Abstract
Enteropathogenic Escherichia coli (EPEC) is a major pathogen for diarrheal diseases among children. Antibiotics, when used appropriately, are effective; however, their overuse and misuse have led to the rise of antibiotic resistance worldwide. Thus, there are renewed efforts into the development of phage therapy as an alternative antibacterial therapy. Because EPEC in vivo models have shortcomings, a surrogate is used to study the mouse pathogen Citrobacter rodentium in animal models. In this study, two new phages CrRp3 and CrRp10, which infect C. rodentium, were isolated and characterized. CrRp3 was found to be a new species within the genus Vectrevirus, and CrRp10 is a new strain within the species Escherichia virus Ime09, in the genus Tequatrovirus. Both phages appear to have independently evolved from E. coli phages, rather than other Citrobacter spp. phages. Neither phage strain carries known genes associated with bacterial virulence, antibiotic resistance, or lysogeny. CrRp3 is more potent, having a 24-fold faster adsorption rate and shorter lytic cycle when compared to the same properties of CrRp10. However, a lysis curve analysis revealed that CrRp10 prevented growth of C. rodentium for 18 h, whereas resistance developed against CrRp3 within 9 h. We also show that hypoxic (5% oxygen) conditions decreased CrRp3 ability to control bacterial densities in culture. In contrast, low oxygen conditions did not affect CrRp10 ability to replicate on C. rodentium. Together, CrRp10 is likely to be the better candidate for future phage therapy investigations.
1. Introduction
Diarrheal diseases continue to be one of the foremost public health issues globally, responsible for more than 1.6 million deaths each year [1]. While mortality rates have reduced substantially (by 34% among children and by 21% among all people) over the last two decades, the incidence of diarrhea has not reduced nearly as much (10% reduction in children and 6% reduction overall) [1]. Enteropathogenic Escherichia coli (EPEC) is a major cause of diarrhea morbidity and mortality among children < 5 years [1,2]. When used appropriately, antibiotics can reduce the severity and duration of diarrheal diseases; however, the overuse and misuse of antibiotics in the treatment of diarrhea has led to an alarming increase in antibiotic resistance (AMR) globally [1,2,3]. These observations illustrate that diarrhea mortality is largely avoidable and renewed efforts to reduce disease the burden with non-antibiotic strategies are urgently needed.
Mouse models for the study of EPEC diseases have had limited utility, because gastrointestinal microbiota defends well against human E. coli infections [4]. Consequently, most mouse models of E. coli gastrointestinal diseases require substantial antibiotic pre-treatment to reduce resident bacteria [5,6,7,8]. The preferred surrogate for EPEC diseases is the mouse-restricted pathogen Citrobacter rodentium [9]. Both EPEC and C. rodentium cause attaching and effacing (A/E) lesions, and share the same pool of locus of enterocyte effacement (LEE)-encoded and non-LEE-encoded effector proteins to subvert and modulate gut epithelial barrier properties [9,10]. Importantly, without the aid of antibiotics, C. rodentium causes pathologies that are indistinguishable from those observed in the human EPEC gut infection [11].
Bacteriophages (phages) are one of the foremost antibacterial agents under development and in clinical testing for treating life-threatening AMR pathogens [12,13], with several recent successful compassionate use phage therapy cases (for examples, see [14,15,16]). Phages are highly abundant and ubiquitous viruses that only infect and kill bacteria. Other promising features of phages include a narrow host range, self-replication at sites of infection, disruption of biofilms, and potential synergy with antibiotics [13,17,18]. Traditionally, virulent double-stranded DNA tailed phages that carry out a strictly lytic infection cycle are considered the ideal viral type for human therapeutic applications [12,13]. However, only four virulent phages that infect C. rodentium have been described to date, including phiCR1 [19], R18C [20], CR8 and CR44b [21]. In addition, C. rodentium phage therapy animal models have not been explored.
In this study, we characterize two new virulent phages, CrRp3 and CrRp10, which infect C. rodentium. Using genome sequence annotations, phenotypic susceptibility under normoxic and hypoxic environments, host cross-genera infection ranges, and phylogenetic analysis, we assess the subtle feature differences that may improve the efficacy of future therapeutic evaluation in an A/E diarrheal disease mouse model.
2. Materials and Methods
2.1. Strains and Culturing
C. rodentium strain ICC180 [22] was grown in Luria–Bertani (LB) broth under both normoxic (atmospheric 21% O2 and 0.04% CO2) and hypoxic (5% O2 and 5% CO2) environments at 37 °C with orbital shaking. For hypoxia culturing, LB was preconditioned at 5% O2 and 5% CO2 for 2 days by using an incubator with O2 control filled with 5% CO2 and balanced with N2 (NBS Galaxy 48R) to remove dissolved oxygen in growth media. Agar (1.5%) was added to LB for solid growth medium.
2.2. Phage Isolation, Cultivation and Purification
C. rodentium phages were isolated from 0.25 μm syringe filtered (Sartorius, Göttingen, Germany) sewage sample collected from water treatment plants around Paris, France. Individual plaque-forming units were isolated from a 10 μL sample spread over LB agar Petri plate, overlaid with 2 mL of mid-log growing ICC180 under normoxic conditions. After overnight incubation, a sterile Pasteur pipette was used to select PFU with different morphologies and phages enriched by culturing with ICC180 in fresh LB. PFU selection and enrichment was repeated 5 times. Final phage lysates were sterilized by high-speed centrifugation, 0.2 µm filtration, further purified by cross-flow filtration (CFF), and cesium chloride (CsCl) density gradient ultracentrifugation, using a methodology previously described [23]. Lastly, phages were stored in phosphate buffer saline (PBS).
2.3. Transmission Electron Microscopy
To visualize viral morphology, purified phages in TN buffer (10 mM Tris-Cl, 10 mM NaCl) were loaded onto carbon-coated copper grids and negatively stained with 2% (wt/vol) uranyl acetate for 30 s. Then, phages were visualized using a FEI Tecnai 120 Biotwin transmission electron microscope (TEM) (FEI, Hillsboro, OR, USA), at an accelerating voltage of 120 kV, and an Orius 1000 digital camera (Gatan, Pleasanton, CA, USA) recorded the micrographs.
2.4. DNA Extraction and Genome Sequencing
Genomic DNA was extracted from sterile DNase and RNase pretreated purified phages by a phenol-chloroform extraction, as previously described [24]. Sequencing libraries with single index were prepared using the NEBNext DNA library prep kit (New England BioLabs, Ipswich, MA, USA) and then sequenced on the Illumina MiSeq sequencing platform (Illumina, San Diego, CA, USA) with paired-end 300 nucleotide reads. Raw reads were trimmed by FastQC v10.1 (www.bioinformatics.babraham.ac.uk/projects/fastqc/) and de novo assembled using the CLC Genomics Assembler (Galaxy Version 4.4.2).
