The Paradoxes of Viral mRNA Translation during Mammalian Orthoreovirus Infection
Abstract
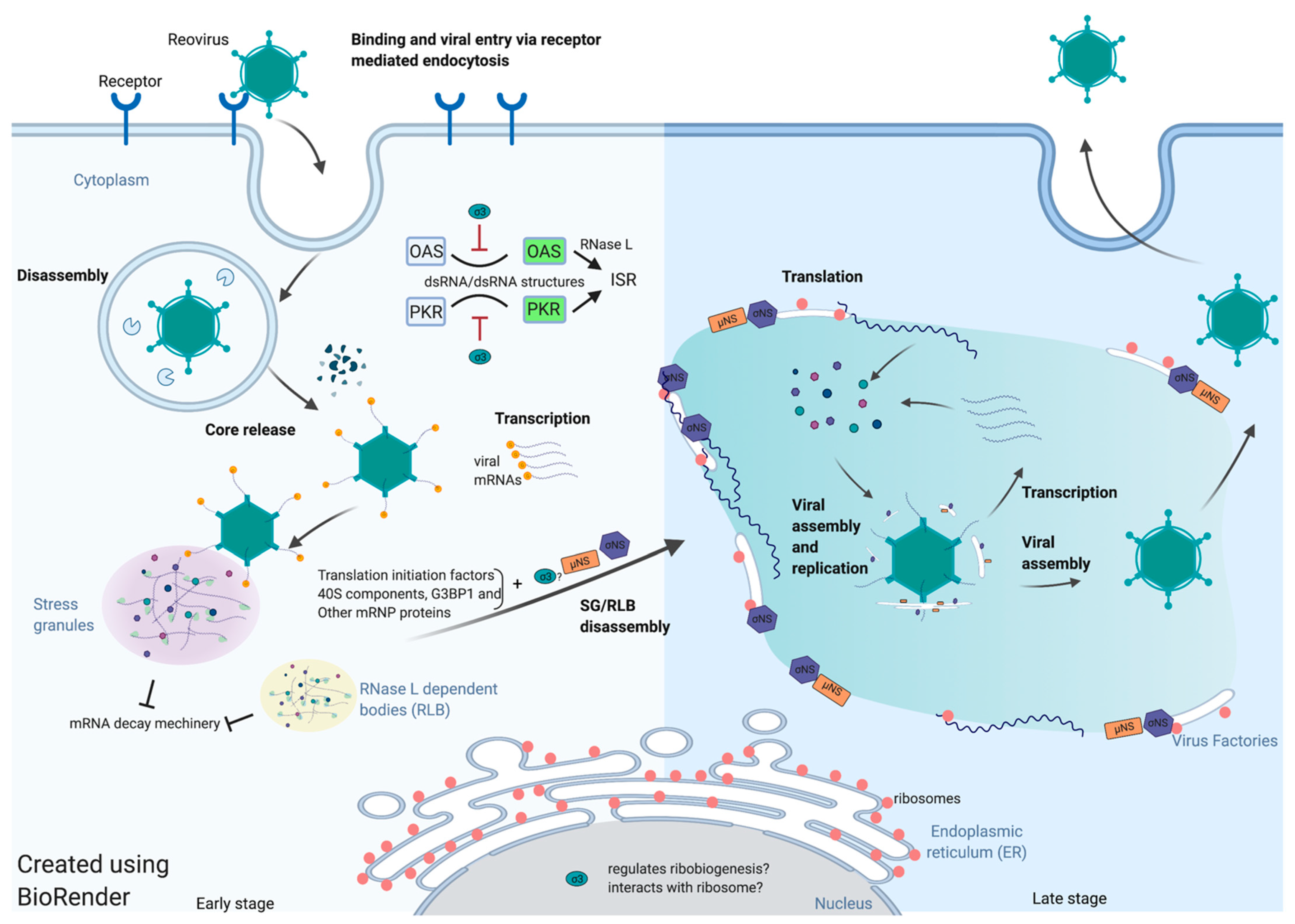
1. Introduction
2. Reovirus mRNA Structure and Stability
3. Challenges for Viral Translation Early in Infection
4. Reovirus and the Integrated Stress Response (ISR)
5. Compartmentalization of Translation
6. Role of PKR and RNase L in Translation Suppression
7. Viral Proteins that Associate with Ribosomes/Polysomes
8. Concluding Remarks
- ○
- What types of RNP granules or RNP granules combinations are formed at early stages after infection, and what function do RNP granules have during viral infection?
- ○
- Are early viral mRNAs translated within RNP granules?
- ○
- What is the mechanism by which reoviruses disrupt SGs/RNP granules?
- ○
- How does σ3 regulate reovirus translation?
- ○
- What is the functional significance of the nuclear localization of σ3?
- ○
- Are uncapped viral mRNAs synthesized late in infection, and if so, how does σ3 promote their translation?
- ○
- How does the activation of the ISR and PKR or RNase L benefit viral replication?
