Differential Behaviours and Preferential Bindings of Influenza Nucleoproteins on Importins-α
Abstract
1. Introduction
2. Materials and Methods
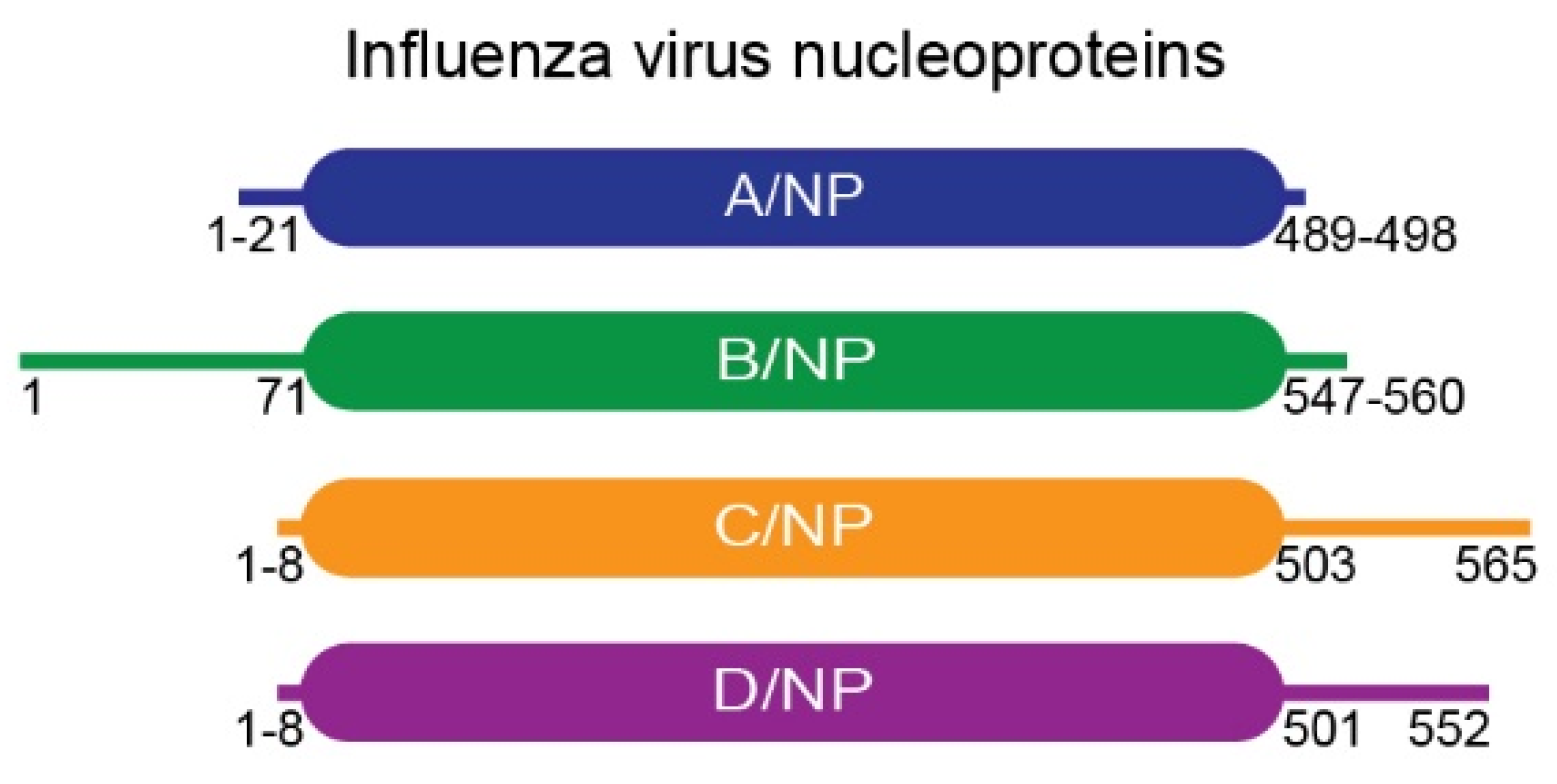
2.1. Molecular Biology and Constructs
2.2. Expression and Purification
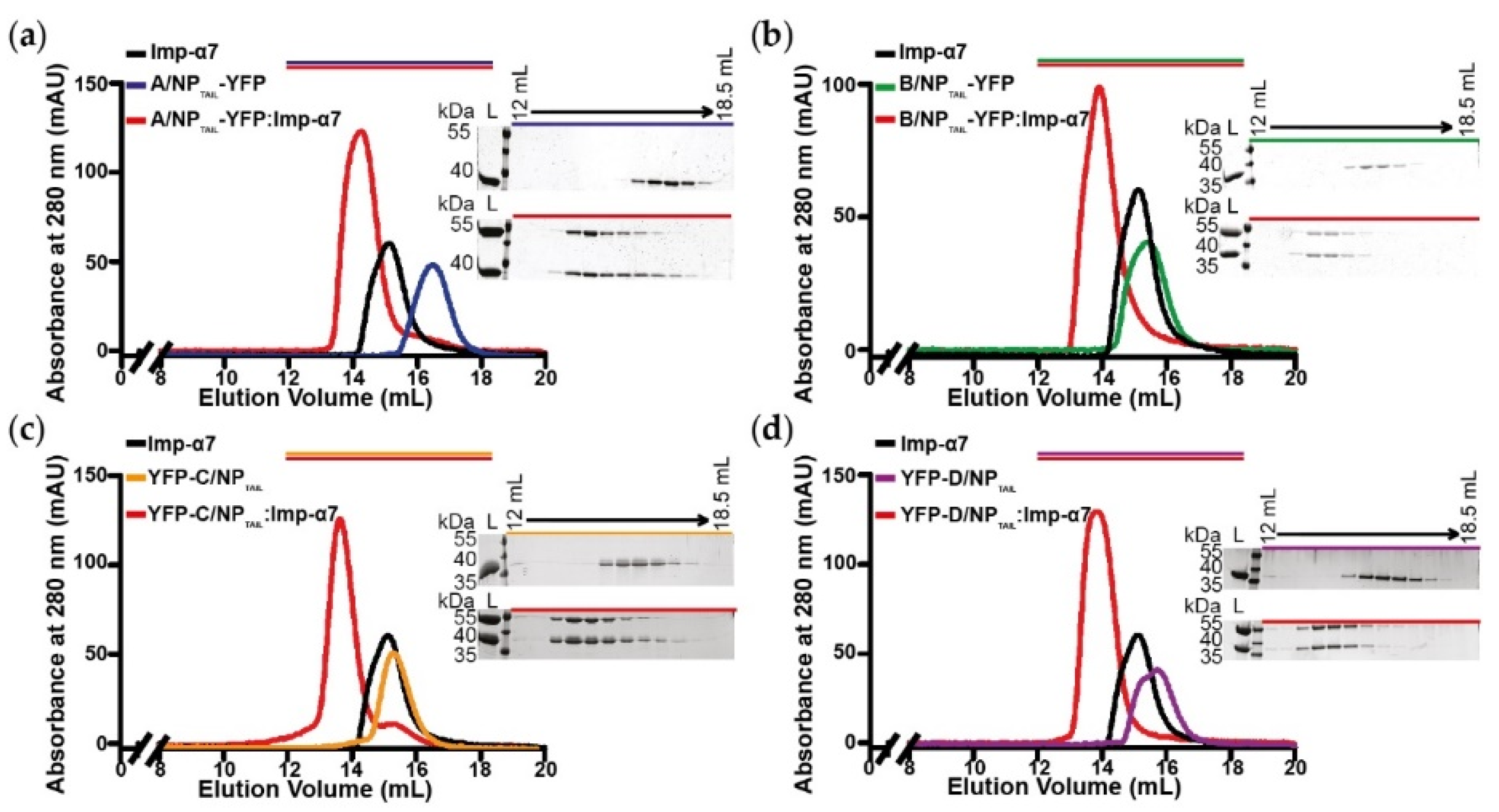
2.3. Interaction Assays by Size Exclusion Chromatography
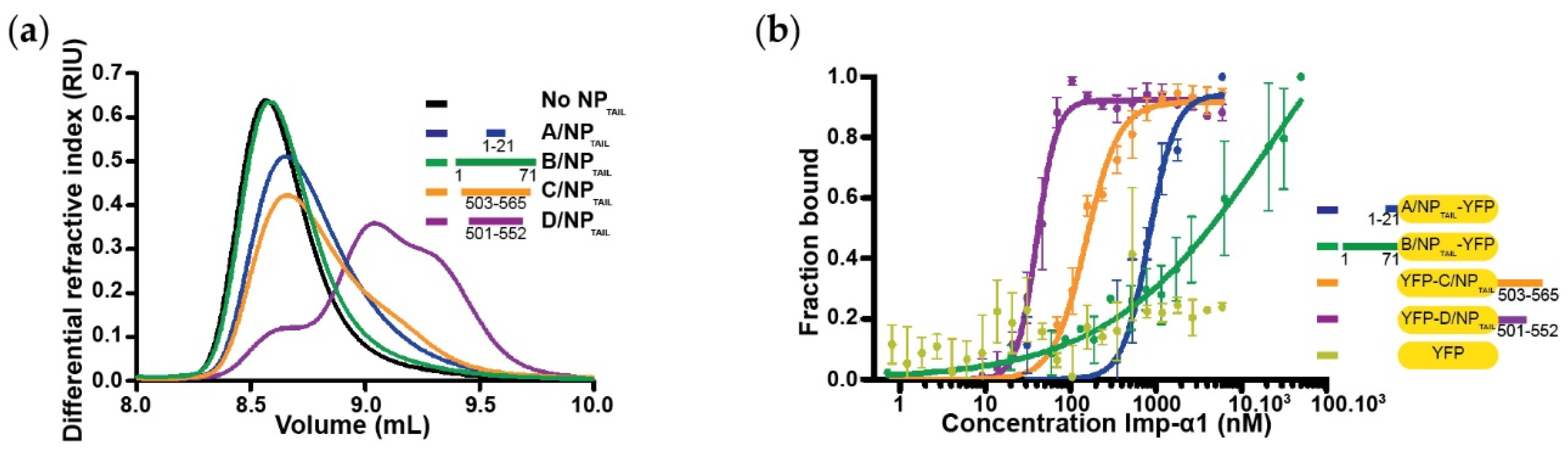
2.4. SEC-MALLS-RI Analysis
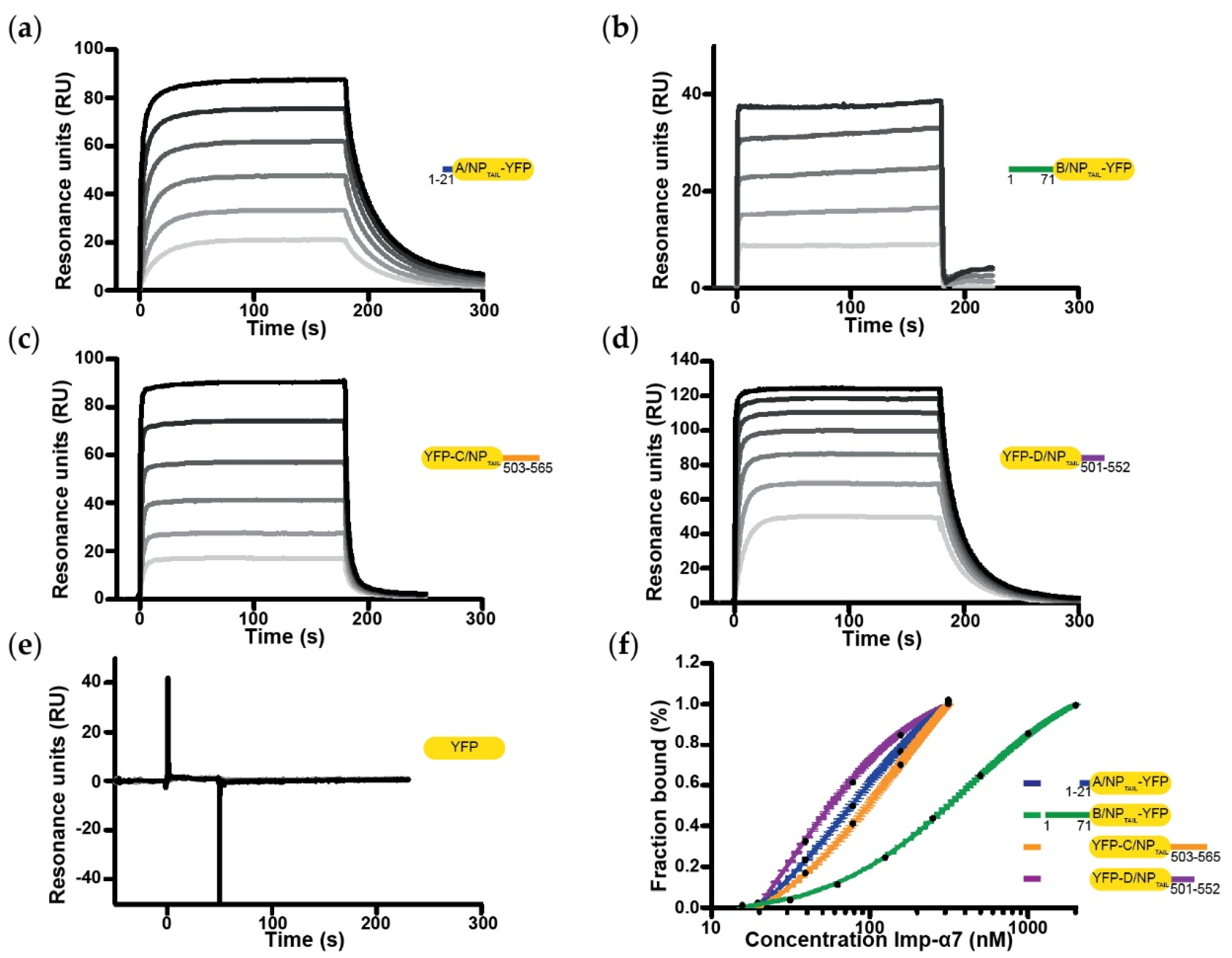
2.5. Surface Plasmon Resonance
2.6. Fluorescence Anisotropy
3. Results
3.1. Biochemical and Biophysical Analysis of the Interaction between NPTAILS and Importin-α7
3.2. Biochemical and Biophysical Analysis of the Interaction between NPTAILS Fused to Yellow Fluorescent Protein (YFP) and Importin-α7
3.3. Systematic Analysis of the Interactions between NPTAILs and Importins-α
3.4. The Case of the Importin-α1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, M.; Lin, X.D.; Chen, X.; Tian, J.H.; Chen, L.J.; Li, K.; Wang, W.; Eden, J.S.; Shen, J.J.; Liu, L.; et al. The evolutionary history of vertebrate RNA viruses. Nature 2018, 556, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, K.; Ruigrok, R.W.; Baudin, F. Roles of the Influenza virus polymerase and nucleoprotein in forming a functional RNP structure. EMBO J. 1997, 16, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Coloma, R.; Arranz, R.; de la Rosa-Trevin, J.M.; Sorzano, C.O.S.; Munier, S.; Carlero, D.; Naffakh, N.; Ortin, J.; Martin-Benito, J. Structural insights into Influenza A virus ribonucleoproteins reveal a processive helical track as transcription mechanism. Nat. Microbiol. 2020, 5, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Feldherr, C.M.; Kallenbach, E.; Schultz, N. Movement of a karyophilic protein through the nuclear pores of oocytes. J. Cell Biol. 1984, 99, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.I. The nuclear pore complex. Annu. Rev. Biochem. 1995, 64, 865–896. [Google Scholar] [CrossRef]
- Mans, B.J.; Anantharaman, V.; Aravind, L.; Koonin, E.V. Comparative genomics, evolution and origins of the nuclear envelope and nuclear pore complex. Cell Cycle 2004, 3, 1612–1637. [Google Scholar] [CrossRef]
- Lin, D.H.; Hoelz, A. The structure of the nuclear pore complex (an update). Annu. Rev. Biochem. 2019, 88, 725–783. [Google Scholar] [CrossRef]
- Bonner, W.M. Protein migration into nuclei. II. Frog oocyte nuclei accumulate a class of microinjected oocyte nuclear proteins and exclude a class of microinjected oocyte cytoplasmic proteins. J. Cell Biol. 1975, 64, 431–437. [Google Scholar] [CrossRef]
- Chook, Y.M.; Suel, K.E. Nuclear import by karyopherin-betas: Recognition and inhibition. Biochim. Biophys. Acta 2011, 1813, 1593–1606. [Google Scholar] [CrossRef]