2.5. Genome Annotations, Alignments, and Phylogenetic Analyses
Annotation of the specific function of ORFs was conducted using rapid annotations of subsystems technology (RAST) [25]. The presence of transfer RNA (tRNA)-encoding genes was determined using the tRNAscan-SE database [26], and protein-coding genes were predicted using Prodigal [27]. The additional annotation of genes was done manually on the HHPRED server [28], and compared against the NCBI NR, COG [29], and TIGRfam [30] databases. A comparative analysis among related complete genomes was performed using tBLASTx or BLASTN [31]. A computational analysis of toxins, antibiotic resistance, and bacterial virulence factors was done with Abricate (https://github.com/tseemann/abricate), which uses ensemble methods to compare sequences to the most up-to-date databases, including the Comprehensive Antibiotic Resistance Database [32], ResFinder [33], NCBI’s AMRFinder [34], Antibiotic Resistance Gene-ANNOTation [35], and the virulence factor database [36]. Phylogenetic relationships of phages CrRp3 and CrRp10 were determined using VICTOR [37], and pairwise comparisons of the nucleotide sequences were conducted using the Genome-BLAST Distance Phylogeny (GBDP) method [38], under settings recommended for prokaryotic viruses [37]. GBDP phylogenetic relationships were constructed from related Citrobacter, Escherichia and Synechococcus podoviruses and myoviruses identified by Blast (downloaded from GenBank 12/21/2017; Table S4). The resulting intergenomic distances (100 replicates each) were used to infer a balanced minimum evolution tree with branch support via FASTME, including SPR post processing [39]. The trees were rooted with Synechococcus phages (outgroup) and visualized with FigTree V1.4.3 (http://tree.bio.ed.ac.uk).
2.6. Phage Adsorption and Growth
A similar protocol [40] was adopted to determine phage adsorption rate. Briefly, 1 mL of phages was mixed with 2.5 × 108 colony forming units (CFU) mL−1 of bacteria in 9 mL at a multiplicity of infection (MOI) of 0.001. The mixture was incubated at 37 °C with shaking and samples withdrawn each 1 min for titration. In addition, we performed a one-step growth curve procedure as described previously [41], with some modifications. Briefly, 1 mL of phages was added to 2.5 × 108 CFU mL−1 of C. rodentium in 9 mL LB, with no divalent cation supplementation, at a MOI of 0.01. At 2 min intervals, two 100 µL samples were taken, with one used to enumerate free phages, and the second was treated with 10% chloroform to release intracellular phages. At 6 min, a 1000x dilution in 100 mL of fresh broth was performed to avoid any potential future re-infection by newly produced phages. Samples were serial diluted in microtiter plates and PFU counted on LB agar seeded with lawns of bacteria at OD600 0.2. Titer comparison between chloroform-treated and non-treated samples was used to estimate eclipse and latent periods, respectively. Adsorption and one-step growth analyses were repeated four times.
2.7. Phage Lysis Curves
C. rodentium strain ICC180 from an overnight culture was used to inoculate fresh LB to create an exponentially growing population with an optical density (OD600) of ~0.5. To maintain hypoxia, bacteria was grown in LB that was preconditioned overnight in 5% O2 and 5% CO2 chamber. Cells were then washed twice by centrifugation at 6000× g for 10 min and then resuspended in chilled H2O. Microplate wells were filled with 100 µL of 2 times LB concentrate (preconditioned if required) before adding washed cells to give a total of 2 × 106 CFU. Phages were added at different MOIs and sterile water added for a total volume of 200 µL. Microplates were immediately placed in a Clariostar microplate reader (BMG Labtech, Cary, NC, USA) and OD600 measured every 6 min for 18 h. Microplate reader O2 and CO2 levels were regulated with an atmospheric control unit (BMG labtech).
2.8. Phage Host Range
We evaluated phage host ranges using a spot test of 4 µL of 107 PFU onto 32 bacterial strains (listed in Table 3), seeded on agar at OD600 0.22, followed by overnight incubation at 37 °C. Bacteria tested included 27 Escherichia coli strains, Erwinia carotovora CFBL2141 [42], Rouxiella chamberiensis [43], Serratia marcescens DB11 [44] and SM365 [45]. For comparison, the host ranges were also determined for E. coli phages LF82_P10 [46], LM33_P1 [47], AL505_P2 [48], and CLB_P2 [49], 536_P7 [50].
2.9. Statistics
Data are shown as means ± standard deviation (SD). Statistical analyses were performed using Prism v5 (GraphPad, San Diego, CA, USA). A Student’s t-test or one-way analysis of variance with Tukey’s multiple-comparison test was used to determine differences between two or multiple groups, respectively. A p < 0.05 was considered significant.
2.10. Accession Numbers
The complete genome sequences of vB_CroP_CrRp3 (CrRp3) and vB_CroM_CrRp10 (CrRp10) are deposited in NCBI GenBank with accession numbers MG775042 and MG775043, respectively.
3. Results
3.1. Isolation, Morphology, Sequencing, and Genome Annotation of CrRp3
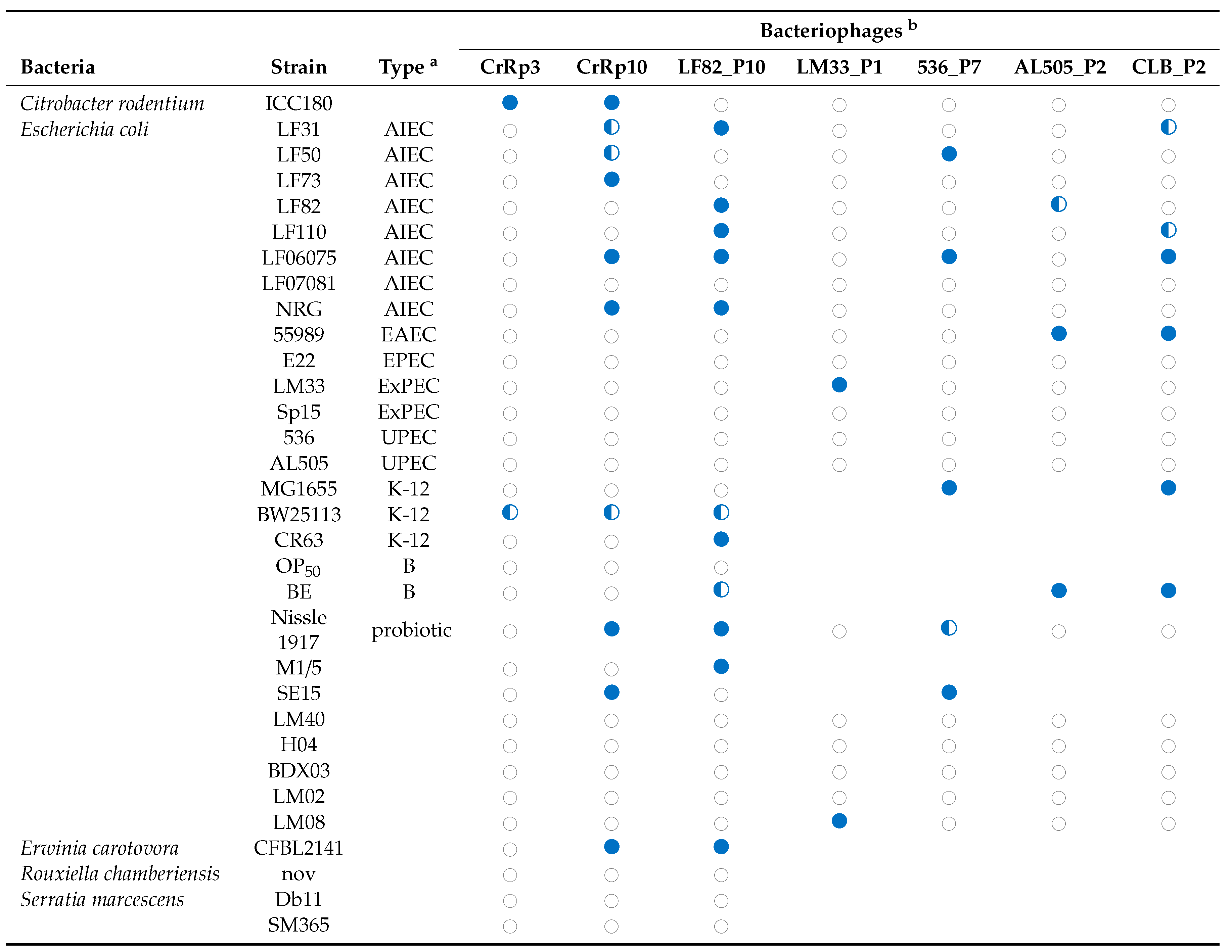
The novel phage CrRp3 (formal name: vB_CroP_CrRp3), which infects C. rodentium strain ICC180, was isolated from sewage. Figure 1a shows that CrRp3 virion has a typical podovirus morphology, with an isometric head, likely icosahedral, and a short tail. Plaques produced by CrRp3 on ICC180 were of 2.36 ± 0.46 mm2, mean ± 95% confidence interval (Figure 1b).
