The Effect of Bovine Viral Diarrhea Virus (BVDV) Strains and the Corresponding Infected-Macrophages’ Supernatant on Macrophage Inflammatory Function and Lymphocyte Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
2.2. Isolation of Monocytes and Differentiation to Monocyte Derived Macrophage (MDM)
2.3. Production and Inactivation of Infected MDM Supernatant
2.4. Phagocytosis
2.5. Bactericidal Activity
2.6. Nitric Oxide Production
2.7. Immunostaining and Flow Cytometry of CD14 and MHC II
2.8. Examining the Indirect or Direct Effect of BVDV Strains on Peripheral Blood Lymphocyte Population and MDBK
2.9. Chromatin Condensation
2.10. Annexin V Staining
2.11. Protein Electrophoresis
2.12. Quantification of Apoptosis-Related Cytokines by qRT-PCR of BVDV-Infected MDMs
2.13. Supernatant Neutralization
2.14. Statistical Analysis
3. Results
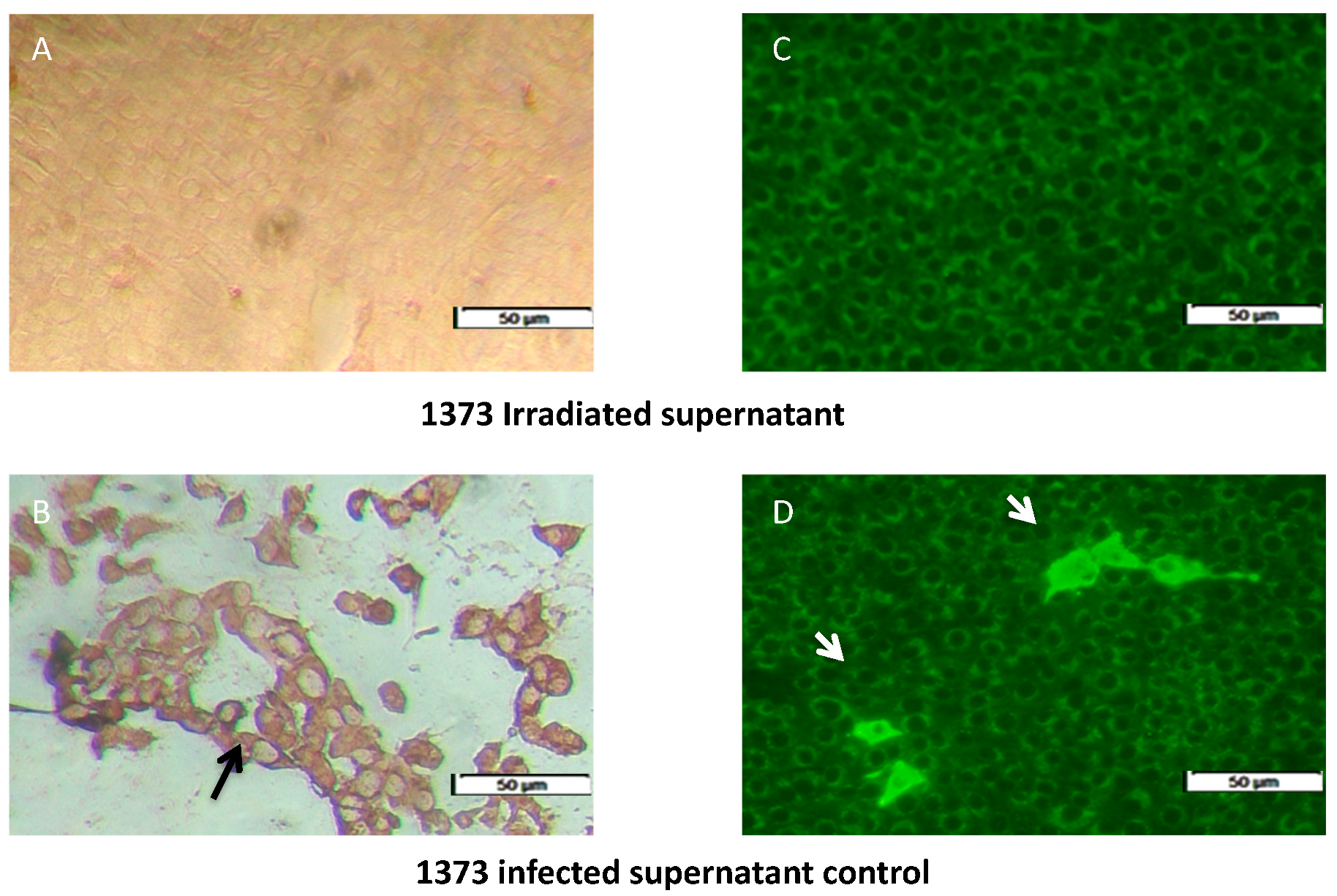
3.1. Inactivation of Infected Supernatant
3.2. Effect of Supernatant from BVDV Infected MDM on Macrophage Phagocytic Activity Compared to the Direct BVDV Effect