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, R.J.; Hellen, C.U.T.; Pestova, T.V. The mechanism of Eukaryotic Translation Initiation and Principles of Its Regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef]
- Schuller, A.P.; Green, R. Roadblocks and Resolutions in Eukaryotic Translation. Nat. Rev. Mol. Cell Biol. 2018, 19, 526–541. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of Translation Initiation in Eukaryotes: Mechanisms and Biological Targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.-D.; Neault, S.; Pelin, A.; Alain, T. Emerging Translation Strategies during Virus–Host Interaction. Wiley Interdiscip. Rev. RNA 2021, 12, e1619. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wu, P.; Deng, S.; Zhang, H.; Hou, Y.; Hu, Z.; Zhang, J.; Chen, X.; Yang, J.-R. Dissimilation of Synonymous Codon Usage Bias in Virus–Host Coevolution Due to Translational Selection. Nat. Ecol. Evol. 2020, 4, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Quax, T.E.F.; Claassens, N.J.; Söll, D.; van der Oost, J. Codon Bias as a Means to Fine-Tune Gene Expression. Mol. Cell 2015, 59, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Mioduser, O.; Goz, E.; Tuller, T. Significant Differences in Terms of Codon Usage Bias between Bacteriophage Early and Late Genes: A Comparative Genomics Analysis. BMC Genom. 2017, 18, 866. [Google Scholar] [CrossRef] [PubMed]
- Fields, B.N.; Knipe, D.M.; David, M.; Howley, P.M. Fields Virology, 6th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; ISBN 978-1-4511-0563-6. [Google Scholar]
- Shatkin, A.J.; LaFiandra, A.J. Transcription by Infectious Subviral Particles of Reovirus. J. Virol. 1972, 10, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Millward, S.; Graham, A.F. Regulation of Transcription of the Reovirus Genome. J. Mol. Biol. 1968, 36, 107–123. [Google Scholar] [CrossRef]
- Ernst, H.; Shatkin, A.J. Reovirus Hemagglutinin mRNA Codes for Two Polypeptides in Overlapping Reading Frames. Proc. Natl. Acad. Sci. USA 1985, 82, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Belli, B.A.; Samuel, C.E. Biosynthesis of Reovirus-Specified Polypeptides: Expression of Reovirus S1-Encoded σ1 NS Protein in Transfected and Infected Cells as Measured with Serotype Specific Polyclonal Antibody. Virology 1991, 185, 698–709. [Google Scholar] [CrossRef]
- Sarkar, G.; Pelletier, J.; Bassel-Duby, R.; Jayasuriya, A.; Fields, B.N.; Sonenberg, N. Identification of a New Polypeptide Coded by Reovirus Gene S1. J. Virol. 1985, 54, 720–725. [Google Scholar] [CrossRef]
- Busch, L.K.; Rodríguez-Grille, J.; Casal, J.I.; Martínez-Costas, J.; Benavente, J. Avian and Mammalian Reoviruses Use Different Molecular Mechanisms to Synthesize Their μNS Isoforms. J. Gen. Virol. 2011, 92, 2566–2574. [Google Scholar] [CrossRef]
- Sagar, V.; Murray, K.E. The Mammalian Orthoreovirus Bicistronic M3 mRNA Initiates Translation Using a 5′ End-Dependent, Scanning Mechanism That Does Not Require Interaction of 5′-3′ Untranslated Regions. Virus Res. 2014. [Google Scholar] [CrossRef]
- Furuichi, Y.; Morgan, M.; Muthukrishnan, S.; Shatkin, A.J. Reovirus Messenger RNA Contains a Methylated, Blocked 5′-Terminal Structure: M-7G(5’)Ppp(5’)G-MpCp-. Proc. Natl. Acad. Sci. USA 1975, 72, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Luongo, C.L.; Reinisch, K.M.; Harrison, S.C.; Nibert, M.L. Identification of the Guanylyltransferase Region and Active Site in Reovirus mRNA Capping Protein Lambda2. J. Biol. Chem. 2000, 275, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Luongo, C.L.; Contreras, C.M.; Farsetta, D.L.; Nibert, M.L. Binding Site for S-Adenosyl-L-Methionine in a Central Region of Mammalian Reovirus Lambda2 Protein. Evidence for Activities in mRNA Cap Methylation. J. Biol. Chem. 1998, 273, 23773–23780. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reinisch, K.M.; Nibert, M.L.; Harrison, S.C. Structure of the Reovirus Core at 3.6 Å Resolution. Nature 2000, 404, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.; Makino, S. Interplay between Viruses and Host mRNA Degradation. Biochim. Biophys. Acta Gene Regul. Mech. 2013, 1829, 732–741. [Google Scholar] [CrossRef]
- Ng, W.C.; Soto-Acosta, R.; Bradrick, S.S.; Garcia-Blanco, M.A.; Ooi, E.E. The 5′ and 3′ Untranslated Regions of the Flaviviral Genome. Viruses 2017, 9, 137. [Google Scholar] [CrossRef]
- Nonoyama, M.; Millward, S.; Graham, A.F. Control of Transcription of the Reovirus Genome. Nucleic Acids Res. 1974, 1, 373–385. [Google Scholar] [CrossRef]
