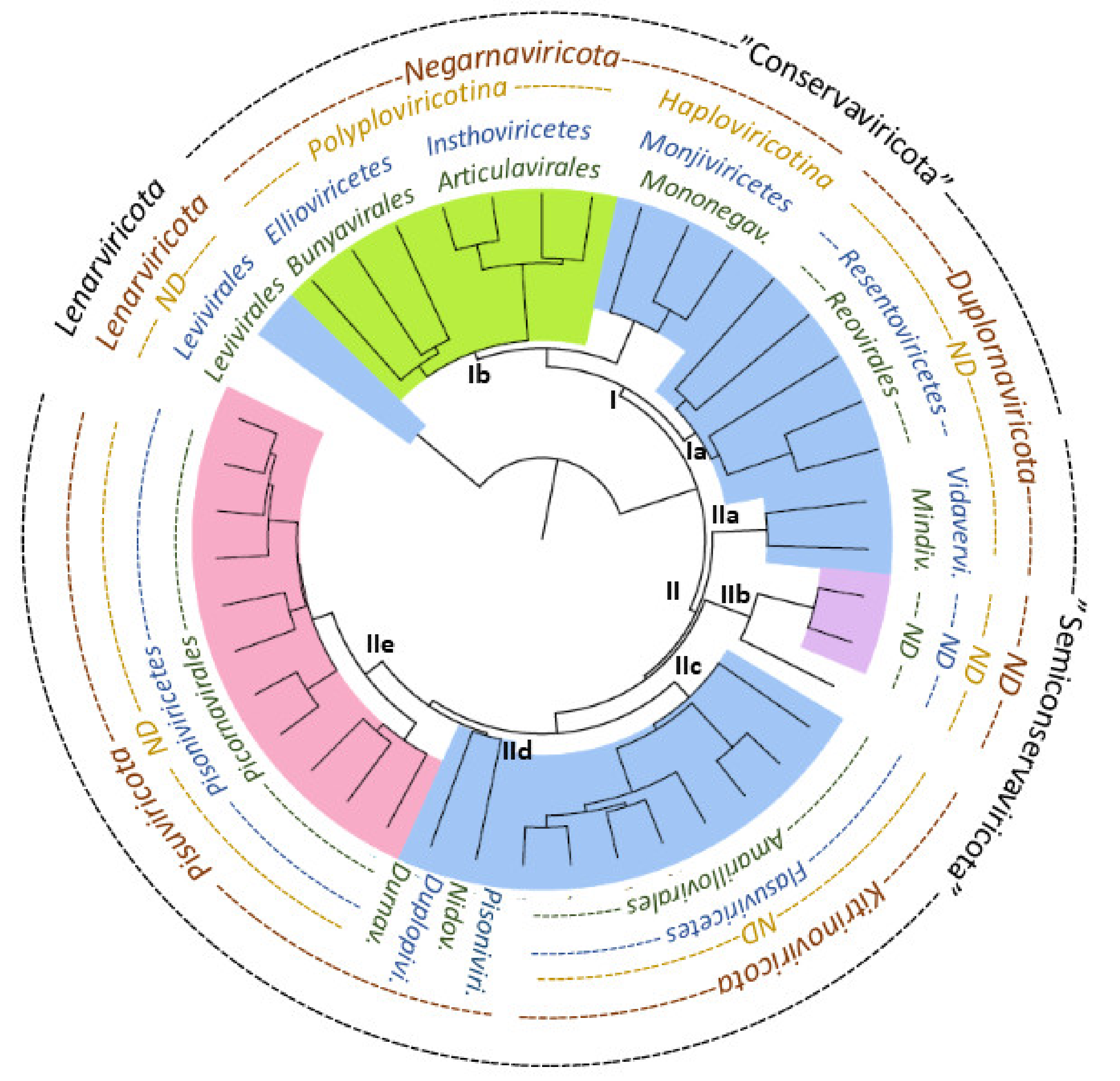
3.5. Early Separation of Semi-Conservative and Conservative Mechanism of Viral RNA Transcription
3.6. Branch I: Origin of (−)RNA Virus RdRps and Development of RNA-Primed Initiation
3.7. Branch II: Development of Protein Priming
3.8. Considerations on the RNA Virus Taxonomy
3.9. Evolutionary Implications and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butcher, S.J.; Grimes, J.M.; Makeyev, E.V.; Bamford, D.H.; Stuart, D.I. A mechanism for initiating RNA-dependent RNA polymerization. Nature 2001, 410, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Pflug, A.; Guilligay, D.; Reich, S.; Cusack, S. Structure of influenza A polymerase bound to the viral RNA promoter. Nature 2014, 516, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.L.; Long, A.M.; Schultz, S.C. Structure of the RNA-dependent RNA polymerase of poliovirus. Structure 1997, 5, 1109–1122. [Google Scholar] [CrossRef]
- Tao, Y.; Farsetta, D.L.; Nibert, M.L.; Harrison, S.C. RNA Synthesis in a cage—structural studies of reovirus polymerase λ3. Cell 2002, 111, 733–745. [Google Scholar] [CrossRef]
- Duffy, S.; Shackelton, L.A.; Holmes, E.C. Rates of evolutionary change in viruses: Patterns and determinants. Nat. Rev. Genet. 2008, 9, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Poch, O.; Sauvaget, I.; Delarue, M.; Tordo, N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989, 8, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Bruenn, J.A. A structural and primary sequence comparison of the viral RNA-dependent RNA polymerases. Nucleic Acids Res. 2003, 31, 1821–1829. [Google Scholar] [CrossRef]