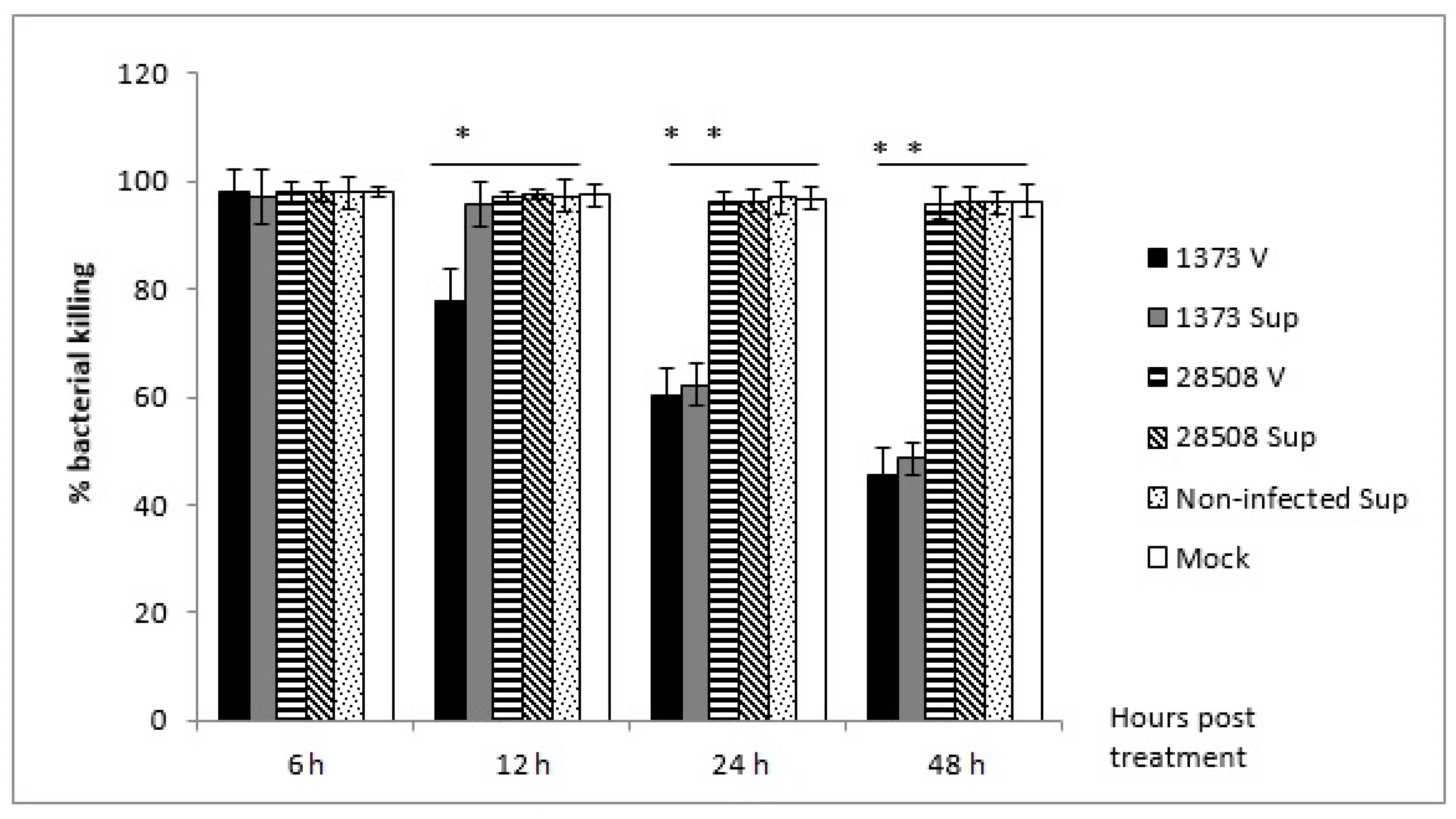
3.3. Effect of Supernatant from BVDV-Infected MDM on Macrophage Bactericidal Activity Compared to the Direct BVDV Effect
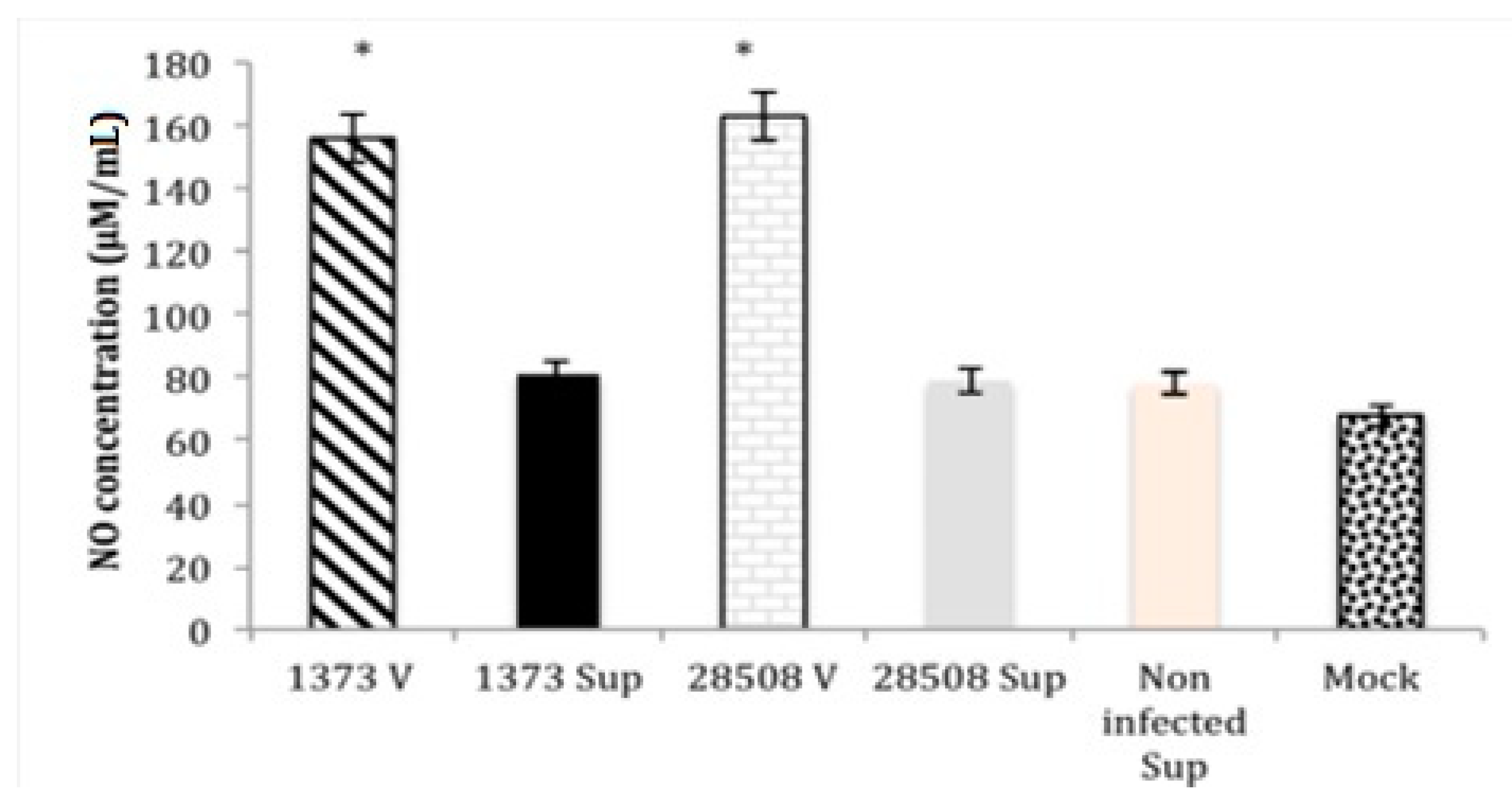
3.4. Effect of Supernatant from BVDV Infected MDM on Macrophage Nitric Oxide Production Compared to the Direct BVDV Effect