- Timney, B.L.; Raveh, B.; Mironska, R.; Trivedi, J.M.; Kim, S.J.; Russel, D.; Wente, S.R.; Sali, A.; Rout, M.P. Simple rules for passive diffusion through the nuclear pore complex. J. Cell Biol. 2016, 215, 57–76. [Google Scholar] [CrossRef]
- Gorlich, D.; Vogel, F.; Mills, A.D.; Hartmann, E.; Laskey, R.A. Distinct functions for the two importin subunits in nuclear protein import. Nature 1995, 377, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Chook, Y.M.; Blobel, G. Karyopherins and nuclear import. Curr. Opin. Struct. Biol. 2001, 11, 703–715. [Google Scholar] [CrossRef]
- Lange, A.; Mills, R.E.; Lange, C.J.; Stewart, M.; Devine, S.E.; Corbett, A.H. Classical nuclear localization signals: Definition, function, and interaction with importin alpha. J. Biol. Chem. 2007, 282, 5101–5105. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Cansizoglu, A.E.; Suel, K.E.; Louis, T.H.; Zhang, Z.; Chook, Y.M. Rules for nuclear localization sequence recognition by karyopherin beta 2. Cell 2006, 126, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Bono, F.; Cook, A.G.; Grunwald, M.; Ebert, J.; Conti, E. Nuclear import mechanism of the EJC component Mago-Y14 revealed by structural studies of importin 13. Mol. Cell 2010, 37, 211–222. [Google Scholar] [CrossRef]
- Padavannil, A.; Sarkar, P.; Kim, S.J.; Cagatay, T.; Jiou, J.; Brautigam, C.A.; Tomchick, D.R.; Sali, A.; D’Arcy, S.; Chook, Y.M. Importin-9 wraps around the H2A-H2B core to act as nuclear importer and histone chaperone. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Swale, C.; Da Costa, B.; Sedano, L.; Garzoni, F.; McCarthy, A.A.; Berger, I.; Bieniossek, C.; Ruigrok, R.W.H.; Delmas, B.; Crepin, T. X-ray structure of the human karyopherin RanBP5, an essential factor for Influenza polymerase nuclear trafficking. J. Mol. Biol. 2020, 432, 3353–3359. [Google Scholar] [CrossRef]
- Pumroy, R.A.; Cingolani, G. Diversification of importin-alpha isoforms in cellular trafficking and disease states. Biochem. J. 2015, 466, 13–28. [Google Scholar] [CrossRef]
- Oka, M.; Yoneda, Y. Importin alpha: Functions as a nuclear transport factor and beyond. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2018, 94, 259–274. [Google Scholar] [CrossRef]
- Martin, K.; Helenius, A. Transport of incoming Influenza virus nucleocapsids into the nucleus. J. Virol. 1991, 65, 232–244. [Google Scholar] [CrossRef]
