CCR5+ T-Cells Homed to the Liver Exhibit Inflammatory and Profibrogenic Signatures in Chronic HIV/HCV-Coinfected Patients
Abstract
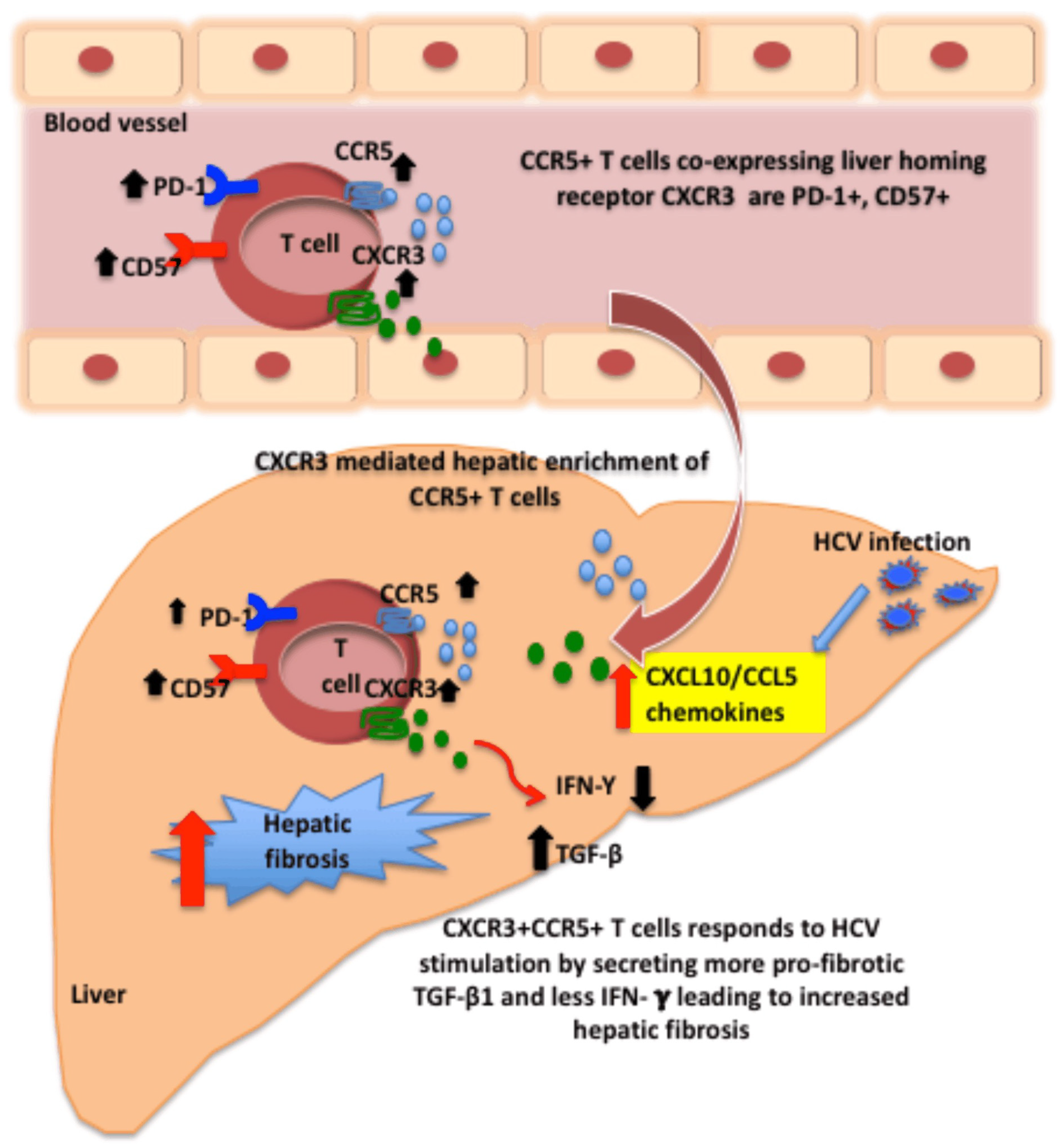
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Immunophenotyping and CCR5 Cell Surface Density Determination in Peripheral Blood Mononuclear Cells (PBMCs)
2.3. Isolation of Liver Infiltrating Lymphocytes (LILs) and Intrahepatic CCR5 Frequency Determination
2.4. HCV Peptide Reconstitution
2.5. Cell Sorting and HCV Peptide Specific T Cell Functions
2.6. Analysis of Cytokine Production by Multiplex ELISA
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
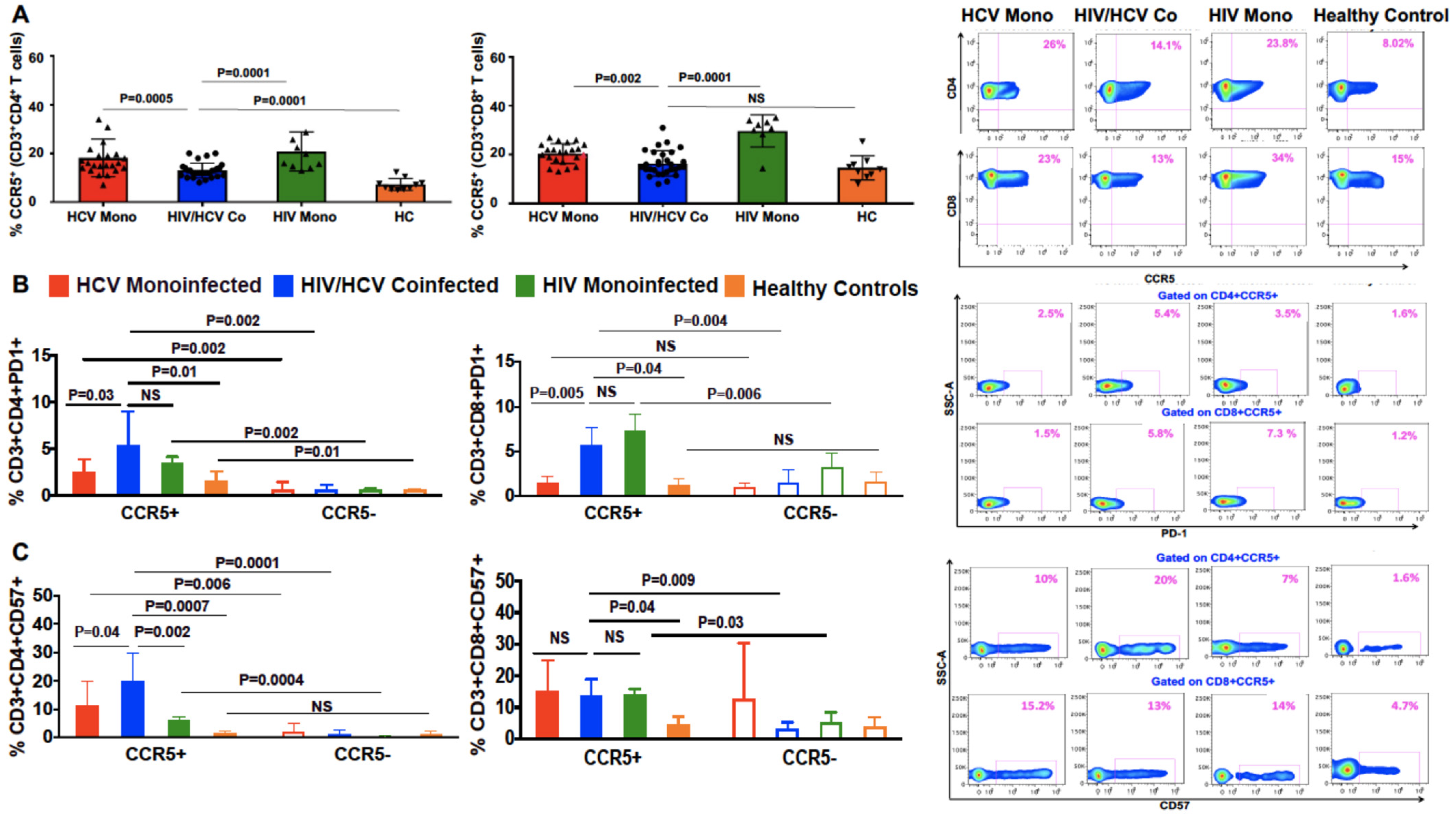
3.2. Lower Frequency of CCR5+ CD4 and CD8 T Cells in the Peripheral Blood of Patients with HIV/HCV Coinfection Compared to HCV Monoinfection
3.3. Peripheral CCR5+ T Cells from HIV/HCV-Coinfected Patients Express More PD1 and CD57 Than Cells from HCV Monoinfected Patients
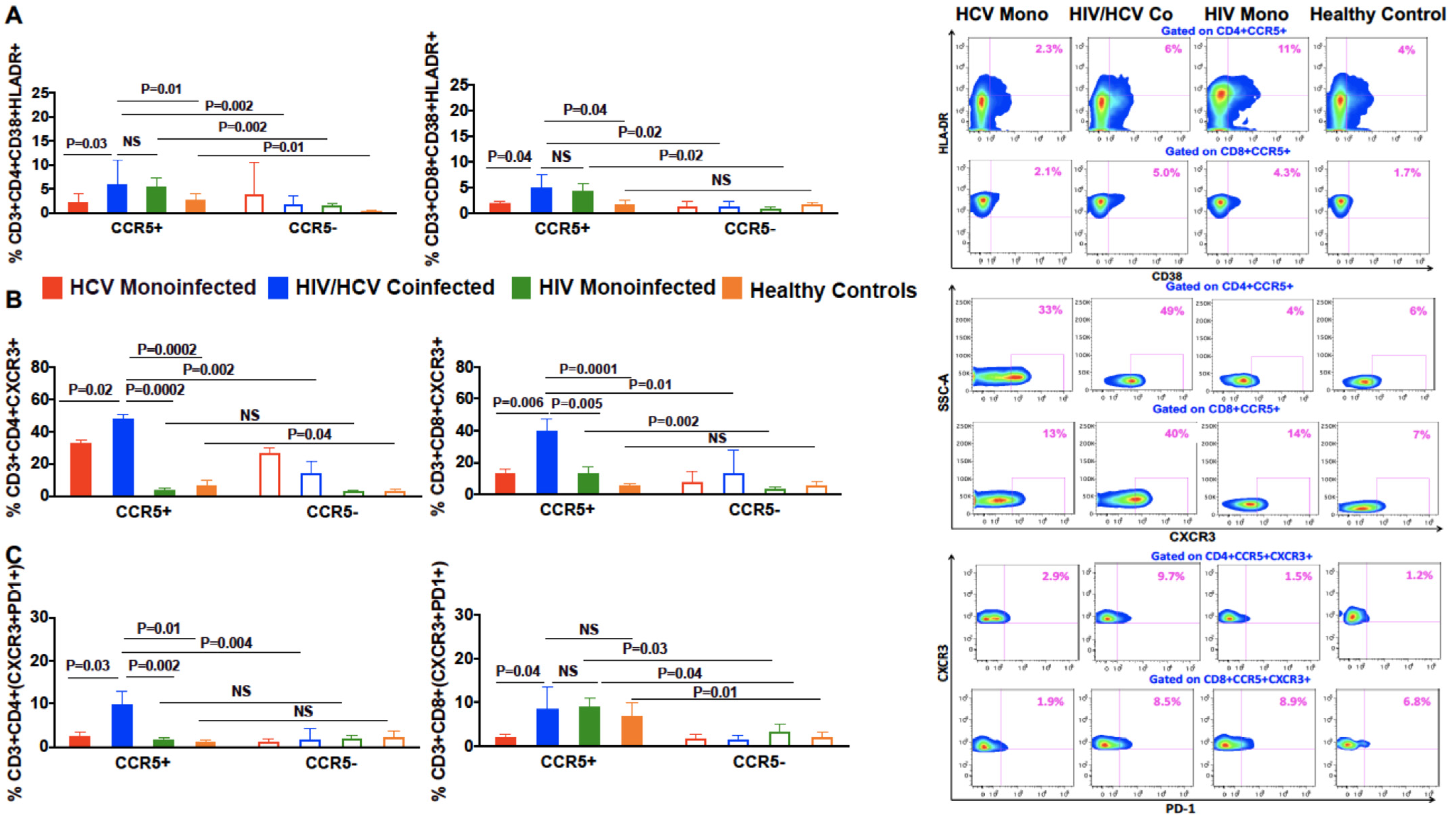
3.4. Increased Co-Expression of Markers of Chronic Immune Activation, HLA-DR and CD38, on CCR5+ Compared to CCR5 Negative T Cells in Coinfected Patients
3.5. In Patients with HIV/HCV Coinfection, Peripheral CCR5+ T Cells Had a Higher Frequency of the T Cell Trafficking Receptor, CXCR3, Compared to CCR5 Negative T Cells
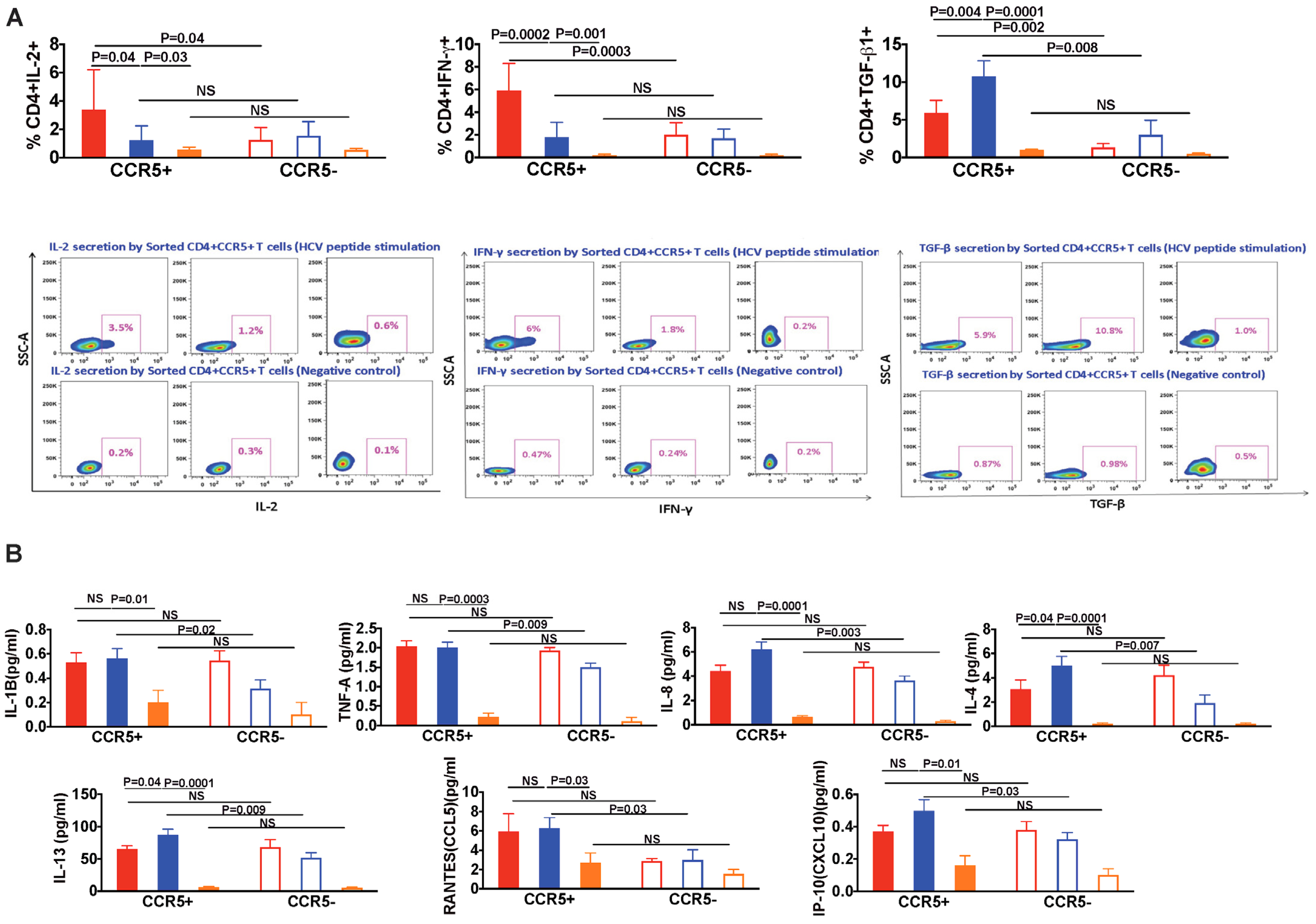
3.6. In HIV/HCV Coinfection, HCV-Specific CCR5+ T Cells Secrete Less IL-2 and IFN-γ, and More TGF-β as Compared with HCV Monoinfection
3.7. Pro-Inflammatory and Pro-Fibrotic Cytokines and Chemokines Are Highly Secreted by CCR5+ T Cells in HIV/HCV Coinfection