3.5. Effect of Supernatant on MHC II Expression Compared to the Direct BVDV Effect
3.6. Effect of Supernatant on CD14 Expression Compared to the Direct BVDV Effect
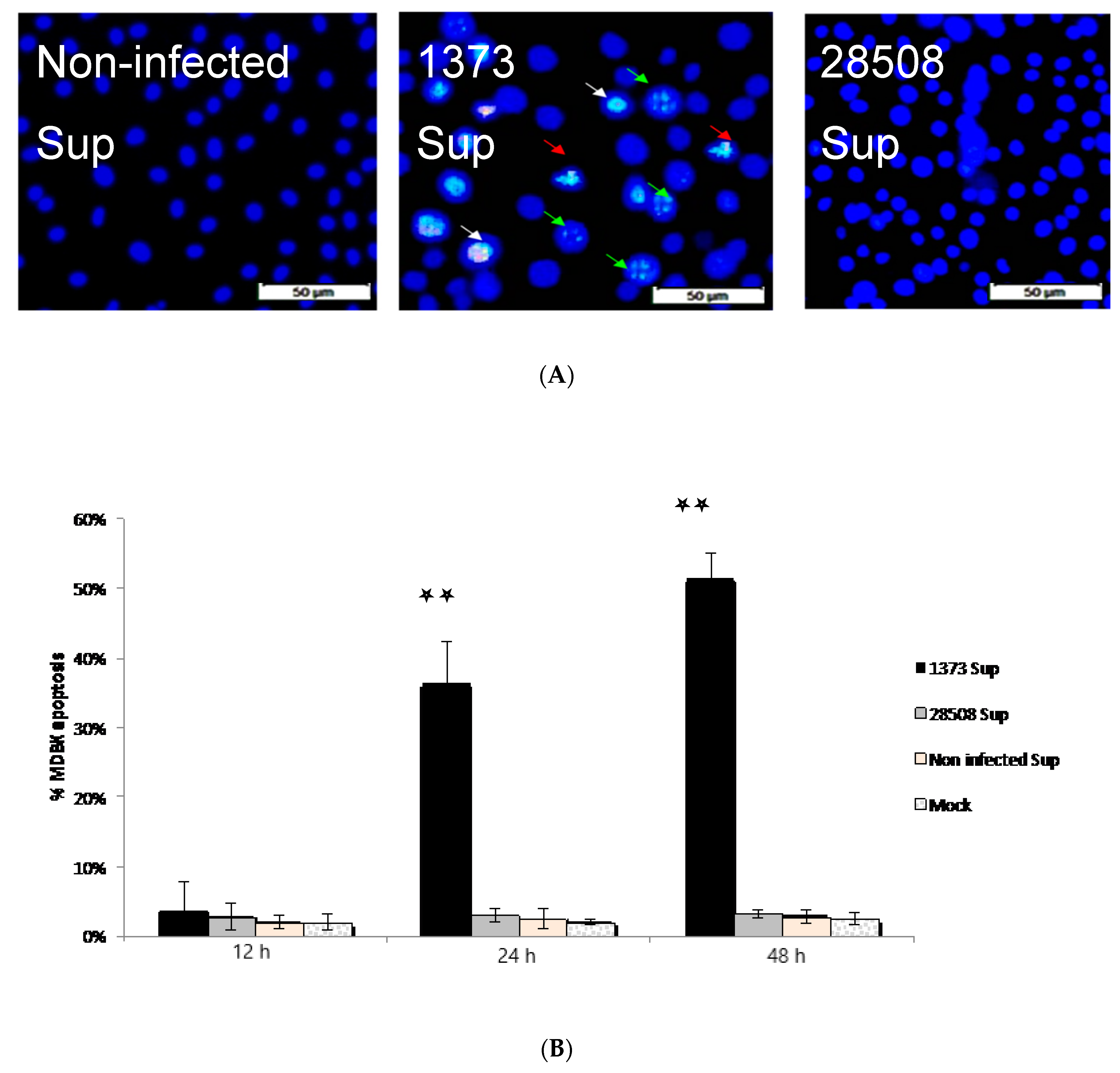
3.7. Effect of Supernatant from BVDV Infected Macrophages on MDBK Cells
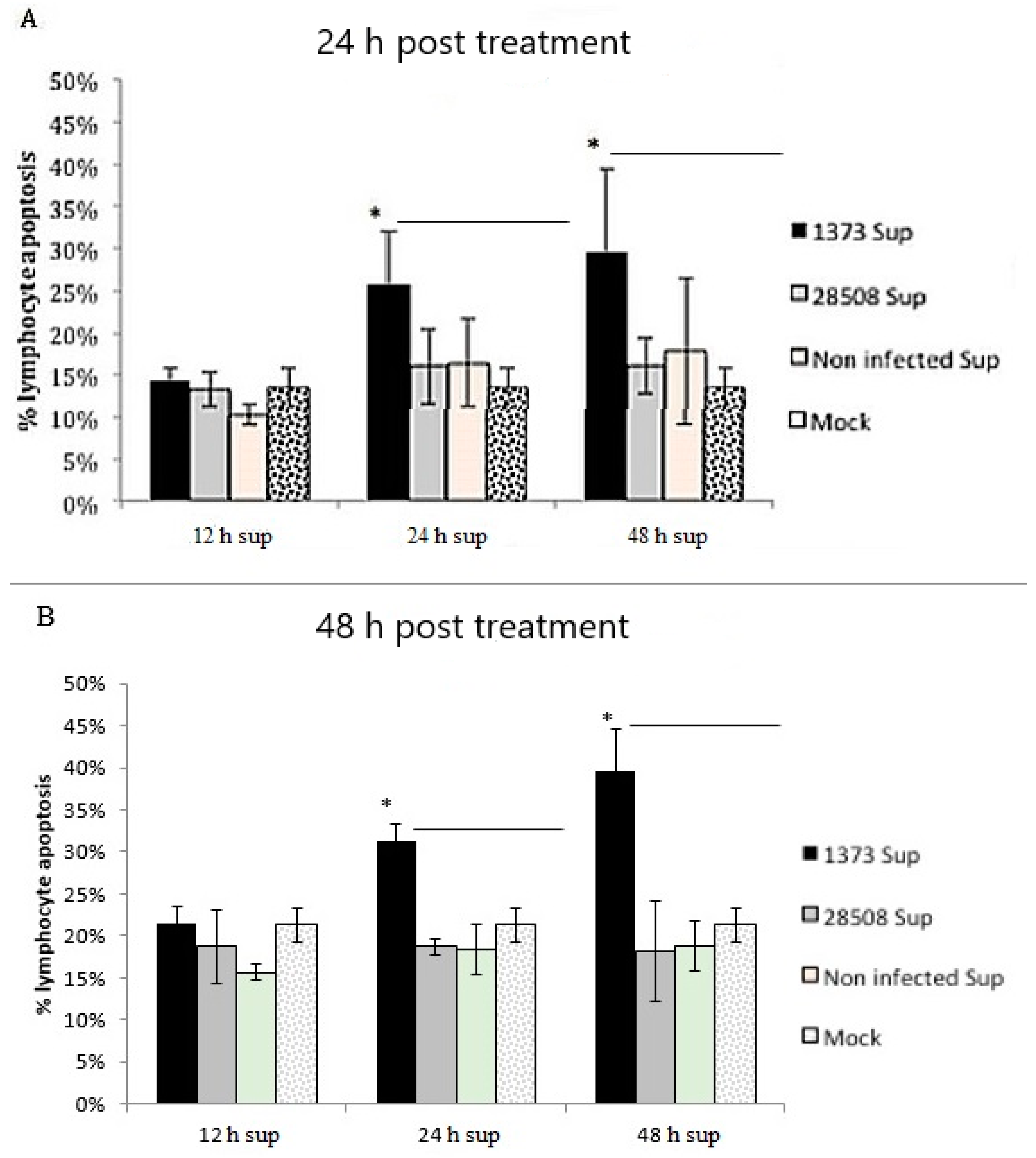
3.8. Effect of Supernatant from BVDV-Infected Macrophages on Different Lymphocyte Populations