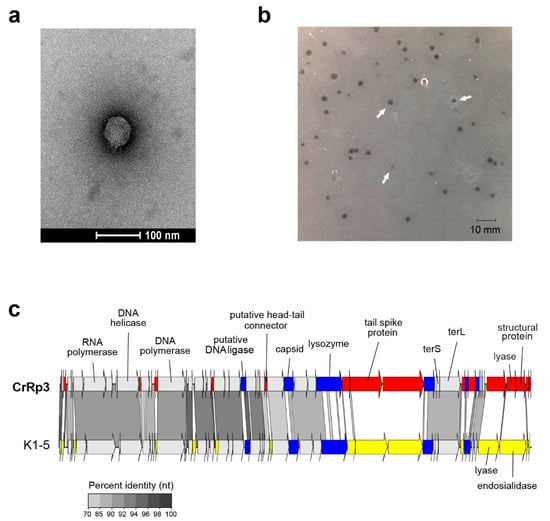
Figure 1.
Morphology, genome organization and gene functional comparison of CrRp3. (a) Electron micrographs of CrRp3 negatively stained with uranyl acetate. (b) Plaque forming units (highlighted by arrows) produced by CrRp3 on lawns of C. rodentium ICC180 after 24 h of growth. (c) Genome alignment of CrRp3 and its closest taxonomic relative E. coli phage K1-5. Genes are colored according to nucleotide sequence homology, with genes exclusive to CrRp3 labeled red, genes exclusive to K1-5 labeled yellow, and homologous genes with <70% identity labeled blue.
Mapping next generation sequence reads showed that CrRp3 has a terminally repetitive dsDNA genome of 44.3 kb, with 54 predicted coding sequences (CDSs) (Figure 1c and Table 1). However, only 19 (35%) could be assigned a putative function (Table S1). Interestingly, CrRp3 has a GC content of 45%, which is 10% lower than that in its host C. rodentium, which has a GC content of 54.5%. The genome does not encode proteins with identifiable sequence homology to known lysogeny-associated proteins, suggesting that CrRp3 is virulent with a strictly lytic lifecycle. In addition, CrRp3 genome does not carry recognizable virulence-associated genes or antibiotic resistance genes.

Table 1.
Citrobacter phages and their genome features.
At the time of analysis, the closest taxonomic relative of CrRp3 was the E. coli phage K1-5 from the Vectrevirus genus in the recently created family Autographivirinae. The two phages share both gene content and genome organization with 90% (over 76% of the genome) and 77% (over 75% of the genome) pairwise nucleotide identity, respectively (Figure 1c). Nearly half of the CrRp3 gene products have the best hits with K1-5 genes, including the DNA and RNA polymerases, DNA ligase and the major capsid protein, as well as many hypothetical proteins (Table S3). Accordingly, CrRp3 is a tentative new species within the genus Vectrevirus in the family Autographiviridae [58]. For most phage genera, >95% DNA sequence identity is used by the International Committee on Taxonomy of Viruses (ICTV), as the species demarcation criterion [58]. Gene products unique to CrRp3 include the head-tail connector protein, endolysin, tailspike protein, lyase, minor structural protein, and several other proteins with unknown functions (Figure 1c and Table S1).
3.2. Morphology and Genome of Phage CrRp10
The second isolated phage, C. rodentium phage CrRp10 (formal name: vB_CroM_CrRp10) displays an elongated (prolate) icosahedral head connected to a long tail covered with a discernable sheath (Figure 2a). These features are characteristic of T4-like myoviruses. Plaques produced by CrRp10 on ICC180 were of <1 mm2 (Figure 2b). This suggests that phage strain has a significant effect on the plaque size, when compared to plaques produced by CrRp3 (Figure 1b vs. Figure 2b).
Figure 2.
Morphology, genome organization and gene functional comparison of CrRp10. (a) Electron micrographs of CrRp10 negatively stained with uranyl acetate. (b) Plaque forming units (highlighted by arrows) on lawns of C. rodentium ICC180 after 24 h or growth. (c) Genome alignment of CrRp10 and its closest taxonomic relative E. coli phage ime09. Genes are colored according to nucleotide sequence homology, with genes exclusive to CrRp10 labeled red, genes exclusive to ime09 labeled yellow, and homologous genes with <70% identity labeled blue.
Genome sequencing showed that CrRp10 has a large circularly permuted dsDNA genome of 171.5 kb with 267 CDSs, and harbors 10 tRNA genes (Figure 2c and Table 1). Only 133 (~50%) of CDS have putative functions (Table S2). Similar to CrRp3, CrRp10 has a GC content of 35.5%, which is 20% lower than that of C. rodentium. The genome does not carry sequence homology to lysogeny-associated genes, which suggests that CrRp10 is a virulent phage. The genomes also exhibit no sequence homologies to known virulence-associated or antibiotic resistance genes.
At the time of analysis, the closest taxonomic relative of CrRp10 was the E. coli phage Ime09, which belongs to the Tequatrovirus genus in the subfamily Tevenvirinae (Figure 2c and Table S3). They share significant synteny, with 98% nucleotide identity over the complete length, and thus, CrRp10 is considered a new strain. However, little is known about phage Ime09, with details restricted to genomic analysis [59]. CrRp10 has divergence from Ime09 within its tail fiber gene, with 3% amino acid dissimilarity over 80% of the corresponding protein. Another notable feature of the CrRp10 genome is what appears to be a recombination event, which resulted in the gain of dUTPase with high sequence similarity to that encoded by phage e11/2. Interestingly, phage e11/2 infects the enterohemorrhagic E. coli (EHEC); also an A/E pathogen [60]. Other recombination events appear to have added several putative endonucleases, with high similarity to homologs in other related Enterobacteriaceae phages (Figure 2c and Table S2).
3.3. Comparison of Citrobacter Phage Proteins
We performed amino acid (AA) sequence alignments to compare proteins among phages that infect C. rodentium and other Citrobacter species (Figure S1). Surprisingly, CrRp3 has <40% AA homology to either C. rodentium phages (CR8 and CR44b) or C. freundii phages phiCFP-1 and Sh4, with the exception of homology between a putative lyase (68%) and minor structural protein (75%) of Citrobacter phage CR8 (Figure S1a and Table S3). Several CrRp10 proteins exhibit low AA homology to the C. rodentium phage Moon, and even less homology with C. freundii phage CfP1 (Figure S1b and Table S3).