- Lesburg, C.A.; Cable, M.B.; Ferrari, E.; Hong, Z.; Mannarino, A.F.; Weber, P.C. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nat. Struct. Biol. 1999, 6, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Pringle, F.M.; Zeddam, J.-L.; Luke, B.T.; Cameron, C.E.; Kalmakoff, J.; Hanzlik, T.N.; Gordon, K.H.J.; Ward, V.K. The palm subdomain-based active site is internally permuted in viral RNA-dependent RNA polymerases of an ancient lineage. J. Mol. Biol. 2002, 324, 47–62. [Google Scholar] [CrossRef]
- Lang, D.M.; Zemla, A.T.; Zhou, C.L.E. Highly similar structural frames link the template tunnel and NTP entry tunnel to the exterior surface in RNA-dependent RNA polymerases. Nucleic Acids Res. 2013, 41, 1464–1482. [Google Scholar] [CrossRef]
- Venkataraman, S.; Prasad, B.; Selvarajan, R. RNA dependent RNA polymerases: Insights from structure, function and evolution. Viruses 2018, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.I.; Kazlauskas, D.; Iranzo, J.; Lucía-Sanz, A.; Kuhn, J.H.; Krupovic, M.; Dolja, V.V.; Koonin, E.V. Origins and evolution of the global RNA virome. mBio 2018, 9, e02329-18. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Wolf, Y.I.; Krupovic, M.; Zhang, Y.-Z.; Maes, P.; Dolja, V.V.; Koonin, E.V. Classify viruses — the gain is worth the pain. Nature 2019, 566, 318–320. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M.; Varsani, A.; Wolf, Y.I.; Yutin, N.; Zerbini, F.M.; Kuhn, J.H. Global organization and proposed megataxonomy of the virus world. Microbiol. Mol. Biol. Rev. 2020, 84. [Google Scholar] [CrossRef] [PubMed]
- The 10th ICTV Report on Virus Taxonomy. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/ (accessed on 17 February 2021).