- Neumann, G.; Castrucci, M.R.; Kawaoka, Y. Nuclear import and export of Influenza virus nucleoprotein. J. Virol. 1997, 71, 9690–9700. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Engelhardt, O.G.; Thomas, B.; Akoulitchev, A.V.; Brownlee, G.G.; Fodor, E. Role of ran binding protein 5 in nuclear import and assembly of the Influenza virus RNA polymerase complex. J. Virol. 2006, 80, 11911–11919. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, E.C.; Orr, O.E.; Man Liu, S.; Engelhardt, O.G.; Fodor, E. Characterization of the interaction between the Influenza A virus polymerase subunit PB1 and the host nuclear import factor Ran-binding protein 5. J. Gen. Virol. 2011, 92, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Swale, C.; Monod, A.; Tengo, L.; Labaronne, A.; Garzoni, F.; Bourhis, J.M.; Cusack, S.; Schoehn, G.; Berger, I.; Ruigrok, R.W.; et al. Structural characterization of recombinant IAV polymerase reveals a stable complex between viral PA-PB1 heterodimer and host RanBP5. Sci. Rep. 2016, 6, 24727. [Google Scholar] [CrossRef] [PubMed]
- Tarendeau, F.; Boudet, J.; Guilligay, D.; Mas, P.; Bougault, C.; Boulo, S.; Baudin, F.; Ruigrok, R.W.H.; Daigle, N.; Ellenberg, J.; et al. Structure and nuclear import function of the C-terminal domain of Influenza virus polymerase PB2 subunit. Nat. Struct. Mol. Biol. 2007, 14, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Boivin, S.; Hart, D.J. Interaction of the Influenza A virus polymerase PB2 C-terminal region with importin alpha isoforms provides insights into host adaptation and polymerase assembly. J. Biol. Chem. 2011, 286, 10439–10448. [Google Scholar] [CrossRef] [PubMed]
- Hudjetz, B.; Gabriel, G. Human-like PB2 627K Influenza virus polymerase activity is regulated by importin-alpha1 and -alpha7. PLoS Pathog. 2012, 8, e1002488. [Google Scholar] [CrossRef]
- Pumroy, R.A.; Ke, S.; Hart, D.J.; Zachariae, U.; Cingolani, G. Molecular determinants for nuclear import of Influenza A PB2 by importin alpha isoforms 3 and 7. Structure 2015, 23, 374–384. [Google Scholar] [CrossRef]
- Cros, J.F.; García-Sastre, A.; Palese, P. An unconventional NLS is critical for the nuclear import of the Influenza A virus nucleoprotein and ribonucleoprotein. Traffic 2005, 6, 205–213. [Google Scholar] [CrossRef]
- Weber, F.; Kochs, G.; Gruber, S.; Haller, O. A classical bipartite nuclear localization signal on Thogoto and Influenza A virus nucleoproteins. Virology 1998, 250, 9–18. [Google Scholar] [CrossRef]
- Wu, W.; Sankhala, R.S.; Florio, T.J.; Zhou, L.; Nguyen, N.L.T.; Lokareddy, R.K.; Cingolani, G.; Pante, N. Synergy of two low-affinity NLSs determines the high avidity of Influenza A virus nucleoprotein NP for human importin alpha isoforms. Sci. Rep. 2017, 7, 11381. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.E.; Palese, P. NPI-1, the human homolog of SRP-1, interacts with Influenza virus nucleoprotein. Virology 1995, 206, 116–125. [Google Scholar] [CrossRef]