3.4. CrRp3 Is Faster at Infecting a Host Cell, but Has a Smaller Burst Size
With an excess of host cells, the estimated adsorption rates for phages CrRp3 and CrRp10 are 3.5 ± 3.2 × 10−10 and 8.52 ± 2.8 × 10−11 mL−1 min−1, respectively (Table 2). That is, when compared, CrRp3 would ‘infect a host’ ~24x faster than CrRp10. This also suggests that these two phages have different cell surface binding receptors. In a well-mixed culture, the CrRp3 replication cycle (latent period) takes approximately 15 min (Table 2 and Figure S2). CrRp10 however, had a slightly longer latent period of about 17 min. The eclipse periods could not be determined because C. rodentium appears to be resistant to chloroform lysis (Figure S2). The shorter replication cycle for CrRp3 correlated had a reduced burst size of 43 phage particles per cell, compared to CrRp10 that produced a burst of 85 phage particles (Table 2).

Table 2.
CrPp3 and CrRp10 replication characteristics on C. rodentium strain ICC180.
3.5. Hypoxia Reduces Lysis at Low MOIs but Not Resistance
Healthy gastrointestinal luminal oxygen levels decreased from 7% (58 mmHg) in the stomach to 0.5% in the colon, and mucosa oxygen levels ranged between 2–6% oxygen [61]. Both CrRp3 and CrRp10 were able to reduce C. rodentium population densities compared to untreated controls at different MOIs under both normoxic (~21% O2) and hypoxic (5% O2) culturing conditions (Figure 3). Under normoxia, CrRp3 took less time than CrRp10 to reverse bacterial population growth (~2.5 h post treatment), and reduced bacterial density below OD limits of detection (LOD) within 3 h (Figure 3a). For CrRp10, the same results took 3.5 and 5.5 h, respectively (Figure 3b). Of these two phages, CrRp3 appears to be more ‘potent’. Under hypoxia, however, CrRp3 at MOIs <0.01 failed to reduce bacterial density below the LOD, whereas CrRp10 could (Figure 3). Indeed, hypoxia also dampened exponential bacterial growth (Figure 3) in the absence of phages, but final cell densities were similar after 18 h. In addition, the regrowth of C. rodentium occurred between 8–9 h after inoculation with CrRp3. In contrast, no observable bacterial regrowth occurred for CrRp10 after 18 h.
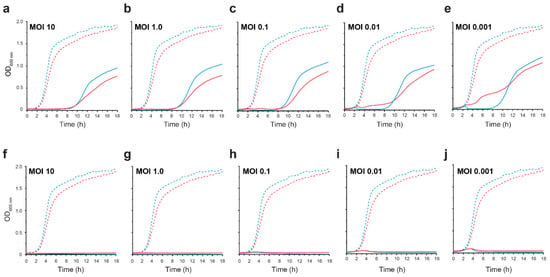
Figure 3.
CrRp3 or CrRp10 lysis curves of C. rodentium under normoxic and hypoxic conditions. Growth curves of C. rodentium ICC180 infected with CrRp3 (a–e) or CrRp10 (f–j) at MOI ranging between 10–0.001 (solid line) or no phage control (dashed line). Bacteria were growth under either 21% (blue line) or 5% (red line) oxygen and 5% CO2 conditions. N = 6 per condition.
3.6. Host Range
Next, we tested the host range of the virulent phages CrRp3 and CrRp10, along with other representative phages against several bacterial strains (Table 3). In addition to their isolation strain of C. rodentium, CrRp3 could infect only the E. coli strain K-12, while CrRp10 displays a much broader host range, including K-12 and several pathotypes of E. coli, as well as the E. carotovora strain CFBP2141. Although the E. coli phage LF82_P10 [46] also exhibits a relatively broad host range, it cannot infect C. rodentium. Moreover, most of the E. coli, as well as the Serratia marcescens strains tested, were resistant to both CrRp3 and CrRp10.

Table 3.
Phage strain host range.
3.7. Phage Phylogenetic Relationships
To investigate phylogenetic relationships among Citrobacter and Escherichia podoviruses including CrRp3, we constructed a genome-blast distance phylogeny tree using 37 complete genome nucleotide sequences from GenBank (Figure 5 and Table S4). We found that these phages display the heterogeneous clustering of closely related Autographiviridae into three distinct clades with maximal bootstrap support (Figure 4a). CrRp3 has closer relationships with podoviruses infecting Escherichia (Figure 4b) than those also infecting C. rodentium (CR8 and CR44b) (Figure 4a; see C1 vs. C3). Rather, CR8 and CR44b clusters with some phages that infect the human pathogen C. freundii (SH3 and SH4). Other C. freundii podoviruses (phiCFP1, SH1, and SH2) cluster in clade 2 (Figure 4c), and phage CVT2 branches separately. The latter was not unexpected, because CVT2 was isolated from the gut of termites with an uncharacterized Citrobacter species [51].
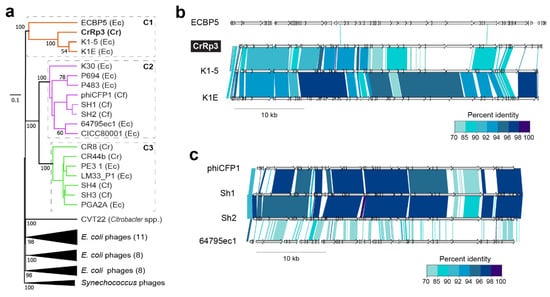
Figure 4.
Phylogenetic relationships among Citrobacter and Escherichia podoviruses. (a) Tree assembled with complete sequences of podoviruses that infect C. rodentium (Cr), C. freundii (Cf) and related E. coli (Ec). The tree is rooted using Synechococcus phages and bootstrap values at nodes defining percent confidence of 100 replicates. Phylogenetic clades (C1–3) have strong bootstrap support of >70%. (b) Complete genome nucleotide alignment of members in clade 1, which includes CrRp3. (c) Genome alignments of members in C2, including related Citrobacter phages, Sh1, Sh2 and phiCFP1 that infect C. freundii.
To determine the relationship of CrRp10 to other Citrobacter and Escherichia Myoviridae, we constructed a genome-blast distance tree using 69 complete genome sequences from GenBank (Figure 5 and Table S4), also showing heterogeneous clustering (Figure 5a and Table S3). CrRp10 clusters in a clade with several E. coli myoviruses (Figure 5). Other myoviruses that infect C. freundii cluster in clades 2–4. Within clade 2 (C2), C. freundii phages Merlin and Moon are further distantly related to 8 Escherichia phages (Figure 5c). In contrast, clade 3 (C3) is almost exclusively composed of C. freundii phages (IME CF2, Miller, CfP1, and Margaery).
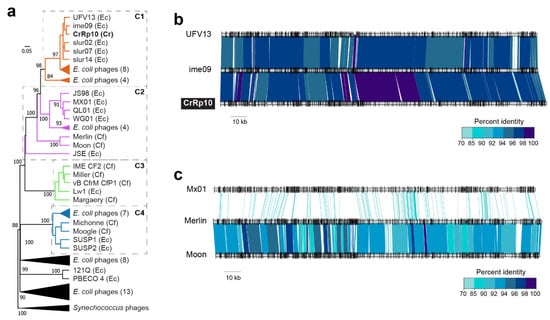
Figure 5.