- Wolf, Y.I.; Silas, S.; Wang, Y.; Wu, S.; Bocek, M.; Kazlauskas, D.; Krupovic, M.; Fire, A.; Dolja, V.V.; Koonin, E.V. Doubling of the known set of RNA viruses by metagenomic analysis of an aquatic virome. Nat. Microbiol. 2020, 5, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Duchêne, S. Can sequence phylogenies safely infer the origin of the global virome? mBio 2019, 10, e00289-19. [Google Scholar] [CrossRef] [PubMed]
- Chothia, C.; Lesk, A.M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986, 5, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Illergård, K.; Ardell, D.H.; Elofsson, A. Structure is three to ten times more conserved than sequence—A study of structural response in protein cores. Proteins 2009, 77, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Mönttinen, H.A.M.; Ravantti, J.J.; Poranen, M.M. Common structural core of three-dozen residues reveals intersuperfamily relationships. Mol. Biol. Evol. 2016, 33, 1697–1710. [Google Scholar] [CrossRef] [PubMed]
- Mönttinen, H.A.M.; Ravantti, J.J.; Poranen, M.M. Structural comparison strengthens the higher-order classification of proteases related to chymotrypsin. PLoS ONE 2019, 14, e0216659. [Google Scholar] [CrossRef] [PubMed]
- Abrescia, N.G.A.; Bamford, D.H.; Grimes, J.M.; Stuart, D.I. Structure unifies the viral universe. Annu. Rev. Biochem. 2012, 81, 795–822. [Google Scholar] [CrossRef] [PubMed]
- Krupovic, M.; Bamford, D.H. Protein conservation in virus evolution. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; p. a0023265. ISBN 978-0-470-01617-6. [Google Scholar]
- Černý, J.; Černá Bolfíková, B.; Valdés, J.J.; Grubhoffer, L.; Růžek, D. Evolution of tertiary structure of viral RNA dependent polymerases. PLoS ONE 2014, 9, e96070. [Google Scholar] [CrossRef]
- Mönttinen, H.A.M.; Ravantti, J.J.; Stuart, D.I.; Poranen, M.M. Automated structural comparisons clarify the phylogeny of the right-hand-shaped polymerases. Mol. Biol. Evol 2014, 31, 2741–2752. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Li, Z.; Jenni, S.; Rahmeh, A.A.; Morin, B.M.; Grant, T.; Grigorieff, N.; Harrison, S.C.; Whelan, S.P.J. Structure of the L protein of vesicular stomatitis virus from electron cryomicroscopy. Cell 2015, 162, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Xu, X.; Jing, J.; Wang, M.; Peng, Q.; Liu, S.; Wu, Y.; Bao, X.; Wang, P.; Qi, J.; et al. Structural insight into arenavirus replication machinery. Nature 2020, 579, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, P.; Malet, H.; Cusack, S.; Reguera, J. Structural insights into bunyavirus replication and its regulation by the vRNA promoter. Cell 2015, 161, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Qian, X.; Lattmann, S.; El Sahili, A.; Yeo, T.H.; Jia, H.; Cressey, T.; Ludeke, B.; Noton, S.; Kalocsay, M.; et al. Structure of the human metapneumovirus polymerase phosphoprotein complex. Nature 2020, 577, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.; Thorkelsson, S.R.; Quemin, E.R.J.; Meier, K.; Kouba, T.; Gogrefe, N.; Busch, C.; Reindl, S.; Günther, S.; Cusack, S.; et al. Structural and functional characterization of the severe fever with thrombocytopenia syndrome virus L protein. Nucleic Acids Res. 2020, 48, 5749–5765. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Hillen, H.S.; Kokic, G.; Farnung, L.; Dienemann, C.; Tegunov, D.; Cramer, P. Structure of replicating SARS-CoV-2 polymerase. Nature 2020, 584, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Ravantti, J.; Bamford, D.; Stuart, D.I. Automatic comparison and classification of protein structures. J. Struct. Biol. 2013, 183, 47–56. [Google Scholar] [CrossRef]