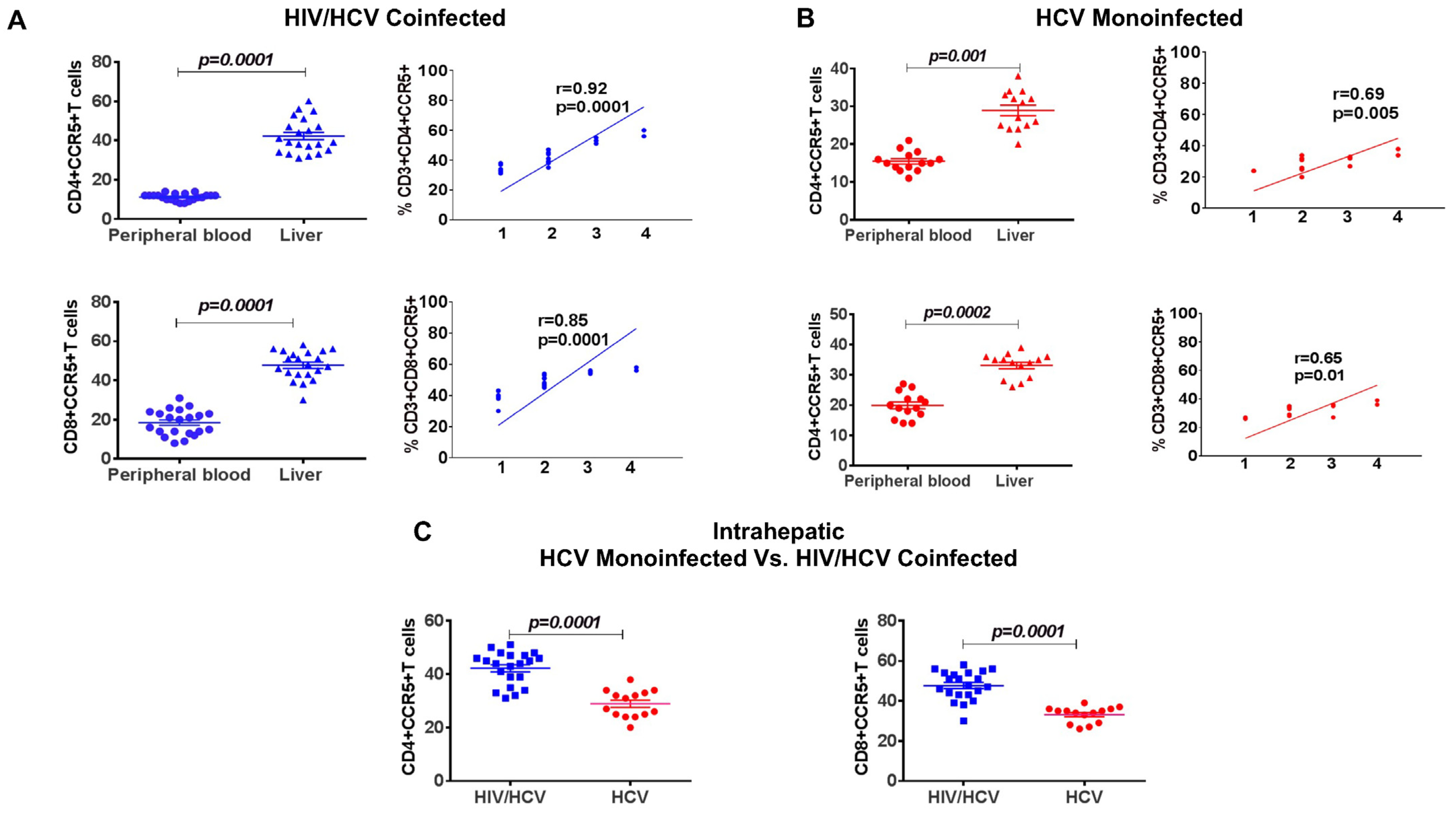
3.8. Increased Frequency of Intrahepatic CCR5+ T Cells Compared to Periphery, Correlated with Increased Fibrosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sherman, K.E.; Rouster, S.D.; Chung, R.T.; Rajicic, N. Hepatitis C Virus Prevalence among Patients Infected with Human Immunodeficiency Virus: A Cross-Sectional Analysis of the US Adult AIDS Clinical Trials Group. Clin. Infect. Dis. 2002, 34, 831–837. [Google Scholar] [CrossRef]
- Backus, L.I.; Boothroyd, D.; Deyton, L.R. HIV, hepatitis C and HIV/hepatitis C virus co-infection in vulnerable populations. AIDS 2005, 19, S13–S19. [Google Scholar] [CrossRef]
- Graham, C.S.; Baden, L.R.; Yu, E.; Mrus, J.M.; Carnie, J.; Heeren, T.; Koziel, M.J. Influence of Human Immunodeficiency Virus Infection on the Course of Hepatitis C Virus Infection: A Meta-Analysis. Clin. Infect. Dis. 2001, 33, 562–569. [Google Scholar] [CrossRef]
- Lo Re, V., 3rd; Kallan, M.J.; Tate, J.P.; Localio, A.R.; Lim, J.K.; Goetz, M.B.; Klein, M.B.; Rimland, D.; Rodriguez-Barradas, M.C.; Butt, A.A.; et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: A cohort study. Ann. Intern. Med. 2014, 160, 369–379. [Google Scholar]
- Hernando, V.; Cachafeiro, S.P.; Lewden, C.; Gonzalez, J.; Segura, F.; Oteo, J.A.; Rubio, R.; Dalmau, D.; Moreno, S.; del Amo, J. All-cause and liver-related mortality in HIV positive subjects compared to the general population: Differences by HCV co-infection. J. Hepatol. 2012, 57, 743–751. [Google Scholar] [CrossRef]