- Farsetta, D.L.; Chandran, K.; Nibert, M.L. Transcriptional Activities of Reovirus RNA Polymerase in Recoated Cores. Initiation and Elongation Are Regulated by Separate Mechanisms. J. Biol. Chem. 2000, 275, 39693–39701. [Google Scholar] [CrossRef] [PubMed]
- Zamora, P.F.; Hu, L.; Knowlton, J.J.; Lahr, R.M.; Moreno, R.A.; Berman, A.J.; Prasad, B.V.V.; Dermody, T.S. Reovirus Nonstructural Protein σNS Acts as an RNA-Stability Factor Promoting Viral Genome Replication. J. Virol. 2018, 92, e00563-18. [Google Scholar] [CrossRef] [PubMed]
- Broering, T.J.; Kim, J.; Miller, C.L.; Piggott, C.D.S.; Dinoso, J.B.; Nibert, M.L.; Parker, J.S.L. Reovirus Nonstructural Protein μNS Recruits Viral Core Surface Proteins and Entering Core Particles to Factory-like Inclusions. J. Virol. 2004, 78, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Hastings, C.; Miller, C.L. Mammalian Orthoreovirus Particles Induce and Are Recruited into Stress Granules at Early Times Postinfection. J. Virol. 2009, 83, 11090–11101. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.E.; Hillner, P.E.; Vale, R.D.; Sachs, A.B. Circularization of mRNA by Eukaryotic Translation Initiation Factors. Mol. Cell 1998, 2, 135–140. [Google Scholar] [CrossRef]
- Groft, C.M.; Burley, S.K. Recognition of EIF4G by Rotavirus NSP3 Reveals a Basis for mRNA Circularization. Mol. Cell 2002, 9, 1273–1283. [Google Scholar] [CrossRef]
- Deo, R.C.; Groft, C.M.; Rajashankar, K.R.; Burley, S.K. Recognition of the Rotavirus mRNA 3’ Consensus by an Asymmetric NSP3 Homodimer. Cell 2002, 108, 71–81. [Google Scholar] [CrossRef]
- Gillian, A.L.; Schmechel, S.C.; Livny, J.; Schiff, L.A.; Nibert, M.L. Reovirus Protein σNS Binds in Multiple Copies to Single-Stranded RNA and Shares Properties with Single-Stranded DNA Binding Proteins. J. Virol. 2000, 74, 5939–5948. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gillian, A.L.; Nibert, M.L. Amino Terminus of Reovirus Nonstructural Protein σNS Is Important for ssRNA Binding and Nucleoprotein Complex Formation. Virology 1998, 240, 1–11. [Google Scholar] [CrossRef]
- Kozak, M.; Shatkin, A.J. Characterization of Ribosome-Protected Fragments from Reovirus Messenger RNA. J. Biol. Chem. 1976, 251, 4259–4266. [Google Scholar] [CrossRef]
- Kozak, M. Binding of Wheat Germ Ribosomes to Fragmented Viral mRNA. J. Virol. 1980, 35, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M. Binding of Wheat Germ Ribosomes to Bisulfite-Modified Reovirus Messenger RNA: Evidence for a Scanning Mechanism. J. Mol. Biol. 1980, 144, 291–304. [Google Scholar] [CrossRef]
- Kozak, M. Analysis of Ribosome Binding Sites from the S1 Message of Reovirus. Initiation at the First and Second AUG Codons. J. Mol. Biol. 1982, 156, 807–820. [Google Scholar] [CrossRef]
- Shatkin, A.J. Methylated Messenger RNA Synthesis In Vitro by Purified Reovirus. Proc. Natl. Acad. Sci. USA 1974, 71, 3204–3207. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, Y.; Muthukrishnan, S.; Shatkin, A.J. 5’-Terminal m-7G(5’)Ppp(5’)G-m-p in Vivo: Identification in Reovirus Genome RNA. Proc. Natl. Acad. Sci. USA 1975, 72, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Ingolia, N.T. Ribosome Profiling: New Views of Translation, from Single Codons to Genome Scale. Nat. Rev. Genet. 2014. [Google Scholar] [CrossRef] [PubMed]
- Castelló, A.; Fischer, B.; Eichelbaum, K.; Horos, R.; Beckmann, B.M.; Strein, C.; Davey, N.E.; Humphreys, D.T.; Preiss, T.; Steinmetz, L.M.; et al. Insights into RNA Biology from an Atlas of Mammalian mRNA-Binding Proteins. Cell 2012, 149, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Barna, M. Translating the Genome in Time and Space: Specialized Ribosomes, RNA Regulons, and RNA-Binding Proteins. Annu. Rev. Cell Dev. Biol. 2015, 31, 31–54. [Google Scholar] [CrossRef]
- Bischoff, J.R.; Samuel, C.E. Mechanism of Interferon Action. Activation of the Human P1/EIF-2 Alpha Protein Kinase by Individual Reovirus s-Class mRNAs: S1 mRNA Is a Potent Activator Relative to S4 mRNA. Virology 1989, 172, 106–115. [Google Scholar] [CrossRef]
- Ivanov, P.; Kedersha, N.; Anderson, P. Stress Granules and Processing Bodies in Translational Control. Cold Spring Harb. Perspect. Biol. 2019, 11, a032813. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Lloyd, R.E. Cytoplasmic RNA Granules and Viral Infection. Annu. Rev. Virol. 2014, 1, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Treeck, B.V.; Protter, D.S.W.; Matheny, T.; Khong, A.; Link, C.D.; Parker, R. RNA Self-Assembly Contributes to Stress Granule Formation and Defining the Stress Granule Transcriptome. Proc. Natl. Acad. Sci. USA 2018, 115, 2734–2739. [Google Scholar] [CrossRef] [PubMed]