- Andreeva, A.; Howorth, D.; Chothia, C.; Kulesha, E.; Murzin, A.G. SCOP2 prototype: A new approach to protein structure mining. Nucleic Acids Res. 2014, 42, D310–D314. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP-phylogeny inference package (Version 3.2). Cladistics 1989, 5, 164–166. [Google Scholar]
- Huson, D.H.; Scornavacca, C. Dendroscope 3: An interactive tool for rooted phylogenetic trees and networks. Syst. Biol. 2012, 61, 1061–1067. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (ITOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Serra, F.; Bork, P. ETE 3: Reconstruction, analysis, and visualization of phylogenomic data. Mol. Biol. Evol. 2016, 33, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- De Farias, S.T.; dos Santos Junior, A.P.; Rêgo, T.G.; José, M.V. Origin and evolution of RNA-dependent RNA polymerase. Front. Genet. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-NCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, D.S.; Buxaderas, M.; Rodríguez, J.F.; Verdaguer, N. The structure of the RNA-dependent RNA polymerase of a permutotetravirus suggests a link between primer-dependent and primer-independent polymerases. PLoS Pathog. 2015, 11, e1005265. [Google Scholar] [CrossRef]
- Jácome, R.; Becerra, A.; Ponce de León, S.; Lazcano, A. Structural analysis of monomeric RNA-dependent polymerases: Evolutionary and therapeutic implications. PLoS ONE 2015, 10, e0139001. [Google Scholar] [CrossRef] [PubMed]
- Poranen, M.M.; Bamford, D.H. Assembly of large icosahedral double-stranded RNA viruses. Adv. Exp. Med. Biol. 2012, 726, 379–402. [Google Scholar] [CrossRef]
- Luque, D.; Mata, C.; Suzuki, N.; Ghabrial, S.; Castón, J. Capsid structure of dsRNA fungal viruses. Viruses 2018, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- El Omari, K.; Sutton, G.; Ravantti, J.J.; Zhang, H.; Walter, T.S.; Grimes, J.M.; Bamford, D.H.; Stuart, D.I.; Mancini, E.J. Plate tectonics of virus shell assembly and reorganization in phage Φ8, a distant relative of mammalian reoviruses. Structure 2013, 21, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.M.; Burroughs, J.N.; Gouet, P.; Diprose, J.M.; Malby, R.; Ziéntara, S.; Mertens, P.P.; Stuart, D.I. The atomic structure of the bluetongue virus core. Nature 1998, 395, 470–478. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M. Origins and evolution of viruses of eukaryotes: The ultimate modularity. Virology 2015, 479–480, 2–25. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, K.; Yu, X.; Chang, W.; Sun, J.; Zhou, Z.H. In situ structures of the segmented genome and RNA polymerase complex inside a dsRNA virus. Nature 2015, 527, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ilca, S.L.; Huiskonen, J.T.; Poranen, M.M. Dual role of a viral polymerase in viral genome replication and particle self-assembly. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shivakoti, S.; Ding, K.; Cui, Y.; Roy, P.; Zhou, Z.H. In situ structures of RNA-dependent RNA polymerase inside bluetongue virus before and after uncoating. Proc. Natl. Acad. Sci. USA 2019, 116, 16535–16540. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.C.; Sarin, L.P.; Bahar, M.W.; Myers, R.A.; Stuart, D.I.; Bamford, D.H.; Grimes, J.M. The N-terminus of the RNA polymerase from infectious pancreatic necrosis virus is the determinant of genome attachment. PLoS Pathog. 2011, 7, e1002085. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, F.; Chevalier, C.; Gutsche, I.; Pous, J.; Navaza, J.; Bressanelli, S.; Delmas, B.; Rey, F.A. The birnavirus crystal structure reveals structural relationships among icosahedral viruses. Cell 2005, 120, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Petitpas, I.; Navaza, J.; Lepault, J.; Kohli, E.; Pothier, P.; Prasad, B.V.; Cohen, J.; Rey, F.A. Atomic structure of the major capsid protein of rotavirus: Implications for the architecture of the virion. EMBO J. 2001, 20, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; El Omari, K.; Sun, X.; Ilca, S.L.; Kotecha, A.; Stuart, D.I.; Poranen, M.M.; Huiskonen, J.T. Double-stranded RNA virus outer shell assembly by bona fide domain-swapping. Nat. Commun. 2017, 8, 14814. [Google Scholar] [CrossRef] [PubMed]
- Poranen, M.M.; Daugelavicius, R.; Ojala, P.M.; Hess, M.W.; Bamford, D.H. A novel virus-host cell membrane interaction. Membrane voltage-dependent endocytic-like entry of bacteriophage Φ6 nucleocapsid. J. Cell Biol. 1999, 147, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Vakharia, V.N.; Tao, Y.J. The structure of a birnavirus polymerase reveals a distinct active site topology. Proc. Natl. Acad. Sci. USA 2007, 104, 7385–7390. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Zhou, K.; Sun, J.; Zhou, Z.H. Conservative transcription in three steps visualized in a double-stranded RNA virus. Nat. Struct. Mol. Biol. 2019, 26, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Celma, C.C.; Zhang, X.; Chang, T.; Shen, W.; Atanasov, I.; Roy, P.; Zhou, Z.H. In situ structures of rotavirus polymerase in action and mechanism of mRNA transcription and release. Nat. Commun. 2019, 10, 2216. [Google Scholar] [CrossRef]
- Van Etten, J.L.; Burbank, D.E.; Cuppels, D.A.; Lane, L.C.; Vidaver, A.K. Semiconservative synthesis of single-stranded RNA by bacteriophage Φ6 RNA polymerase. J. Virol. 1980, 33, 769–773. [Google Scholar] [CrossRef]
- Collier, A.M.; Lyytinen, O.L.; Guo, Y.R.; Toh, Y.; Poranen, M.M.; Tao, Y.J. Initiation of RNA polymerization and polymerase encapsidation by a small dsRNA virus. PLoS Pathog. 2016, 12, e1005523. [Google Scholar] [CrossRef]
- Reguera, J.; Gerlach, P.; Cusack, S. Towards a structural understanding of RNA synthesis by negative strand RNA viral polymerases. Curr. Opin. Struct. Biol. 2016, 36, 75–84. [Google Scholar] [CrossRef]
- Dubankova, A.; Boura, E. Structure of the yellow fever NS5 protein reveals conserved drug targets shared among flaviviruses. Antiviral Res. 2019, 169, 104536. [Google Scholar] [CrossRef]
- Ferrer-Orta, C.; Arias, A.; Escarmís, C.; Verdaguer, N. A Comparison of viral RNA-dependent RNA polymerases. Curr. Opin. Struct. Biol. 2006, 16, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Vives-Adrian, L.; Lujan, C.; Oliva, B.; van der Linden, L.; Selisko, B.; Coutard, B.; Canard, B.; van Kuppeveld, F.J.M.; Ferrer-Orta, C.; Verdaguer, N. The crystal structure of a cardiovirus RNA-dependent RNA polymerase reveals an unusual conformation of the polymerase active site. J. Virol. 2014, 88, 5595–5607. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.W.G.; Westaway, E.G. Replication strategy of Kunjin virus: Evidence for recycling role of replicative form RNA as template in semiconservative and asymmetric replication. Virology 1985, 140, 68–79. [Google Scholar] [CrossRef]
- Baltimore, D. Structure of the poliovirus replicative intermediate RNA. J. Mol. Biol. 1968, 32, 359–368. [Google Scholar] [CrossRef]