- Chen, J.Y.; Feeney, E.; Chung, R.T. HCV and HIV co-infection: Mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Ding, E.; Iii, G.R.S.; Kim, A.Y. Meta-Analysis: Increased Mortality Associated with Hepatitis C in HIV-Infected Persons Is Unrelated to HIV Disease Progression. Clin. Infect. Dis. 2009, 49, 1605–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Weinberg, E.; Chung, R.T. Pathogenesis of Accelerated Fibrosis in HIV/HCV Co-infection. J. Infect. Dis. 2013, 207, S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, C.M.; Lichtner, M.; Mascia, C.; Zuccalà, P.; Vullo, V. Molecular Mechanisms of Liver Fibrosis in HIV/HCV Coinfection. Int. J. Mol. Sci. 2014, 15, 9184–9208. [Google Scholar] [CrossRef]
- Glässner, A.; Eisenhardt, M.; Kokordelis, P.; Krämer, B.; Wolter, F.; Nischalke, H.D.; Boesecke, C.; Sauerbruch, T.; Rockstroh, J.K.; Spengler, U.; et al. Impaired CD4+ T cell stimulation of NK cell anti-fibrotic activity may contribute to accelerated liver fibrosis progression in HIV/HCV patients. J. Hepatol. 2013, 59, 427–433. [Google Scholar] [CrossRef]
- Dragic, T.; Litwin, V.; Allaway, G.P.; Martin, S.R.; Huang, Y.; Nagashima, K.A.; Cayanan, C.; Maddon, P.J.; Koup, R.A.; Moore, J.P.; et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 1996, 381, 667–673. [Google Scholar] [CrossRef]
- Alkhatib, G.; Combadiere, C.; Broder, C.C.; Kennedy, P.E.; Murphy, P.M.; Berger, E.A. CC CKR5: A RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 1996, 272, 1955–1958. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Bataller, R.; Brenner, D. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am. J. Physiol. Liver Physiol. 2003, 285, G949–G958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seki, E.; De Minicis, S.; Gwak, G.-Y.; Kluwe, J.; Inokuchi, S.; Bursill, C.A.; Llovet, J.M.; Brenner, D.A.; Schwabe, R.F. CCR1 and CCR5 promote hepatic fibrosis in mice. J. Clin. Investig. 2009, 119, 1858–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wald, O.; Weiss, I.D.; Galun, E.; Peled, A. Chemokines in hepatitis C virus infection: Pathogenesis, prognosis and therapeutics. Cytokine 2007, 39, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Sherman, K.E.; Abdel-Hameed, E.; Rouster, S.D.; Shata, M.T.M.; Blackard, J.T.; Safaie, P.; Kroner, B.; Preiss, L.; Horn, P.S.; Kottilil, S. Improvement in Hepatic Fibrosis Biomarkers Associated With Chemokine Receptor Inactivation Through Mutation or Therapeutic Blockade. Clin. Infect. Dis. 2019, 68, 1911–1918. [Google Scholar] [CrossRef]
- Friedman, S.L.; Ratziu, V.; Harrison, S.A.; Abdelmalek, M.; Aithal, G.; Caballeria, J.; Francque, S.; Farrell, G.; Kowdley, K.V.; Craxi, A.; et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018, 67, 1754–1767. [Google Scholar] [CrossRef] [Green Version]
- Shields, P.L.; Morland, C.M.; Salmon, M.; Qin, S.; Hubscher, S.G.; Adams, D. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J. Immunol. 1999, 163, 6236–6243. [Google Scholar]
- Shrivastava, S.; Wilson, E.; Poonia, B.; Tang, L.; Osinusi, A.; Kohli, A.; Kottilil, S. Augmentation of hepatitis C virus-specific immunity and sustained virologic response. J. Viral Hepat. 2017, 24, 742–749. [Google Scholar] [CrossRef]