- Van Treeck, B.; Parker, R. Emerging Roles for Intermolecular RNA-RNA Interactions in RNP Assemblies. Cell 2018, 174, 791–802. [Google Scholar] [CrossRef]
- Protter, D.S.W.; Parker, R. Principles and Properties of Stress Granules. Trends Cell Biol. 2016, 26, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Mathieu, C.; Kolaitis, R.-M.; Zhang, P.; Messing, J.; Yurtsever, U.; Yang, Z.; Wu, J.; Li, Y.; Pan, Q.; et al. G3BP1 Is a Tunable Switch That Triggers Phase Separation to Assemble Stress Granules. Cell 2020, 181, 325–345.e28. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Boixet, J.; Kopach, A.; Holehouse, A.S.; Wittmann, S.; Jahnel, M.; Schlüßler, R.; Kim, K.; Trussina, I.R.E.A.; Wang, J.; Mateju, D.; et al. RNA-Induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation. Cell 2020, 181, 346–361.e17. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.; Panas, M.D.; Achorn, C.A.; Lyons, S.; Tisdale, S.; Hickman, T.; Thomas, M.; Lieberman, J.; McInerney, G.M.; Ivanov, P.; et al. G3BP–Caprin1–USP10 Complexes Mediate Stress Granule Condensation and Associate with 40S Subunits. J. Cell Biol. 2016, 212. [Google Scholar] [CrossRef]
- Kedersha, N.; Anderson, P. Stress Granules: Sites of mRNA Triage That Regulate mRNA Stability and Translatability. Biochem. Soc. Trans. 2002, 30, 963–969. [Google Scholar] [CrossRef]
- Edupuganti, R.R.; Geiger, S.; Lindeboom, R.G.H.; Shi, H.; Hsu, P.J.; Lu, Z.; Wang, S.-Y.; Baltissen, M.P.A.; Jansen, P.W.T.C.; Rossa, M.; et al. N 6 -Methyladenosine (m 6 A) Recruits and Repels Proteins to Regulate mRNA Homeostasis. Nat. Struct. Mol. Biol. 2017, 24, 870–878. [Google Scholar] [CrossRef]
- White, J.P.; Lloyd, R.E. Regulation of Stress Granules in Virus Systems. Trends Microbiol. 2012, 20, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Hosmillo, M.; Lu, J.; McAllaster, M.R.; Eaglesham, J.B.; Wang, X.; Emmott, E.; Domingues, P.; Chaudhry, Y.; Fitzmaurice, T.J.; Tung, M.K.; et al. Noroviruses Subvert the Core Stress Granule Component G3BP1 to Promote Viral VPg-Dependent Translation. eLife 2019, 8, e46681. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.M.; Worth, M.P.; Hinchman, M.M.; Parker, J.S.L.; Ledgerwood, E.D. Mammalian Orthoreovirus Infection Is Enhanced in Cells Pre-Treated with Sodium Arsenite. Viruses 2019, 11, 563. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Reynaud, J.M.; Rasalouskaya, A.; Akhrymuk, I.; Mobley, J.A.; Frolov, I.; Frolova, E.I. New World and Old World Alphaviruses Have Evolved to Exploit Different Components of Stress Granules, FXR and G3BP Proteins, for Assembly of Viral Replication Complexes. PLoS Pathog. 2016, 12, e1005810. [Google Scholar] [CrossRef] [PubMed]
- Chernov, K.G.; Barbet, A.; Hamon, L.; Ovchinnikov, L.P.; Curmi, P.A.; Pastré, D. Role of Microtubules in Stress Granule Assembly: Microtubule Dynamical Instability Favors the Formation of Micrometric Stress Granules in Cells. J. Biol. Chem. 2009, 284, 36569–36580. [Google Scholar] [CrossRef] [PubMed]
- McEwen, E.; Kedersha, N.; Song, B.; Scheuner, D.; Gilks, N.; Han, A.; Chen, J.-J.; Anderson, P.; Kaufman, R.J. Heme-Regulated Inhibitor Kinase-Mediated Phosphorylation of Eukaryotic Translation Initiation Factor 2 Inhibits Translation, Induces Stress Granule Formation, and Mediates Survival upon Arsenite Exposure. J. Biol. Chem. 2005, 280, 16925–16933. [Google Scholar] [CrossRef]
- Mateju, D.; Eichenberger, B.; Voigt, F.; Eglinger, J.; Roth, G.; Chao, J.A. Single-Molecule Imaging Reveals Translation of mRNAs Localized to Stress Granules. Cell 2020, 183, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.; Hastings, C.; Miller, C.L. Amino Acids 78 and 79 of Mammalian Orthoreovirus Protein μNS Are Necessary for Stress Granule Localization, Core Protein λ2 Interaction, and de Novo Virus Replication. Virology 2014, 448, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mattioli, M.; Walter, P. The Integrated Stress Response: From Mechanism to Disease. Science 2020, 368. [Google Scholar] [CrossRef]
- Smith, J.A.; Schmechel, S.C.; Raghavan, A.; Abelson, M.; Reilly, C.; Katze, M.G.; Kaufman, R.J.; Bohjanen, P.R.; Schiff, L.A. Reovirus Induces and Benefits from an Integrated Cellular Stress Response. J. Virol. 2006, 80, 2019–2033. [Google Scholar] [CrossRef]