- Wang, P.; Palese, P.; O’Neill, R.E. The NPI-1/NPI-3 (karyopherin alpha) binding site on the Influenza a virus nucleoprotein NP is a nonconventional nuclear localization signal. J. Virol. 1997, 71, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Melen, K.; Fagerlund, R.; Franke, J.; Kohler, M.; Kinnunen, L.; Julkunen, I. Importin alpha nuclear localization signal binding sites for STAT1, STAT2, and Influenza A virus nucleoprotein. J. Biol. Chem. 2003, 278, 28193–28200. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.; Klingel, K.; Otte, A.; Thiele, S.; Hudjetz, B.; Arman-Kalcek, G.; Sauter, M.; Shmidt, T.; Rother, F.; Baumgarte, S.; et al. Differential use of importin-alpha isoforms governs cell tropism and host adaptation of Influenza virus. Nat. Commun. 2011, 2, 156. [Google Scholar] [CrossRef]
- Sasaki, Y.; Hagiwara, K.; Kakisaka, M.; Yamada, K.; Murakami, T.; Aida, Y. Importin alpha3/Qip1 is involved in multiplication of mutant Influenza virus with alanine mutation at amino acid 9 independently of nuclear transport function. PLoS ONE 2013, 8, e55765. [Google Scholar] [CrossRef]
- Donchet, A.; Oliva, J.; Labaronne, A.; Tengo, L.; Miloudi, M.; Gerard, F.C.A.; Mas, C.; Schoehn, G.; Ruigrok, R.W.H.; Ducatez, M.; et al. The structure of the nucleoprotein of Influenza D shows that all Orthomyxoviridae nucleoproteins have a similar NPCORE, with or without a NPTAIL for nuclear transport. Sci. Rep. 2019, 9, 600. [Google Scholar] [CrossRef]
- Labaronne, A.; Milles, S.; Donchet, A.; Jensen, M.R.; Blackledge, M.; Bourhis, J.M.; Ruigrok, R.W.H.; Crepin, T. Structural analysis of the complex between Influenza B nucleoprotein and human importin-alpha. Sci. Rep. 2017, 7, 17164. [Google Scholar] [CrossRef]
- Ozawa, M.; Fujii, K.; Muramoto, Y.; Yamada, S.; Yamayoshi, S.; Takada, A.; Goto, H.; Horimoto, T.; Kawaoka, Y. Contributions of two nuclear localization signals of Influenza A virus nucleoprotein to viral replication. J. Virol. 2007, 81, 30–41. [Google Scholar] [CrossRef]
- Ng, A.K.; Zhang, H.; Tan, K.; Li, Z.; Liu, J.H.; Chan, P.K.; Li, S.M.; Chan, W.Y.; Au, S.W.; Joachimiak, A.; et al. Structure of the Influenza virus A H5N1 nucleoprotein: Implications for RNA binding, oligomerization, and vaccine design. FASEB J. 2008, 22, 3638–3647. [Google Scholar] [CrossRef]
- Boulo, S.; Akarsu, H.; Lotteau, V.; Muller, C.W.; Ruigrok, R.W.; Baudin, F. Human importin alpha and RNA do not compete for binding to Influenza A virus nucleoprotein. Virology 2011, 409, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Nakada, R.; Hirano, H.; Matsuura, Y. Structure of importin-alpha bound to a non-classical nuclear localization signal of the Influenza A virus nucleoprotein. Sci. Rep. 2015, 5, 15055. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.S.; Lo, C.Y.; Mok, C.K.; Chan, P.K.; Shaw, P.C. The extended C-terminal region of Influenza C virus nucleoprotein is important for nuclear import and ribonucleoprotein activity. J. Virol. 2019, 93, e02048-18. [Google Scholar] [CrossRef] [PubMed]