- Reynes, J.; Portales, P.; Segondy, M.; Baillat, V.; André, P.; Réant, B.; Avinens, O.; Couderc, G.; Benkirane, M.; Clot, J.; et al. CD4+T Cell Surface CCR5 Density as a Determining Factor of Virus Load in Persons Infected with Human Immunodeficiency Virus Type 1. J. Infect. Dis. 2000, 181, 927–932. [Google Scholar] [CrossRef] [Green Version]
- Barrett, L.; Trehanpati, N.; Poonia, S.; Daigh, L.; Sarin, S.K.; Masur, H.; Kottilil, S. Hepatic compartmentalization of exhausted and regulatory cells in HIV/HCV-coinfected patients. J. Viral Hepat. 2015, 22, 281–288. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Karandikar, N.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Crotty, L.E.; Casazza, J.P.; Kuruppu, J.; Migueles, S.A.; Connors, M.; et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 2003, 101, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Papagno, L.; Spina, C.A.; Marchant, A.; Salio, M.; Rufer, N.; Little, S.; Dong, T.; Chesney, G.; Waters, A.; Easterbrook, P.; et al. Immune Activation and CD8+ T-Cell Differentiation towards Senescence in HIV-1 Infection. PLoS Biol. 2004, 2, E20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman, J.; Trimble, L.A.; Friedman, R.; Lisziewicz, J.; Lori, F.; Shankar, P.; Jessen, H. Expansion of CD57 and CD62L-CD45RA+ CD8 T lymphocytes correlates with reduced viral plasma RNA after primary HIV infection. AIDS 1999, 13, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Kern, F.; Khatamzas, E.; Surel, I.; Frömmel, C.; Reinke, P.; Waldrop, S.L.; Picker, L.J.; Volk, H.-D. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur. J. Immunol. 1999, 29, 2908–2915. [Google Scholar] [CrossRef]