- Qin, Q.; Carroll, K.; Hastings, C.; Miller, C.L. Mammalian Orthoreovirus Escape from Host Translational Shutoff Correlates with Stress Granule Disruption and Is Independent of eIF2α Phosphorylation and PKR. J. Virol. 2011, 85, 8798–8810. [Google Scholar] [CrossRef]
- Choudhury, P.; Bussiere, L.D.; Miller, C.L. Mammalian Orthoreovirus Factories Modulate Stress Granule Protein Localization by Interaction with G3BP1. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Fros, J.J.; Domeradzka, N.E.; Baggen, J.; Geertsema, C.; Flipse, J.; Vlak, J.M.; Pijlman, G.P. Chikungunya Virus nsP3 Blocks Stress Granule Assembly by Recruitment of G3BP into Cytoplasmic Foci. J. Virol. 2012, 86, 10873–10879. [Google Scholar] [CrossRef] [PubMed]
- Scholte, F.E.M.; Tas, A.; Albulescu, I.C.; Žusinaite, E.; Merits, A.; Snijder, E.J.; van Hemert, M.J. Stress Granule Components G3BP1 and G3BP2 Play a Proviral Role Early in Chikungunya Virus Replication. J. Virol. 2015, 89, 4457–4469. [Google Scholar] [CrossRef] [PubMed]
- Götte, B.; Panas, M.D.; Hellström, K.; Liu, L.; Samreen, B.; Larsson, O.; Ahola, T.; McInerney, G.M. Separate Domains of G3BP Promote Efficient Clustering of Alphavirus Replication Complexes and Recruitment of the Translation Initiation Machinery. PLOS Pathog. 2019, 15, e1007842. [Google Scholar] [CrossRef] [PubMed]
- Giantini, M.; Shatkin, A.J. Stimulation of Chloramphenicol Acetyltransferase mRNA Translation by Reovirus Capsid Polypeptide σ3 in Cotransfected COS Cells. J. Virol. 1989, 63, 2415–2421. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.M.; Peters, T.R.; Dermody, T.S. Reovirus σNS and μNS Proteins Form Cytoplasmic Inclusion Structures in the Absence of Viral Infection. J. Virol. 2003, 77, 5948–5963. [Google Scholar] [CrossRef] [PubMed]
- Dales, S.; Omatos, P.J.; Hsu, K.C. The Uptake and Development of Reovirus in Strain L Cells Followed with Labeled Viral Ribonucleic Acid and Ferritin-Antibody Conjugates. Virology 1965, 25, 193–211. [Google Scholar] [CrossRef]
- Silverstein, S.C.; Schur, P.H. Immunofluorescent Localization of Double-Stranded RNA in Reovirus-Infected Cells. Virology 1970, 41, 564–566. [Google Scholar] [CrossRef]
- Silverstein, S.C.; Dales, S. The Penetration of Reovirus RNA and Initiation of Its Genetic Function In L-strain Fibroblasts. J. Cell Biol. 1968, 36, 197–230. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Chen, L.B.; Fields, B.N. The Interaction of Mammalian Reoviruses with the Cytoskeleton of Monkey Kidney CV-1 Cells. Virology 1982, 120, 399–411. [Google Scholar] [CrossRef]
- Rhim, J.S.; Jordan, L.E.; Mayor, H.D. Cytochemical, Fluorescent-Antibody and Electron Microscopic Studies on the Growth of Reovirus (ECHO 10) in Tissue Culture. Virology 1962, 17, 342–355. [Google Scholar] [CrossRef]
- Parker, J.S.L.; Broering, T.J.; Kim, J.; Higgins, D.E.; Nibert, M.L. Reovirus Core Protein μ2 Determines the Filamentous Morphology of Viral Inclusion Bodies by Interacting with and Stabilizing Microtubules. J. Virol. 2002, 76, 4483–4496. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.L.; Broering, T.J.; Parker, J.S.L.; Arnold, M.M.; Nibert, M.L. Reovirus σNS Protein Localizes to Inclusions through an Association Requiring the μNS Amino Terminus. J. Virol. 2003, 77, 4566–4576. [Google Scholar] [CrossRef] [PubMed]
- Mbisa, J.L.; Becker, M.M.; Zou, S.; Dermody, T.S.; Brown, E.G. Reovirus μ2 Protein Determines Strain-Specific Differences in the Rate of Viral Inclusion Formation in L929 Cells. Virology 2000, 272, 16–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Broering, T.J.; Parker, J.S.L.; Joyce, P.L.; Kim, J.; Nibert, M.L. Mammalian Reovirus Nonstructural Protein μNS Forms Large Inclusions and Colocalizes with Reovirus Microtubule-Associated Protein μ2 in Transfected Cells. J. Virol. 2002, 76, 8285–8297. [Google Scholar] [CrossRef]
- Becker, M.M.; Goral, M.I.; Hazelton, P.R.; Baer, G.S.; Rodgers, S.E.; Brown, E.G.; Coombs, K.M.; Dermody, T.S. Reovirus σNS Protein Is Required for Nucleation of Viral Assembly Complexes and Formation of Viral Inclusions. J. Virol. 2001, 75, 1459–1475. [Google Scholar] [CrossRef] [PubMed]