Phylogenetic relationships among Citrobacter and Escherichia myoviruses. (a) Tree assembled with complete genome sequences of myoviruses that infect C. rodentium myoviruses (Cr), C. freundii (Cf) and related E. coli (Ec). The tree is rooted using Synechococcus phages and bootstrap values at nodes defining percent confidence of 100 replicates. Phylogenetic clades (C1–4) have strong bootstrap support of >70%. (b) Complete genome nucleotide alignment of members in C1, which includes CrRp10. (c) Genome alignment of members in C2, including the closest related Citrobacter phages, Merlin and Moon, which infect C. freundii.
4. Discussion
EPEC, EHEC, and the mouse pathogen C. rodentium, are all attaching/effacing pathogens and causative agents of diarrheal disease. In this study, we isolated and characterized two new phages, CrRp3 and CrRp10, which infect C. rodentium. We show that the podovirus CrRp3 is a tentative new species within the genus Vectrevirus in the family Autographiviridae, while the myovirus CrRp10 is a new strain within the Tequatrovirus genus in the family Myoviridae. Between CrRp3 and CrRp10, only 48% of their combined gene repertoire have assigned putative functions. Of the assignments, we found that neither phage harbor known genes associated with bacterial virulence or antibiotic resistance. The latter is consistent with previous findings that antibiotic resistance genes are rarely carried in phage genomes [62]. In addition, we show that CrRp3 and CrRp10 genomes lack identifiable integrase genes. This implies that both phages carry out strictly lytic replication cycles. Indeed, with more than 50% of genes having an unknown function, these findings emphasize the critical need for significant gene functional studies before any certainty that CrRp3 and CrRp10 do not carry harmful genes.
While these phages expand our knowledge of viral biodiversity, we found that neither CrRp3 nor CrRp10 genomes exhibit nucleotide sequence homology with other C. rodentium phages, including CR8 and CR44b from the genus Caroctavirus [21]. In addition, CrRp3 and CrRp10 are distantly related to phages that infect C. freundii, including members of the genera Moonvirus (Merlin, Miller, Moon) [52,53,54], Mooglevirus (Moogle, Michonne, Mordin) [55,56,63], Tlsvirus (Stevie) [57], and Teetrevirus (phiCFP-1, SH1, SH2, SH3, SH4, SH5) [64,65]. C. freundii can cause a variety of nosocomial acquired extraintestinal human diseases, such as the urinary tract, respiratory tract, and wound infections [66]. Thus, we show that CrRp3 and CrRp10 appear to have independently evolved from closely related E. coli phages, presumably because it was advantageous to expand the host range to infect C. rodentium, and as a result, occupy new niches. Petty et al. showed that the genome of C. rodentium exhibits several features typical of recently passing through an evolutionary bottleneck, including several large-scale genomic rearrangements and functional gene loss in the core genomic regions [67,68,69]. This led the authors to hypothesize that C. rodentium evolved from a human E. coli strain [69]. Our results strengthen this hypothesis by showing that phages that infect C. rodentium appear to have also evolved from phages that infect E. coli. Nonetheless, CrRp3 carries genes that have diverged significantly from presumably ancestral genes of E. coli K1-5-like phage, in particular, gene products responsible for receptor recognition (tail fibers) and cell lysis (endolysin). Interestingly, phage K1-5 exhibits two tail fiber genes, which allow it to be promiscuous between E. coli strains with different capsule compositions [70]. CrRp3 endolysin gene has homology to other Citrobacter phage endolysin genes. This suggests a mosaic genome structure driven by recombination events from diverse viruses. This is consistent with other autographiviruses that exhibit a high genetic identity, structure, and specific RNA polymerase, with the modest differences observed in genes implicated in adaptation to host constraints [71].
Lysis of bacterial cells provides insight into the dynamics between individual phages and their host bacteria. We found that CrRp3 appears to be the more ‘potent’ virus of the two. First, it was determined that CrRp3 would be 24x faster at infecting a host cell based on adsorption rates compared to CrRp10. Either this could be due to cell surface receptors for CrRp3 outnumbering receptors for CrRp10, or CrRp3 tail fibers have a higher affinity to a shared receptor. Second, in well-mixed cultures, CrRp3 took approximately 2 min less than CrRp10 to complete a single lytic replication cycle. This short cycle time correlated to CrRp3 reversing C. rodentium exponential growth sooner than CrRp10 (Figure 3). CrRp3 also exhibited, on average, a 51% reduced progeny burst compared to CrRp10′s burst size (Table 2). This raises the question of whether shorter replication cycles are favorable to higher phage progeny production to eliminate bacterial infections.
However, CrRp10 was more resilient to resistance. We found that CrRp10 did not allow the population growth of C. rodentium after 18 h (end of study), even at an initial MOI of 0.001 (i.e., 1 virion to 1000 cells) (Figure 3b). In contrast, C. rodentium exhibited regrowth after 9 h of co-incubation with CrRp3. Bacteria thwart phage attack through an arsenal of antiviral mechanisms targeting most steps of the phage replication cycle. The transition from phage-sensitive to phage resistant is often due to spontaneous chromosomal mutations that modify cell surface receptors for the phages [72]. Resistance mutations may be expected to impart a fitness cost because they target important biological functions in the cell [73]. This suggests that the mutation of the specific receptors used by CrRp10 to bind to bacterial hosts imposes much higher fitness costs than resistance to CrRp3′s receptors. Furthermore, second-site compensatory mutations did not lessen or alleviate the fitness costs associated with resistance to CrRp10. In relation to phage therapy, El Haddad et al. found that 7 out of 12 clinical studies confirmed that resistance had emerged during phage treatment [74]. Although the consequences of phage resistance in the clinic are under-studied, they could be the root cause of phage therapy clinical trial failures, for example [75]. Thus, selecting therapeutic agents like phage CrRp10, which was resilient to resistance in vitro, may lead to improvements in phage therapy efficacy.
To the best of our knowledge, this is the first investigation of phage infection under a controlled low-oxygen atmosphere (<10 mmHg); herein described as “physiologic hypoxia”. Hypoxia is a common feature during inflammation associated with bacterial infection and the intestinal luminal environment [61,76]. In addition, host intestinal epithelial cells maintain physiologic hypoxia by counter-current blood flow [77]. Enteropathogenic A/E pathogens secrete the virulence protein (effector) NleB, which alters the function of a master regulator of cellular O2 homeostasis, HIF-1α, thereby increasing O2 levels between 2–5% for glycolysis [77]. We show that 5% oxygen caused a marked delay in the ability of CrRp3 to create a bacterial population decline compared to infections under normoxia. With the higher concentration of oxygen, the physiologic conditions of the host bacterium may have been at a more favorable metabolic state for a higher productive phage burst or faster lytic cycle completions [78,79]. By contrast, enteric pathogens adapt to oxygen limitations by entering into a metabolically reduced state [80], which was observed as a slightly slower growth rate (Figure 3). The reduced growth rate may have affected phage-bacteria interactions and/or reduced the expression of phage receptors. This contrasts with our knowledge that other phages do not replicate in slow-growing bacteria, for example [81,82]. Our results warrant further investigations into the effects of hypoxia on phage-bacteria interactions.