- Weng, S.-Y.; Wang, X.; Vijayan, S.; Tang, Y.; Kim, Y.O.; Padberg, K.; Regen, T.; Molokanova, O.; Chen, T.; Bopp, T.; et al. IL-4 Receptor Alpha Signaling through Macrophages Differentially Regulates Liver Fibrosis Progression and Reversal. EBioMedicine 2018, 29, 92–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apolinario, A.; Majano, P.L.; Alvarez-Pérez, E.A.; Saez, A.; Lozano, C.; Vargas, J.; García-Monzón, C. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: Relationship with the histological activity of liver disease. Am. J. Gastroenterol. 2002, 97, 2861–2870. [Google Scholar] [CrossRef]
- Larrubia, J.-R.; Calvino, M.; Benito, S.; Sanz-De-Villalobos, E.; Perna, C.; Pérez-Hornedo, J.; González-Mateos, F.; García-Garzón, S.; Bienvenido, A.; Parra, T. The role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infection. J. Hepatol. 2007, 47, 632–641. [Google Scholar] [CrossRef]
- Berres, M.-L.; Koenen, R.; Rueland, A.; Zaldivar, M.M.; Heinrichs, D.; Sahin, H.; Schmitz, P.; Streetz, K.L.; Berg, T.; Gassler, N.; et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J. Clin. Investig. 2010, 120, 4129–4140. [Google Scholar] [CrossRef] [Green Version]