- de Castro, I.F.; Volonté, L.; Risco, C. Virus Factories: Biogenesis and Structural Design. Cell. Microbiol. 2013, 15, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Netherton, C.L.; Wileman, T. Virus Factories, Double Membrane Vesicles and Viroplasm Generated in Animal Cells. Curr. Opin. Virol. 2011, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Novoa, R.R.; Calderita, G.; Arranz, R.; Fontana, J.; Granzow, H.; Risco, C. Virus Factories: Associations of Cell Organelles for Viral Replication and Morphogenesis. Biol. Cell 2005, 97, 147–172. [Google Scholar] [CrossRef]
- Den Boon, J.A.; Ahlquist, P. Organelle-Like Membrane Compartmentalization of Positive-Strand RNA virus Replication Factories. Annu. Rev. Microbiol. 2010, 64, 241–256. [Google Scholar] [CrossRef]
- Nagy, P.D.; Pogany, J. The Dependence of Viral RNA Replication on Co-Opted Host Factors. Nat. Rev. Microbiol. 2012, 10, 137–149. [Google Scholar] [CrossRef]
- Stanifer, M.L.; Kischnick, C.; Rippert, A.; Albrecht, D.; Boulant, S. Reovirus Inhibits Interferon Production by Sequestering IRF3 into Viral Factories. Sci. Rep. 2017, 7, 10873. [Google Scholar] [CrossRef] [PubMed]
- Lemay, G. Synthesis and Translation of Viral mRNA in Reovirus-Infected Cells: Progress and Remaining Questions. Viruses 2018, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, R.; Fernández de Castro, I.; Knowlton, J.J.; Zamora, P.F.; Sutherland, D.M.; Risco, C.; Dermody, T.S. Function, Architecture, and Biogenesis of Reovirus Replication Neoorganelles. Viruses 2019, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.L.; Arnold, M.M.; Broering, T.J.; Hastings, C.E.; Nibert, M.L. Localization of Mammalian Orthoreovirus Proteins to Cytoplasmic Factory-Like Structures via Nonoverlapping Regions of μNS. J. Virol. 2010, 84, 867–882. [Google Scholar] [CrossRef]
- Desmet, E.A.; Anguish, L.J.; Parker, J.S.L. Virus-Mediated Compartmentalization of the Host Translational Machinery. MBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- De Castro, I.F.; Zamora, P.F.; Ooms, L.; Fernández, J.J.; Lai, C.M.-H.; Mainou, B.A.; Dermody, T.S.; Risco, C. Reovirus Forms Neo-Organelles for Progeny Particle Assembly within Reorganized Cell Membranes. MBio 2014, 5. [Google Scholar] [CrossRef]
- Tenorio, R.; de Castro, I.F.; Knowlton, J.J.; Zamora, P.F.; Lee, C.H.; Mainou, B.A.; Dermody, T.S.; Risco, C. Reovirus σNS and μNS Proteins Remodel the Endoplasmic Reticulum to Build Replication Neo-Organelles. MBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Bussiere, L.D.; Choudhury, P.; Bellaire, B.; Miller, C.L. Characterization of a Replicating Mammalian Orthoreovirus with Tetracysteine-Tagged μNS for Live-Cell Visualization of Viral Factories. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Yin, P.; Keirstead, N.D.; Broering, T.J.; Arnold, M.M.; Parker, J.S.L.; Nibert, M.L.; Coombs, K.M. Comparisons of the M1 Genome Segments and Encoded μ2 Proteins of Different Reovirus Isolates. J. Virol. 2004, 1, 6. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chappell, J.D.; Danthi, P.; Dermody, T.S. Gene-Specific Inhibition of Reovirus Replication by RNA Interference. J. Virol. 2006, 80, 9053–9063. [Google Scholar] [CrossRef] [PubMed]
- Enam, S.U.; Zinshteyn, B.; Goldman, D.H.; Cassani, M.; Livingston, N.M.; Seydoux, G.; Green, R. Puromycin Reactivity Does Not Accurately Localize Translation at the Subcellular Level. eLife 2020, 9, e60303. [Google Scholar] [CrossRef] [PubMed]
- Huismans, H.; Joklik, W.K. Reovirus-Coded Polypeptides in Infected Cells: Isolation of Two Native Monomeric Polypeptides with Affinity for Single-Stranded and Double-Stranded RNA, Respectively. Virology 1976, 70, 411–424. [Google Scholar] [CrossRef]
- Reid, D.W.; Nicchitta, C.V. Primary Role for Endoplasmic Reticulum-Bound Ribosomes in Cellular Translation Identified by Ribosome Profiling. J. Biol. Chem. 2012, 287, 5518–5527. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.B.; Dodd, R.D.; Brewer, J.W.; Lager, P.J.; Keene, J.D.; Nicchitta, C.V. Stable Ribosome Binding to the Endoplasmic Reticulum Enables Compartment-Specific Regulation of mRNA Translation. Mol. Biol. Cell 2005, 16, 5819–5831. [Google Scholar] [CrossRef] [PubMed]
- Unworth, H.; Raguz, S.; Edwards, H.J.; Higgins, C.F.; Yagüe, E. mRNA Escape from Stress Granule Sequestration Is Dictated by Localization to the Endoplasmic Reticulum. FASEB J. 2010, 24, 3370–3380. [Google Scholar] [CrossRef]
- Lerner, R.S.; Nicchitta, C.V. mRNA Translation Is Compartmentalized to the Endoplasmic Reticulum Following Physiological Inhibition of Cap-Dependent Translation. RNA 2006, 12, 775–789. [Google Scholar] [CrossRef]