Another criterion for the selection of therapeutic phages is the spectrum of bacterial species or strains lysed. We found that CrRp3 and CrRp10 exhibit polyvalence in hosts across genera within the Enterobacteriaceae. We show that, while CrRp3 produced plaques on lawns of C. rodentium and the non-pathogenic E. coli strain K12, CrRp10 also produced plaques on eight other E. coli strains (pathogenic and non-pathogenic) and the plant pathogen E. carotovora. In contrast, most phages are confined to a single host species and often to a subset of strains [47,83]. For example, the C. rodentium phage phiCR1 was unable to produce plaques on E. coli [19]. Considering the well-documented, collateral effects of broad-spectrum antibiotics, which are notorious for secondary outcomes such as antibiotic-associated diarrhea [84], a narrow phage spectrum may be advantageous during therapy. However, species specificity comes with inherent constraints. By selecting a phage that is limited to a single species or limited number of strains, treatment is likely to be less effective against multispecies or polystrain infections [85].
The overuse and misuse of antibiotics in the treatment of diarrhea have led to an alarming increase of AMR in diarrheagenic bacteria [1,2,3]. Phage therapy has been effective at reducing E. coli burden in the murine gut with antibiotic pretreatment [5,6,7,8]. Although C. rodentium is widely used as an exemplary in vivo model system for gastrointestinal bacterial diseases [9,86], there are no reports using this species for phage therapy development. Prior to animal modeling, the careful selection of phages for therapeutic applications is especially important. Indeed, it is important to select phages that do not undergo lysogeny and do not carry toxin and antibiotic resistance genes. The selection of phage strains that are resilient to resistance has received little attention, which could be due to the unproven premise that phage cocktails will prevent resistance development [23,87]. Among 12 phage therapy human clinical studies that implemented phage cocktails, 7 cases confirmed the emergence of phage resistance during treatment [74]. For example, Acinetobacter baumannii developed resistance to all eight phages used to treat bacteremia after just 1 week of treatment [88]. Another criterion for phage selection that is lacking exploration is phage infection performance under human physiologic hypoxia conditions. Together, the features of CrRp10 suggest that it could be a promising therapeutic agent in mouse models of diarrheal diseases.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/12/7/737/s1, Figure S1: Amino acid sequence alignments to compare the compositions of Citrobacter phages (a) podoviruses and (b) myoviruses, Figure S2: One-step growth curves of phage (a) CrRp3 and (b) CrRp10, Table S1: Citrobacter phage CrRp3 annotations, Table S2: Citrobacter phage CrRp10 annotations, Table S3: Classification of the best hits of phages CrRp3 and CrRp10, Table S4: E. coli phage complete genomes used in genome-blast distance phylogeny analyses.
Author Contributions
Formal analysis, M.K. and L.D.; Investigation, C.M.M., T.L. and R.C.; Methodology, C.M.M. and D.R.R.; Supervision, D.R.R.; Writing—original draft, C.M.M.; Writing—review and editing, T.L., M.K., L.D. and D.R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by an EMBO fellowship (ALTF 1562-2015) and Marie Curie Actions award (LTFCOFUND2013, GA-2013-609409) to C.M.M and European Respiratory Society grant (RESPIRE2–2015–8416) to D.R.R.
Acknowledgments
The authors are grateful to Luisa De Sordi and Marta Mansos Lourenço for their advice, acknowledge contributions by Elena Rensen and grateful to the Ultrastructural BioImaging unit at Institut Pasteur for access to the TEM.
Conflicts of Interest
The authors declare that they have no competing financial interests.
References
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- Qu, M.; Lv, B.; Zhang, X.; Yan, H.; Huang, Y.; Qian, H.; Pang, B.; Jia, L.; Kan, B.; Wang, Q. Prevalence and antibiotic resistance of bacterial pathogens isolated from childhood diarrhea in Beijing, China (2010–2014). Gut Pathog. 2016, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Ran, L.; Wu, S.; Deng, X.; Ke, C.; Feng, Z.; Ma, L.; Varma, J.K. Survey of physician diagnostic and treatment practices for patients with acute ciarrhea in Guangdong province, China. Foodborne Pathog. Dis. 2012, 9, 47–53. [Google Scholar] [CrossRef]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef]
- Maura, D.; Galtier, M.; Le Bouguénec, C.; Debarbieux, L. Virulent bacteriophages can target O104:H4 enteroaggregative Escherichia coli in the mouse intestine. Antimicrob. Agents Chemother. 2012, 56, 6235–6242. [Google Scholar] [CrossRef] [PubMed]
- Galtier, M.; De Sordi, L.; Sivignon, A.; De Vallée, A.; Maura, D.; Christel, N.; Rahmouni, O.; Wannerberger, K.; Darfeuille-Michaud, A.; Desreumaux, P.; et al. Bacteriophages targeting adherent invasive Escherichia coli strains as a promising new treatment for Crohn’s disease. J. Crohn’s Colitis 2017, 11, 840–847. [Google Scholar] [CrossRef]
- Dissanayake, U.; Ukhanova, M.; Moye, Z.D.; Sulakvelidze, A.; Mai, V. Bacteriophages reduce pathogenic Escherichia coli counts in mice without distorting gut microbiota. Front. Microbiol. 2019, 10, 1984. [Google Scholar] [CrossRef]
- Cepko, L.C.S.; Garling, E.E.; Dinsdale, M.J.; Scott, W.P.; Bandy, L.; Nice, T.J.; Faber-Hammond, J.; Mellies, J.L. Myoviridae phage PDX kills enteroaggregative Escherichia coli without human microbiome dysbiosis. J. Med. Microbiol. 2020, 69, 309–323. [Google Scholar] [CrossRef]
- Crepin, V.F.; Collins, J.W.; Habibzay, M.; Frankel, G. Citrobacter rodentium mouse model of bacterial infection. Nat. Protoc. 2016, 11, 1851–1876. [Google Scholar] [CrossRef]
- Hu, J.; Torres, A.G. Enteropathogenic Escherichia coli: Foe or innocent bystander? Clin. Microbiol. Infect. 2015, 21, 729–734. [Google Scholar] [CrossRef]
- Bouladoux, N.; Harrison, O.; Belkaid, Y. The mouse model of infection with Citrobacter rodentium. Curr. Protoc. Immunol. 2017, 119, 19.15.1–19.15.25. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.R.; Debarbieux, L. Phage therapy: Awakening a sleeping giant. Emerg. Top. Life Sci. 2017, 1, 93–103. [Google Scholar] [CrossRef]
- Altamirano, F.L.G.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, S.; Hamilton, T.; Biswas, B.; Kumaraswamy, M.; Schooley, R.T.; Wooten, D. Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. Open Forum Infect. Dis. 2018, 5, ofy064. [Google Scholar] [CrossRef] [PubMed]
- Nir-Paz, R.; Gelman, D.; Khouri, A.; Sisson, B.M.; Fackler, J.; Alkalay-Oren, S.; Khalifa, L.; Rimon, A.; Yerushalmy, O.; Bader, R.; et al. Successful treatment of antibiotic-resistant, poly-microbial bone infection with bacteriophages and antibiotics combination. Clin. Infect. Dis. 2019, 69, 2015–2018. [Google Scholar] [CrossRef]