- Buck, K.W. Semi-conservative replication of double-stranded RNA by a virion-associated RNA polymerase. Biochem. Biophys. Res. Commun. 1978, 84, 639–645. [Google Scholar] [CrossRef]
- Michel, M.R.; Gomatos, P.J. Semliki forest virus-specific RNAs synthesized in vitro by enzyme from infected BHK cells. J. Virol. 1973, 11, 900–914. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Mizumoto, K.; Ishihama, A. Minimum molecular architectures for transcription and replication of the influenza virus. Proc. Natl. Acad. Sci. USA 2002, 99, 13166–13171. [Google Scholar] [CrossRef] [PubMed]
- Morin, B.; Kranzusch, P.J.; Rahmeh, A.A.; Whelan, S.P.J. The polymerase of negative-stranded RNA viruses. Curr. Opin. Virol. 2013, 3, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Morin, B.; Rahmeh, A.A.; Whelan, S.P.J. Mechanism of RNA synthesis initiation by the vesicular stomatitis virus polymerase. EMBO J. 2012, 31, 1320–1329. [Google Scholar] [CrossRef]
- Plotch, S.J.; Bouloy, M.; Ulmanen, I.; Krug, R.M. A unique cap(M7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell 1981, 23, 847–858. [Google Scholar] [CrossRef]
- Reguera, J.; Weber, F.; Cusack, S. Bunyaviridae RNA polymerases (L-protein) have an N-terminal, influenza-like endonuclease domain, essential for viral cap-dependent transcription. PLoS Pathog. 2010, 6, e1001101. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.; Guilligay, D.; Pflug, A.; Malet, H.; Berger, I.; Crépin, T.; Hart, D.; Lunardi, T.; Nanao, M.; Ruigrok, R.W.H.; et al. Structural insight into cap-snatching and RNA synthesis by influenza polymerase. Nature 2014, 516, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J.W.; Fodor, E. Influenza virus RNA polymerase: Insights into the mechanisms of viral RNA synthesis. Nat. Rev. Microbiol. 2016, 14, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Ogino, M.; Gupta, N.; Green, T.J.; Ogino, T. A dual-functional priming-capping loop of rhabdoviral RNA polymerases directs terminal de novo initiation and capping intermediate formation. Nucleic Acids Res. 2019, 47, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Delmas, B.; Attoui, H.; Ghosh, S.; Malik, Y.S.; Mundt, E.; Vakharia, V.N. ICTV report consortium ICTV virus taxonomy profile: Birnaviridae. J. Gen. Virol. 2019, 100, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Rohayem, J.; Robel, I.; Jäger, K.; Scheffler, U.; Rudolph, W. Protein-primed and de novo initiation of RNA synthesis by norovirus 3Dpol. J. Virol. 2006, 80, 7060–7069. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Adams, M.J.; Benkő, M.; Breitbart, M.; Brister, J.R.; Carstens, E.B.; Davison, A.J.; Delwart, E.; Gorbalenya, A.E.; Harrach, B.; et al. Virus taxonomy in the age of metagenomics. Nat. Rev. Microbiol. 2017, 15, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E. Increasing the number of available ranks in virus taxonomy from five to ten and adopting the Baltimore classes as taxa at the basal rank. Arch. Virol. 2018, 163, 2933–2936. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, P.M.; Gibbs, M.J.; Gould, E.A.; Holmes, E.C. A reevaluation of the higher taxonomy of viruses based on RNA polymerases. J. Virol. 1996, 70, 6083–6096. [Google Scholar] [CrossRef] [PubMed]
- De Farias, S.T.; Rêgo, T.G.; José, M.V. tRNA core hypothesis for the transition from the RNA world to the ribonucleoprotein world. Life 2016, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. ‘It will change everything’: DeepMind’s AI makes gigantic leap in solving protein structures. Nature 2020, 588, 203–204. [Google Scholar] [CrossRef] [PubMed]