- Palade, G. Intracellular Aspects of the Process of Protein Synthesis. Science 1975, 189, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Palade, G.E. A Small Particulate Component of the Cytoplasm. J. Cell Biol. 1955, 1, 59–68. [Google Scholar] [CrossRef]
- Reid, D.W.; Nicchitta, C.V. Diversity and Selectivity in mRNA Translation on the Endoplasmic Reticulum. Nat. Rev. Mol. Cell Biol. 2015, 16, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, S.; Reid, D.W.; Cox, A.H.; Nicchitta, C.V. De Novo Translation Initiation on Membrane-Bound Ribosomes as a Mechanism for Localization of Cytosolic Protein mRNAs to the Endoplasmic Reticulum. RNA 2014, 20, 1489–1498. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of Type I Interferon Responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Arias, C.F.; López, S. Protein Kinase R Is Responsible for the Phosphorylation of eIF2α in Rotavirus Infection. J. Virol. 2010, 84, 10457–10466. [Google Scholar] [CrossRef] [PubMed]
- Sherry, B. Rotavirus and Reovirus Modulation of the Interferon Response. J. Interferon Cytokine Res. 2009, 29, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Lostalé-Seijo, I.; Martínez-Costas, J.; Benavente, J. Interferon Induction by Avian Reovirus. Virology 2016, 487, 104–111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohamed, A.; Konda, P.; Eaton, H.E.; Gujar, S.; Smiley, J.R.; Shmulevitz, M. Closely Related Reovirus Lab Strains Induce Opposite Expression of RIG-I/IFN-Dependent versus -Independent Host Genes, via Mechanisms of Slow Replication versus Polymorphisms in dsRNA Binding σ3 Respectively. PLoS Pathog. 2020, 16, e1008803. [Google Scholar] [CrossRef] [PubMed]
- Sadler, A.J.; Williams, B.R.G. Interferon-Inducible Antiviral Effectors. Nat. Rev. Immunol. 2008, 8, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.R. Signal Integration via PKR. Sci. STKE 2001. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.; Rullas, J.; García, M.A.; Alcamí, J.; Esteban, M. The Catalytic Activity of dsRNA-Dependent Protein Kinase, PKR, Is Required for NF-KappaB Activation. Oncogene 2001, 20, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Zamanian-Daryoush, M.; Mogensen, T.H.; DiDonato, J.A.; Williams, B.R.G. NF-ΚB Activation by Double-Stranded-RNA-Activated Protein Kinase (PKR) Is Mediated through NF-ΚB-Inducing Kinase and IκB Kinase. Mol. Cell. Biol. 2000, 20, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H. Viral Encounters with 2′,5′-Oligoadenylate Synthetase and RNase L during the Interferon Antiviral Response. J. Virol. 2007, 81, 12720–12729. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Schmechel, S.C.; Williams, B.R.G.; Silverman, R.H.; Schiff, L.A. Involvement of the Interferon-Regulated Antiviral Proteins PKR and RNase L in Reovirus-Induced Shutoff of Cellular Translation. J. Virol. 2005, 79, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Fields, B.N. Reovirus Inhibition of Cellular RNA and Protein Synthesis: Role of the S4 Gene. Virology 1982, 122, 381–391. [Google Scholar] [CrossRef]
- Yue, Z.; Shatkin, A.J. Double-Stranded RNA-Dependent Protein Kinase (PKR) Is Regulated by Reovirus Structural Proteins. Virology 1997, 234, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Denzler, K.L.; Jacobs, B.L. Site-Directed Mutagenic Analysis of Reovirus σ3 Protein Binding to dsRNA. Virology 1994, 204, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Imani, F.; Jacobs, B.L. Inhibitory Activity for the Interferon-Induced Protein Kinase Is Associated with the Reovirus Serotype 1 Sigma 3 Protein. Proc. Natl. Acad. Sci. USA 1988, 85, 7887–7891. [Google Scholar] [CrossRef] [PubMed]
- Beattie, E.; Denzler, K.L.; Tartaglia, J.; Perkus, M.E.; Paoletti, E.; Jacobs, B.L. Reversal of the Interferon-Sensitive Phenotype of a Vaccinia Virus Lacking E3L by Expression of the Reovirus S4 Gene. J. Virol. 1995, 69, 499–505. [Google Scholar] [CrossRef]
- Lloyd, R.M.; Shatkin, A.J. Translational Stimulation by Reovirus Polypeptide Sigma 3: Substitution for VAI RNA and Inhibition of Phosphorylation of the Alpha Subunit of Eukaryotic Initiation Factor 2. J. Virol. 1992, 66, 6878–6884. [Google Scholar] [CrossRef]
- Schmechel, S.; Chute, M.; Skinner, P.; Anderson, R.; Schiff, L. Preferential Translation of Reovirus mRNA by a σ3-Dependent Mechanism. Virology 1997, 232, 62–73. [Google Scholar] [CrossRef]