- Timney, B.L.; Tetenbaum-Novatt, J.; Agate, D.S.; Williams, R.; Zhang, W.; Chait, B.T.; Rout, M.P. Simple kinetic relationships and nonspecific competition govern nuclear import rates in vivo. J. Cell Biol. 2006, 175, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Mackmull, M.T.; Klaus, B.; Heinze, I.; Chokkalingam, M.; Beyer, A.; Russell, R.B.; Ori, A.; Beck, M. Landscape of nuclear transport receptor cargo specificity. Mol. Syst. Biol. 2017, 13, 962. [Google Scholar] [CrossRef]
- Sumarheni, S.; Hong, S.S.; Josserand, V.; Coll, J.L.; Boulanger, P.; Schoehn, G.; Fender, P. Human full-length coagulation factor X and a GLA domain-derived 40-mer polypeptide bind to different regions of the adenovirus serotype 5 hexon capsomer. Hum. Gene Ther. 2014, 25, 339–349. [Google Scholar] [CrossRef]
- Wyatt, P.J. Submicrometer particle sizing by multiangle light scattering following fractionation. J. Colloid Interface Sci. 1998, 197, 9–20. [Google Scholar] [CrossRef]
- Labaronne, A.; Swale, C.; Monod, A.; Schoehn, G.; Crepin, T.; Ruigrok, R.W. Binding of RNA by the nucleoproteins of Influenza viruses A and B. Viruses 2016, 8, 247. [Google Scholar] [CrossRef]
- Mason, D.A.; Stage, D.E.; Goldfarb, D.S. Evolution of the metazoan-specific importin alpha gene family. J. Mol. Evol. 2009, 68, 351–365. [Google Scholar] [CrossRef]
- Miyatake, H.; Sanjoh, A.; Unzai, S.; Matsuda, G.; Tatsumi, Y.; Miyamoto, Y.; Dohmae, N.; Aida, Y. Crystal structure of human importin-alpha1 (Rch1), revealing a potential autoinhibition mode involving homodimerization. PLoS ONE 2015, 10, e0115995. [Google Scholar] [CrossRef]
- Resa-Infante, P.; Paterson, D.; Bonet, J.; Otte, A.; Oliva, B.; Fodor, E.; Gabriel, G. Targeting importin-alpha7 as a therapeutic approach against pandemic Influenza viruses. J. Virol. 2015, 89, 9010–9020. [Google Scholar] [CrossRef] [PubMed]
- Ninpan, K.; Suptawiwat, O.; Boonarkart, C.; Phuangphung, P.; Sathirareuangchai, S.; Uiprasertkul, M.; Auewarakul, P. Expression of importin-alpha isoforms in human nasal mucosa: Implication for adaptation of avian Influenza A viruses to human host. Virol. J. 2016, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Sankhala, R.S.; Lokareddy, R.K.; Begum, S.; Pumroy, R.A.; Gillilan, R.E.; Cingolani, G. Three-dimensional context rather than NLS amino acid sequence determines importin alpha subtype specificity for RCC1. Nat. Commun. 2017, 8, 979. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Tsimbalyuk, S.; Edwards, M.R.; Cross, E.M.; Batra, J.; Soares da Costa, T.P.; Aragao, D.; Basler, C.F.; Forwood, J.K. Structural basis for importin alpha 3 specificity of W proteins in Hendra and Nipah viruses. Nat. Commun. 2018, 9, 3703. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.; Dilworth, S.M.; Laskey, R.A.; Dingwall, C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: Identification of a class of bipartite nuclear targeting sequence. Cell 1991, 64, 615–623. [Google Scholar] [CrossRef]
- Fontes, M.R.; Teh, T.; Kobe, B. Structural basis of recognition of monopartite and bipartite nuclear localization sequences by mammalian importin-alpha. J. Mol. Biol. 2000, 297, 1183–1194. [Google Scholar] [CrossRef]
- Hodel, M.R.; Corbett, A.H.; Hodel, A.E. Dissection of a nuclear localization signal. J. Biol. Chem. 2001, 276, 1317–1325. [Google Scholar] [CrossRef]
- Lokareddy, R.K.; Hapsari, R.A.; van Rheenen, M.; Pumroy, R.A.; Bhardwaj, A.; Steen, A.; Veenhoff, L.M.; Cingolani, G. Distinctive properties of the nuclear localization signals of inner nuclear membrane proteins Heh1 and Heh2. Structure 2015, 23, 1305–1316. [Google Scholar] [CrossRef]
- Cardarelli, F.; Bizzarri, R.; Serresi, M.; Albertazzi, L.; Beltram, F. Probing nuclear localization signal-importin alpha binding equilibria in living cells. J. Biol. Chem. 2009, 284, 36638–36646. [Google Scholar] [CrossRef]
- Hutchinson, E.C.; Denham, E.M.; Thomas, B.; Trudgian, D.C.; Hester, S.S.; Ridlova, G.; York, A.; Turrell, L.; Fodor, E. Mapping the phosphoproteome of Influenza a and B viruses by mass spectrometry. PLoS Pathog. 2012, 8, e1002993. [Google Scholar] [CrossRef]
- Zheng, W.; Li, J.; Wang, S.; Cao, S.; Jiang, J.; Chen, C.; Ding, C.; Qin, C.; Ye, X.; Gao, G.F.; et al. Phosphorylation controls the nuclear-cytoplasmic shuttling of Influenza A virus nucleoprotein. J. Virol. 2015, 89, 5822–5834. [Google Scholar] [CrossRef] [PubMed]
- Terry, L.J.; Shows, E.B.; Wente, S.R. Crossing the nuclear envelope: Hierarchical regulation of nucleocytoplasmic transport. Science 2007, 318, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.T.; Clarke, S.G. Protein arginine methylation in mammals: Who, what, and why. Mol. Cell 2009, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nardozzi, J.; Wenta, N.; Yasuhara, N.; Vinkemeier, U.; Cingolani, G. Molecular basis for the recognition of phosphorylated STAT1 by importin alpha5. J. Mol. Biol. 2010, 402, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.E.; Pernet, O.; Lee, B. Regulation of the nucleocytoplasmic trafficking of viral and cellular proteins by ubiquitin and small ubiquitin-related modifiers. Biol. Cell. 2012, 104, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Ptak, C.; Wozniak, R.W. SUMO and nucleocytoplasmic transport. Adv. Exp. Med. Biol. 2017, 963, 111–126. [Google Scholar]