| Genome | Kingdom | Phylum; Subphylum | Class; Order | Family; Subfamily | Genus | Virus |
|---|---|---|---|---|---|---|
| (−)RNA | Orthornavirae | Negarnaviricota; Polyploviricotina | Ellioviricetes; Bunyavirales | Arenaviridae | Mammarenavirus | Machupo mammarenavirus |
| Peribunyaviridae | Orthobunyavirus | La Crosse orthobunyavirus | ||||
| Phenuiviridae | Bandavirus | Huaiyangshan banyangvirus | ||||
| Insthoviricetes; Articulavirales | Orthomyxoviridae | Alphainfluenzavirus | influenza A virus | |||
| Betainfluenzavirus | influenza B virus | |||||
| Gammainfluenzavirus | influenza C virus | |||||
| Deltainfluenzavirus | influenza D virus | |||||
| Negarnaviricota; Haploviricotina | Monjiviricetes; Mononegavirales | Pneumoviridae | Metapneumovirus | human metapneumovirus | ||
| Rhabdoviridae | Lyssavirus | rabies lyssavirus | ||||
| Vesiculovirus | vesicular stomatitis Indian virus | |||||
| dsRNA | Duplornaviricota | Resentoviricetes; Reovirales | Reoviridae; Sedoreovirinae | Rotavirus | rotavirus A | |
| Orbivirus | bluetongue virus | |||||
| Reoviridae; Spinareovirinae | Aquareovirus | aquareovirus G | ||||
| Cypovirus | Bombyx mori cypovirus 1 | |||||
| Orthoreovirus | mammalian orthoreovirus | |||||
| Vidaverviricetes; Mindivirales | Cystoviridae | Cystovirus | Pseudomonas phage phi6 | |||
| Pseudomonas phage phi12 | ||||||
| Not determined (ND) | ND; ND | Birnaviridae | Avibirnavirus | infectious bursal disease virus | ||
| Aquabirnavirus | infectious pancreatic necrosis virus | |||||
| (+)RNA | Permutotetraviridae | Alphapermutotetravirus | thosean asigna virus | |||
| Kitrinoviricota | Flasuviricetes; Amarillovirales | Flaviviridae | Hepacivirus | hepacivirus C | ||
| Pestivirus | pestivirus A | |||||
| pestivirus C | ||||||
| Flavivirus | Dengue virus | |||||
| Zika virus | ||||||
| West Nile virus | ||||||
| Japanese encephalitis virus | ||||||
| Yellow fever virus | ||||||
| ds | Pisuviricota | Duplopiviricetes; Durnavirales | Picobirnaviridae | Picobirnavirus | human picobirnavirus | |
| (+)RNA | Pisoniviricetes; Nidovirales | Coronaviridae; Orthocoronavirinae | Betacoronavirus | SARS-CoV-2 2 | ||
| Pisoniviricetes; Picornavirales | Picornaviridae | Kobuvirus | aichivirus C | |||
| Cardiovirus | cardiovirus A | |||||
| Aphthovirus | foot-and-mouth disease virus | |||||
| Enterovirus | enterovirus A71 (enterovirus A) | |||||
| coxsackievirus B3 (enterovirus B) | ||||||
| poliovirus 1 (enterovirus C) | ||||||
| human rhinovirus A | ||||||
| Sicinivirus | sicinivirus A | |||||
| Caliciviridae | Norovirus | Norwalk virus | ||||
| Lagovirus | rabbit hemorrhagic disease virus | |||||
| Sapovirus | Sapporo virus | |||||
| Lenarviricota | Allassoviricetes; Levivirales | Leviviridae | Allolevivirus | Enterobacteria phage Qβ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mönttinen, H.A.M.; Ravantti, J.J.; Poranen, M.M. Structure Unveils Relationships between RNA Virus Polymerases. Viruses 2021, 13, 313. https://doi.org/10.3390/v13020313
Mönttinen HAM, Ravantti JJ, Poranen MM. Structure Unveils Relationships between RNA Virus Polymerases. Viruses. 2021; 13(2):313. https://doi.org/10.3390/v13020313
Chicago/Turabian StyleMönttinen, Heli A. M., Janne J. Ravantti, and Minna M. Poranen. 2021. "Structure Unveils Relationships between RNA Virus Polymerases" Viruses 13, no. 2: 313. https://doi.org/10.3390/v13020313
APA StyleMönttinen, H. A. M., Ravantti, J. J., & Poranen, M. M. (2021). Structure Unveils Relationships between RNA Virus Polymerases. Viruses, 13(2), 313. https://doi.org/10.3390/v13020313