- Strong, J.E.; Coffey, M.C.; Tang, D.; Sabinin, P.; Lee, P.W.K. The Molecular Basis of Viral Oncolysis: Usurpation of the Ras Signaling Pathway by Reovirus. EMBO J. 1998, 17, 3351–3362. [Google Scholar] [CrossRef]
- Mohamed, A.; Clements, D.R.; Gujar, S.A.; Lee, P.W.; Smiley, J.R.; Shmulevitz, M. Single Amino Acid Differences between Closely Related Reovirus T3D Lab Strains Alter Oncolytic Potency in Vitro and in Vivo. J. Virol. 2019. [Google Scholar] [CrossRef]
- Miller, C.L. Stress Granules and Virus Replication. Future Virol. 2011, 6, 1329–1338. [Google Scholar] [CrossRef]
- Burke, J.M.; Moon, S.L.; Matheny, T.; Parker, R. RNase L Reprograms Translation by Widespread mRNA Turnover Escaped by Antiviral mRNAs. Mol. Cell 2019, 75, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.M.; Lester, E.T.; Tauber, D.; Parker, R. RNase L Promotes the Formation of Unique Ribonucleoprotein Granules Distinct from Stress Granules. J. Biol. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, P.; Siddiqui, M.A.; Malathi, K. RNase L Amplifies Interferon Signaling by Inducing PKR-Mediated Antiviral Stress Granules. J. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Banerjee, S.; Wang, Y.; Goldstein, S.A.; Dong, B.; Gaughan, C.; Silverman, R.H.; Weiss, S.R. Activation of RNase L Is Dependent on OAS3 Expression during Infection with Diverse Human Viruses. Proc. Natl. Acad. Sci. USA 2016, 113, 2241–2246. [Google Scholar] [CrossRef] [PubMed]
- Lemay, G.; Millward, S. Expression of the Cloned S4 Gene of Reovirus Serotype 3 in Transformed Eucaryotic Cells: Enrichment of the Viral Protein in the Crude Initiation Factor Fraction. Virus Res. 1986, 6, 133–140. [Google Scholar] [CrossRef]
- Lemieux, R.; Lemay, G.; Millward, S. The Viral Protein Sigma 3 Participates in Translation of Late Viral mRNA in Reovirus-Infected L Cells. J. Virol. 1987, 61, 2472–2479. [Google Scholar] [CrossRef]
- Skup, D.; Millward, S. mRNA Capping Enzymes Are Masked in Reovirus Progeny Subviral Particles. J. Virol. 1980, 34, 490–496. [Google Scholar] [CrossRef]
- Detjen, B.M.; Walden, W.E.; Thach, R.E. Translational Specificity in Reovirus-Infected Mouse Fibroblasts. J. Biol. Chem. 1982, 257, 9855–9860. [Google Scholar] [CrossRef]
- Yue, Z.; Shatkin, A.J. Regulated, Stable Expression and Nuclear Presence of Reovirus Double-Stranded RNA-Binding Protein Sigma3 in HeLa Cells. J. Virol. 1996, 70, 3497–3501. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, F.-M.; van Koningsbruggen, S.; Navascués, J.; Lamond, A.I. The Multifunctional Nucleolus. Nat. Rev. Mol. Cell Biol. 2007, 8, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Thomson, E.; Ferreira-Cerca, S.; Hurt, E. Eukaryotic Ribosome Biogenesis at a Glance. J. Cell Sci. 2013, 126, 4815–4821. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, T.; Danis, C.; Lemay, G. Two Basic Motifs of Reovirus σ3 Protein Are Involved in Double-Stranded RNA Binding. Biochem. Cell Biol. 2011. [Google Scholar] [CrossRef] [PubMed]
| Genome Segment | mRNA | 5′-UTR and 3′-UTR Length | Viral Proteins |
|---|---|---|---|
| L1 | l1 | 24 nt and 35 nt | λ3 (viral RNA-dependent RNA polymerase) |
| L2 | l2 | 13 nt and 36 nt | λ2 (core structural) |
| L3 | l3 | 13 nt and 63 nt | λ1 (core structural) |
| M1 | m1 | 13 nt and 83 nt | μ2 (polymerase cofactor; microtubule-binding) |
| M2 | m2 | 29 nt and 50 nt | μ1 (outer-capsid structural) |
| M3 | m3 | 18 nt and 60 nt | μNS (nonstructural; viral factory matrix) μNSC (nonstructural) |
| S1 | s1 | 12 nt and 39 nt | σ1 (outer-capsid structural protein; attachment) σ1s (nonstructural) |
| S2 | s2 | 18 nt and 59 nt | σ2 (core structural) |
| S3 | s3 | 27 nt and 73 nt | σNS (nonstructural; single-stranded RNA binding) |
| S4 | s4 | 32 nt and 69 nt | σ3 (outer-capsid structural; dsRNA-binding) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Parker, J.S.L. The Paradoxes of Viral mRNA Translation during Mammalian Orthoreovirus Infection. Viruses 2021, 13, 275. https://doi.org/10.3390/v13020275
Guo Y, Parker JSL. The Paradoxes of Viral mRNA Translation during Mammalian Orthoreovirus Infection. Viruses. 2021; 13(2):275. https://doi.org/10.3390/v13020275
Chicago/Turabian StyleGuo, Yingying, and John S. L. Parker. 2021. "The Paradoxes of Viral mRNA Translation during Mammalian Orthoreovirus Infection" Viruses 13, no. 2: 275. https://doi.org/10.3390/v13020275
APA StyleGuo, Y., & Parker, J. S. L. (2021). The Paradoxes of Viral mRNA Translation during Mammalian Orthoreovirus Infection. Viruses, 13(2), 275. https://doi.org/10.3390/v13020275