- Friedrich, B.; Quensel, C.; Sommer, T.; Hartmann, E.; Kohler, M. Nuclear localization signal and protein context both mediate importin alpha specificity of nuclear import substrates. Mol. Cell. Biol. 2006, 26, 8697–8709. [Google Scholar] [CrossRef]
- Jullien, D.; Gorlich, D.; Laemmli, U.K.; Adachi, Y. Nuclear import of RPA in Xenopus egg extracts requires a novel protein XRIPalpha but not importin alpha. EMBO J. 1999, 18, 4348–4358. [Google Scholar] [CrossRef]
- Narayanan, U.; Ospina, J.K.; Frey, M.R.; Hebert, M.D.; Matera, A.G. SMN, the spinal muscular atrophy protein, forms a pre-import snRNP complex with snurportin1 and importin beta. Hum. Mol. Genet. 2002, 11, 1785–1795. [Google Scholar] [CrossRef]
- Hodges, J.L.; Leslie, J.H.; Mosammaparast, N.; Guo, Y.; Shabanowitz, J.; Hunt, D.F.; Pemberton, L.F. Nuclear import of TFIIB is mediated by Kap114p, a karyopherin with multiple cargo-binding domains. Mol. Biol. Cell 2005, 16, 3200–3210. [Google Scholar] [CrossRef][Green Version]
- Tome-Amat, J.; Ramos, I.; Amanor, F.; Fernandez-Sesma, A.; Ashour, J. Influenza A virus utilizes low-affinity, high-avidity interactions with the nuclear import machinery to ensure infection and immune evasion. J. Virol. 2019, 93, e01046-18. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.S.; Reed, E.H.; Parthasarathy, R.; Jahnke, C.N.; Caldwell, R.M.; Bermudez, J.G.; Ramage, H.; Good, M.C.; Hammer, D.A. Controllable protein phase separation and modular recruitment to form responsive membraneless organelles. Nat. Commun. 2018, 9, 2985. [Google Scholar] [CrossRef] [PubMed]
- Guseva, S.; Milles, S.; Jensen, M.R.; Salvi, N.; Kleman, J.P.; Maurin, D.; Ruigrok, R.W.H.; Blackledge, M. Measles virus nucleo- and phosphoproteins form liquid-like phase-separated compartments that promote nucleocapsid assembly. Sci. Adv. 2020, 6, eaaz7095. [Google Scholar] [CrossRef] [PubMed]
- Milles, S.; Mercadante, D.; Aramburu, I.V.; Jensen, M.R.; Banterle, N.; Koehler, C.; Tyagi, S.; Clarke, J.; Shammas, S.L.; Blackledge, M.; et al. Plasticity of an ultrafast interaction between nucleoporins and nuclear transport receptors. Cell 2015, 163, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Zilman, A. Aggregation, phase separation and spatial morphologies of the assemblies of FG nucleoporins. J. Mol. Biol. 2018, 430, 4730–4740. [Google Scholar] [CrossRef] [PubMed]
- Dormann, D. FG-nucleoporins caught in the act of liquid-liquid phase separation. J. Cell Biol. 2020, 219, e201910211. [Google Scholar] [CrossRef]