- Chan, B.K.; Sistrom, M.; Wertz, J.E.; Kortright, K.E.; Narayan, D.; Turner, P.E. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 26717. [Google Scholar] [CrossRef]
- Segall, A.M.; Roach, D.R.; Strathdee, S.A. Stronger together? Perspectives on phage-antibiotic synergy in clinical applications of phage therapy. Curr. Opin. Microbiol. 2019, 51, 46–50. [Google Scholar] [CrossRef]
- Petty, N.K.; Toribio, A.; Goulding, D.; Foulds, I.; Thomson, N.R.; Dougan, G.; Salmond, G.P.C. A generalized transducing phage for the murine pathogen Citrobacter rodentium. Microbiology 2007, 153, 2984–2988. [Google Scholar] [CrossRef]
- Sváb, D.; Horváth, B.; Rohde, M.; Maróti, G.; Tóth, I. R18C is a new viable P2-like bacteriophage of rabbit origin infecting Citrobacter rodentium and Shigella sonnei strains. Arch. Virol. 2019, 164, 3157–3160. [Google Scholar] [CrossRef]
- Toribio, A.; Pickard, D.; Cerdeño-Tárraga, A.M.; Petty, N.K.; Thomson, N.R.; Salmond, G.; Dougan, G. Complete genome sequences of two Citrobacter rodentium bacteriophages, CR8 and CR44b. Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Wiles, S.; Clare, S.; Harker, J.A.; Huett, A.; Young, D.; Dougan, G.; Frankel, G. Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cell Microbiol. 2004, 6, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Forti, F.; Roach, D.R.; Cafora, M.; Pasini, M.E.; Horner, D.S.; Fiscarelli, E.V.; Rossitto, M.; Cariani, L.; Briani, F.; Debarbieux, L.; et al. Design of a broad-range bacteriophage cocktail that reduces Pseudomonas aeruginosa biofilms and treats acute infections in two animal models. Antimicrob. Agents Chemother. 2018, 62, e02573-17. [Google Scholar] [CrossRef] [PubMed]
- Pickard, D.J.J. Preparation of bacteriophage lysates and pure DNA. Methods Mol. Biol. 2009, 502, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A Program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; Chen, G.; Locascio, P.F.; Land, M.; Larimer, F.W.; Hauser, L. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Soeding, J.; Biegert, A.; Lupas, A.N. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005, 33, W244–W248. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Haft, D.H. TIGRFAMs: A protein family resource for the functional identification of proteins. Nucleic Acids Res. 2001, 29, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2016, 45, D566–D573. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Feldgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, G.H.; Zhao, S.; Hsu, C.-H.; McDermott, P.F.; et al. Validating the AMRFinder tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob. Agents Chemother. 2019, 63, e00483-19. [Google Scholar] [CrossRef]
- Gupta, S.K.; Padmanabhan, B.R.; Diene, S.M.; Lopez-Rojas, R.; Kempf, M.; Landraud, L.; Rolain, J.-M. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob. Agents Chemother. 2013, 58, 212–220. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis-10 years on. Nucleic Acids Res. 2015, 44, D694–D697. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. VICTOR: Genome-based phylogeny and classification of prokaryotic viruses. Bioinformatics 2017, 33, 3396–3404. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A comprehensive, accurate, and fast distance-based phylogeny inference program. Mol. Boil. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef]
- Kropinski, A.M. Measurement of the rate of attachment of bacteriophage to cells. Methods Mol. Biol. 2009, 501, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Kropinski, A.M. Practical advice on the one-step growth curve. Adv. Struct. Saf. Stud. 2017, 1681, 41–47. [Google Scholar] [CrossRef]
- Basset, A.; Khush, R.S.; Braun, A.; Gardan, L.; Boccard, F.; Hoffmann, J.A.; Lemaitre, B. The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc. Natl. Acad. Sci. USA 2000, 97, 3376–3381. [Google Scholar] [CrossRef] [PubMed]
- Le Flèche-Matéos, A.; Levast, M.; Lomprez, F.; Arnoux, Y.; Andonian, C.; Perraud, M.; Vincent, V.; Gouilh, M.A.; Thiberge, J.-M.; Vandenbogaert, M.; et al. Rouxiella chamberiensis gen nov., sp. nov., a member of the family Enterobacteriaceae isolated from parenteral nutrition bags. Int. J. Syst. Evol. Microbiol. 2015, 65, 1812–1818. [Google Scholar] [CrossRef]
- Flyg, C.; Kenne, K.; Boman, H.G. Insect pathogenic properties of Serratia marcescens: Phage-resistant mutants with a decreased resistance to Cecropia immunity and a decreased virulence to Drosophila. Microbiology 1980, 120, 173–181. [Google Scholar] [CrossRef]
- Sapriel, G.; Wandersman, C.; Delepelaire, P. The SecB chaperone is bifunctional in Serratia marcescens: SecB is involved in the Sec pathway and required for HasA secretion by the ABC transporter. J. Bacteriol. 2003, 185, 80–88. [Google Scholar] [CrossRef]
- De Sordi, L.; Khanna, V.; Debarbieux, L. The gut microbiota facilitates drifts in the genetic diversity and infectivity of bacterial viruses. Cell Host Microbe 2017, 22, 801–808. [Google Scholar] [CrossRef]
- Dufour, N.; Clermont, O.; La Combe, B.; Messika, J.; Dion, S.; Khanna, V.; Denamur, E.; Ricard, J.-D.; Debarbieux, L.; on behalf of the ColoColi group. Bacteriophage LM33_P1, a fast-acting weapon against the pandemic ST131-O25b:H4 Escherichia coli clonal complex. J. Antimicrob. Chemother. 2016, 71, 3072–3080. [Google Scholar] [CrossRef]
- Galtier, M.; De Sordi, L.; Maura, D.; Arachchi, H.; Volant, S.; Dillies, M.-A.; Debarbieux, L. Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environ. Microbiol. 2016, 18, 2237–2245. [Google Scholar] [CrossRef]
- Maura, D.; Morello, E.; Du Merle, L.; Bomme, P.; Le Bouguénec, C.; Debarbieux, L. Intestinal colonization by enteroaggregative Escherichia coli supports long-term bacteriophage replication in mice. Environ. Microbiol. 2011, 14, 1844–1854. [Google Scholar] [CrossRef]
- Dufour, N.; Debarbieux, L.; Fromentin, M.; Ricard, J.-D. Treatment of highly virulent extraintestinal pathogenic Escherichia coli pneumonia with bacteriophages. Crit. Care Med. 2015, 43, e190–e198. [Google Scholar] [CrossRef]
- Tikhe, C.; Martin, T.M.; Gissendanner, C.R.; Husseneder, C. Complete genome sequence of Citrobacter phage CVT22 isolated from the gut of the formosan subterranean termite, Coptotermes formosanus Shiraki. Genome Announc. 2015, 3. [Google Scholar] [CrossRef]