- Butera, D.; Marukian, S.; Iwamaye, A.E.; Hembrador, E.; Chambers, T.J.; Di Bisceglie, A.M.; Charles, E.D.; Talal, A.H.; Jacobson, I.M.; Rice, C.M.; et al. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood 2005, 106, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Helbig, K.; Ruszkiewicz, A.; Lanford, R.E.; Berzsenyi, M.D.; Harley, H.A.; McColl, S.R.; Beard, M.R. Differential Expression of the CXCR3 Ligands in Chronic Hepatitis C Virus (HCV) Infection and Their Modulation by HCV In Vitro. J. Virol. 2009, 83, 836–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeremski, M.; Hooker, G.; Shu, M.A.; Winkelstein, E.; Brown, Q.; Jarlais, D.C.D.; Tobler, L.H.; Rehermann, B.; Busch, M.P.; Edlin, B.R.; et al. Induction of CXCR3- and CCR5-associated chemokines during acute hepatitis C virus infection. J. Hepatol. 2011, 55, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorr, P.; Westby, M.; Dobbs, S.; Griffin, P.; Irvine, B.; Macartney, M.; Mori, J.; Rickett, G.; Smith-Burchnell, C.; Napier, C.; et al. Maraviroc (UK-427,857), a Potent, Orally Bioavailable, and Selective Small-Molecule Inhibitor of Chemokine Receptor CCR5 with Broad-Spectrum Anti-Human Immunodeficiency Virus Type 1 Activity. Antimicrob. Agents Chemother. 2005, 49, 4721–4732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fätkenheuer, G.; Pozniak, A.L.; Johnson, M.A.; Plettenberg, A.; Staszewski, S.; Hoepelman, A.I.M.; Saag, M.S.; Goebel, F.D.; Rockstroh, J.K.; Dezube, B.J.; et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat. Med. 2005, 11, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Kruger, A.J.; Fuchs, B.C.; Masia, R.; Holmes, J.A.; Salloum, S.; Sojoodi, M.; Ferreira, D.S.; Rutledge, S.M.; Caravan, P.; Alatrakchi, N.; et al. Prolonged cenicriviroc therapy reduces hepatic fibrosis despite steatohepatitis in a diet-induced mouse model of nonalcoholic steatohepatitis. Hepatol. Commun. 2018, 2, 529–545. [Google Scholar] [CrossRef]