| Construct | Importin-α | Method | Kd | Ref | |
|---|---|---|---|---|---|
| A/NP | R416A | α5 | ITC | 26 nM | [41] |
| A/NP | 2–15 | α1 | SPBA | 1.7 µM | [42] |
| A/NP | 1–13 | α1 | ITC | 5 µM | [31] |
| A/NP | 198–216 | α1 | ITC | 72 µM | [31] |
| B/NP | 1–70 | α7 | ITC | 844 nM | [38] |
| C/NP | 506–565 | α1 | MT | 48 nM | [43] |
| D/NP | 505–552 | α7 | SPR | 100 nM | [37] |
| Imp-α3 | Imp-α5 | Imp-α7 | |
|---|---|---|---|
| A/NPTAIL | 301 ± 21 | 128 ± 20 | 73 ± 4 |
| B/NPTAIL | 308 ± 106 | 770 ± 24 | 405 ± 5 |
| C/NPTAIL | 59 ± 9 | 287 ± 31 | 146 ± 8 |
| D/NPTAIL | 22 ± 15 | 21 ± 5 | 29 ± 1 |
| YFP control | n.i. | n.i. | n.i. |
| Imp-α3 | Imp-α5 | Imp-α7 | ||||
|---|---|---|---|---|---|---|
| kon (×106) | koff (×10−1) | kon (×106) | koff (×10−1) | kon (×106) | koff (×10−1) | |
| A/NPTAIL | 1.31 ± 0.03 | 2.81 ± 0.04 | 1.0 ± 0.6 | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.7 ± 0.1 |
| B/NPTAIL | >10 | >10 | >10 | >10 | >10 | >10 |
| C/NPTAIL | 2.4 ± 1.1 | 1.3 ± 0.4 | 6.1 ± 3.6 | 2.6 ± 0.4 | 2.6 ± 0.4 | 2.5 ± 0.1 |
| D/NPTAIL | 2.8 ± 0.2 | 0.07 ± 0.01 | 1.1 ± 0.1 | 5.0 ± 0.2 | 5.0 ± 0.2 | 1.04 ± 0.03 |
| YFP control | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Imp-α3 | Imp-α5 | Imp-α7 | |
|---|---|---|---|
| B/NPTAIL | 308 ± 106 | 770 ± 24 | 405 ± 5 |
| B/NPTAIL mut1 | >5000 | >5000 | >5000 |
| D/NPTAIL | 22 ± 15 | 21 ± 5 | 29 ± 1 |
| D/NPTAIL mut1 | 922 ± 77 | 1150 ± 154 | 658 ± 208 |
| D/NPTAIL mut2 | 2457 ± 184 | >5000 | >5000 |
| D/NPTAIL mut3 | n.i. | n.i. | n.i. |
 | |||
| A/NPTAIL | B/NPTAIL | C/NPTAIL | D/NPTAIL | YFP Control | |
|---|---|---|---|---|---|
| imp-αl | 621 ± 89 | n.d. | 149 ± 1 | 41 ± 12 | n.i. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donchet, A.; Vassal-Stermann, E.; Gérard, F.C.A.; Ruigrok, R.W.H.; Crépin, T. Differential Behaviours and Preferential Bindings of Influenza Nucleoproteins on Importins-α. Viruses 2020, 12, 834. https://doi.org/10.3390/v12080834
Donchet A, Vassal-Stermann E, Gérard FCA, Ruigrok RWH, Crépin T. Differential Behaviours and Preferential Bindings of Influenza Nucleoproteins on Importins-α. Viruses. 2020; 12(8):834. https://doi.org/10.3390/v12080834
Chicago/Turabian StyleDonchet, Amélie, Emilie Vassal-Stermann, Francine C. A. Gérard, Rob W. H. Ruigrok, and Thibaut Crépin. 2020. "Differential Behaviours and Preferential Bindings of Influenza Nucleoproteins on Importins-α" Viruses 12, no. 8: 834. https://doi.org/10.3390/v12080834
APA StyleDonchet, A., Vassal-Stermann, E., Gérard, F. C. A., Ruigrok, R. W. H., & Crépin, T. (2020). Differential Behaviours and Preferential Bindings of Influenza Nucleoproteins on Importins-α. Viruses, 12(8), 834. https://doi.org/10.3390/v12080834