- Lesage, K.C.; Hargrove, E.C.; Cahill, J.; Rasche, E.S.; Everett, G.F.K. Complete genome sequence of Citrobacter freundii myophage Merlin. Genome Announc. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Luna, A.J.; Hernandez, A.C.; Everett, G.F.K. Complete genome sequence of Citrobacter freundii myophage Miller. Genome Announc. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.B.; Luna, A.J.; Hernández, A.C.; Everett, G.F.K. Complete genome sequence of Citrobacter freundii myophage Moon. Genome Announc. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.L.; Berkowitz, V.E.; Cahill, J.; Rasche, E.S.; Everett, G.F.K. Complete genome sequence of Citrobacter freundii myophage Michonne. Genome Announc. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Luna, A.J.; Hernández, A.C.; Everett, G.F.K. Complete genome sequence of Citrobacter freundii myophage Moogle. Genome Announc. 2015, 3. [Google Scholar] [CrossRef]
- Shaw, J.; Medina, C.A.A.; Chen, Y.; Luna, A.J.; Hernández, A.C.; Everett, G.F.K. Complete genome of Citrobacter freundii siphophage Stevie. Genome Announc. 2015, 3. [Google Scholar] [CrossRef]
- Adriaenssens, E.M.; Sullivan, M.B.; Knezevic, P.; Van Zyl, L.J.; Sarkar, B.L.; Dutilh, B.E.; Alfenas-Zerbini, P.; Lobocka, M.; Tong, Y.; Brister, J.R.; et al. Taxonomy of prokaryotic viruses: 2018–2019 update from the ICTV Bacterial and Archaeal Viruses Subcommittee. Arch. Virol. 2020, 165, 1–8. [Google Scholar] [CrossRef]
- Li, S.; Fan, H.; An, X.; Fan, H.; Jiang, H.; Chen, Y.; Tong, Y. Scrutinizing virus genome termini by high-throughput sequencing. PLoS ONE 2014, 9, e85806. [Google Scholar] [CrossRef]
- Rivas, L.; Coffey, B.; McAuliffe, O.; McDonnell, M.J.; Burgess, C.M.; Coffey, A.; Ross, R.P.; Duffy, G. In vivo and ex vivo evaluations of bacteriophages e11/2 and e4/1c for use in the control of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2010, 76, 7210–7216. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.; Esipova, T.V.; Judge, C.P.; Bittinger, K.; Chen, J.; Laughlin, A.; Grunberg, S.; Baldassano, R.N.; Lewis, J.D.; Li, H.; et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 2014, 147, 1055–1063. [Google Scholar] [CrossRef]
- Enault, F.; Briet, A.; Bouteille, L.; Roux, S.; Sullivan, M.B.; Petit, M.-A. Phages rarely encode antibiotic resistance genes: A cautionary tale for virome analyses. ISME J. 2017, 11, 237–247. [Google Scholar] [CrossRef]
- Guan, J.; Snowden, J.D.; Cahill, J.; Rasche, E.S.; Everett, G.F.K. Complete genome sequence of Citrobacter freundii myophage Mordin. Genome Announc. 2015, 3. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Zhao, J.; He, X.; Li, E.; Li, H.; Liu, W.; Zou, D.; Wei, X.; Wang, X.; et al. Characterization of phiCFP-1, a virulent bacteriophage specific for Citrobacter freundii. J. Med. Virol. 2015, 88, 895–905. [Google Scholar] [CrossRef]
- Hamdi, S.; Rousseau, G.M.; Labrie, S.J.; Kourda, R.S.; Tremblay, D.M.; Moineau, S.; Slama, K.B. characterization of five Podoviridae phages infecting Citrobacter freundii. Front. Microbiol. 2016, 7, 1023. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Hook, E.W.; Smith, A.A.; Plorde, J.J. Citrobacter infections in humans: Experience at the Seattle Veterans Administration Medical Center and a review of the literature. Rev. Infect. Dis. 1980, 2, 746–760. [Google Scholar] [CrossRef]
- Schauer, D.B.; Zabel, B.A.; Pedraza, I.F.; O’Hara, C.M.; Steigerwalt, A.G.; Brenner, D.J. Genetic and biochemical characterization of Citrobacter rodentium sp. nov. J. Clin. Microbiol. 1995, 33, 2064–2068. [Google Scholar] [CrossRef]
- Petty, N.K.; Bulgin, R.; Crepin, V.F.; Cerdeño-Tárraga, A.M.; Schroeder, G.; Quail, M.A.; Lennard, N.; Corton, C.; Barron, A.; Clark, L.; et al. The Citrobacter rodentium genome sequence reveals convergent evolution with human pathogenic Escherichia coli. J. Bacteriol. 2009, 192, 525–538. [Google Scholar] [CrossRef]
- Petty, N.K.; Feltwell, T.; Pickard, D.; Clare, S.; Toribio, A.; Fookes, M.; Roberts, K.; Monson, R.; Nair, S.; Kingsley, R.A.; et al. Citrobacter rodentium is an unstable pathogen showing evidence of significant genomic flux. PLoS Pathog. 2011, 7, e1002018. [Google Scholar] [CrossRef] [PubMed]
- Scholl, D.; Adhya, S.; Merril, C.R. Bacteriophage SP6 is closely related to phages K1-5, K5, and K1E but encodes a tail protein very similar to that of the distantly related P22. J. Bacteriol. 2002, 184, 2833–2836. [Google Scholar] [CrossRef] [PubMed]
- Scholl, D.; Kieleczawa, J.; Kemp, P.; Rush, J.; Richardson, C.; Merril, C.; Adhya, S.; Molineux, I. Genomic analysis of bacteriophages SP6 and K1-5, an estranged subgroup of the T7 supergroup. J. Mol. Boil. 2004, 335, 1151–1171. [Google Scholar] [CrossRef] [PubMed]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Genet. 2010, 8, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.C.T.; Friman, V.-P.; Smith, M.C.M.; Brockhurst, M.A. Resistance evolution against phage combinations depends on the timing and order of exposure. MBio 2019, 10, e01652-19. [Google Scholar] [CrossRef] [PubMed]
- El Haddad, L.; Harb, C.P.; Gebara, M.A.; Stibich, M.A.; Chemaly, R. A systematic and critical review of bacteriophage therapy against multidrug-resistant ESKAPE organisms in humans. Clin. Infect. Dis. 2019, 69, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Hawkins, C.; Änggård, E.; Harper, D. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. J. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Xu, C.; Liu, X.; Zha, H.; Fan, S.; Zhang, D.; Li, S.; Xiao, W. A pathogen-derived effector modulates host glucose metabolism by arginine GlcNAcylation of HIF-1α protein. PLoS Pathog. 2018, 14, e1007259. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, M.; De Sordi, L.; Debarbieux, L. The diversity of bacterial lifestyles hampers bacteriophage tenacity. Viruses 2018, 10, 327. [Google Scholar] [CrossRef]
- You, L.; Suthers, P.F.; Yin, J. Effects of Escherichia coli physiology on growth of phage T7 in vivo and in silico. J. Bacteriol. 2002, 184, 1888–1894. [Google Scholar] [CrossRef]
- McDaniel, L.E.; Bailey, E.G.; Zimmerli, A. Effect of oxygen-supply rates on growth of Escherichia coli. Ii. Comparison of results in shake flasks and 50-liter fermenter. Appl. Microbiol. 1965, 13, 115–119. [Google Scholar] [CrossRef]
- Swift, B.M.C.; Gerrard, Z.E.; Huxley, J.; Rees, C. Factors affecting phage D29 infection: A tool to investigate different growth states of mycobacteria. PLoS ONE 2014, 9, e106690. [Google Scholar] [CrossRef] [PubMed]
- Hadas, H.; Einav, M.; Fishov, I.; Zaritsky, A. Bacteriophage T4 development depends on the physiology of its host Escherichia coli. Microbiology 1997, 143, 179–185. [Google Scholar] [CrossRef]
- Hyman, P.; Abedon, S.T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 2010, 70, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Canani, R.B.; Guarino, A.; Hojsak, I.; Indrio, F.; Kolaček, S.; Orel, R.; Shamir, R.; Vandenplas, Y.; Van Goudoever, J.B.; et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 495–506. [Google Scholar] [CrossRef]
- Servick, K. Beleaguered phage therapy trial presses on. Science 2016, 352, 1506. [Google Scholar] [CrossRef]
- Croxen, M.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Abedon, S.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).