- Ratziu, V.; Sanyal, A.; Harrison, S.A.; Wong, V.W.S.; Francque, S.; Goodman, Z.; Aithal, G.P.; Kowdley, K.V.; Seyedkazemi, S.; Fischer, L.; et al. Cenicriviroc Treatment for Adults with Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology 2020, 72, 892–905. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrivastava, S.; Kottilil, S.; Sherman, K.E.; Masur, H.; Tang, L. CCR5+ T-Cells Homed to the Liver Exhibit Inflammatory and Profibrogenic Signatures in Chronic HIV/HCV-Coinfected Patients. Viruses 2021, 13, 2074. https://doi.org/10.3390/v13102074
Shrivastava S, Kottilil S, Sherman KE, Masur H, Tang L. CCR5+ T-Cells Homed to the Liver Exhibit Inflammatory and Profibrogenic Signatures in Chronic HIV/HCV-Coinfected Patients. Viruses. 2021; 13(10):2074. https://doi.org/10.3390/v13102074
Chicago/Turabian StyleShrivastava, Shikha, Shyam Kottilil, Kenneth E. Sherman, Henry Masur, and Lydia Tang. 2021. "CCR5+ T-Cells Homed to the Liver Exhibit Inflammatory and Profibrogenic Signatures in Chronic HIV/HCV-Coinfected Patients" Viruses 13, no. 10: 2074. https://doi.org/10.3390/v13102074
APA StyleShrivastava, S., Kottilil, S., Sherman, K. E., Masur, H., & Tang, L. (2021). CCR5+ T-Cells Homed to the Liver Exhibit Inflammatory and Profibrogenic Signatures in Chronic HIV/HCV-Coinfected Patients. Viruses, 13(10), 2074. https://doi.org/10.3390/v13102074