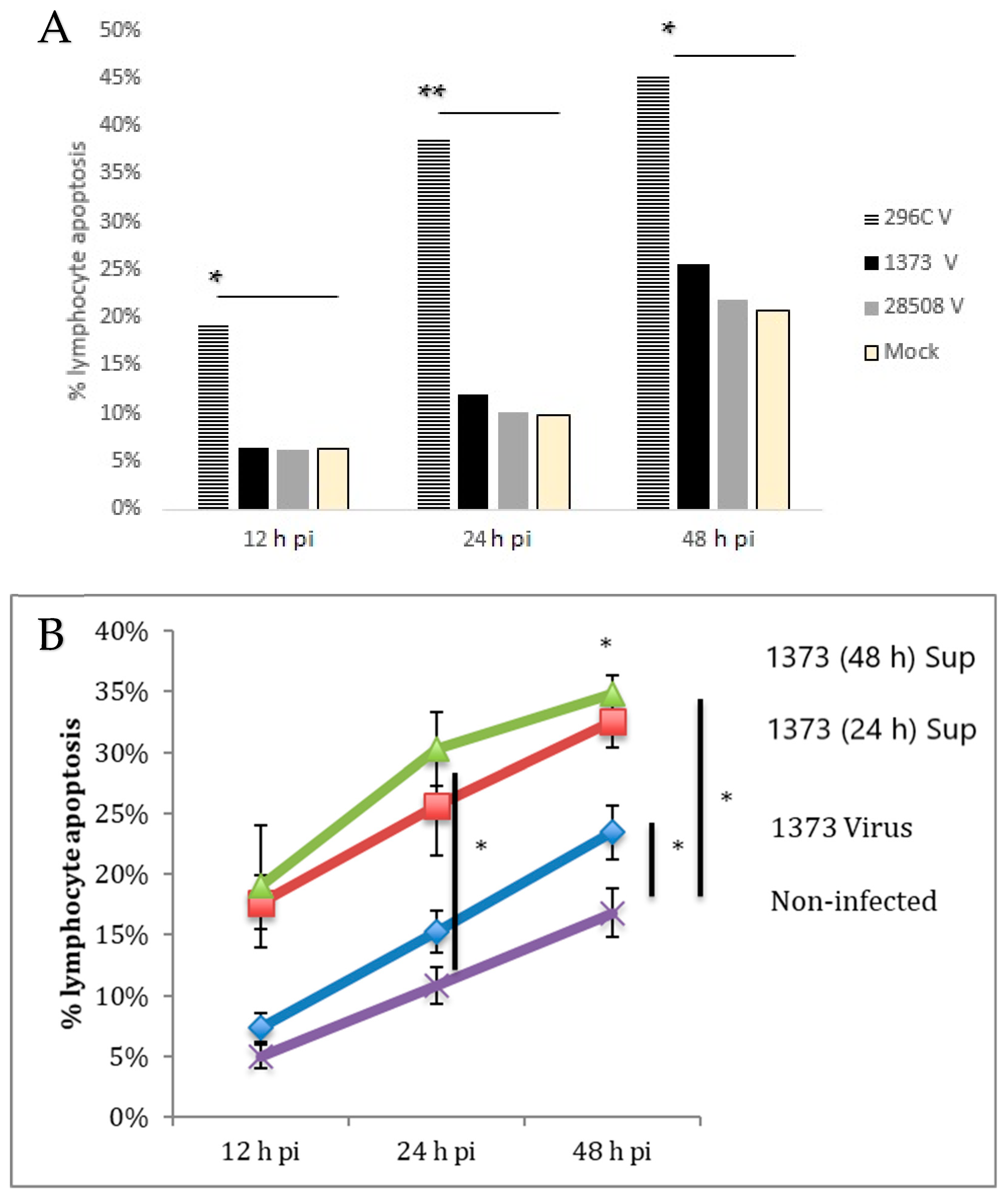
3.9. Effect of Direct Infection of Lymphocyte with BVDV Strains
3.10. Supernatant Protein Analysis
3.11. Role of Cytokines in the Indirect Lymphocyte Apoptosis
3.12. Role of Viral Factors in the Indirect Lymphocyte Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ammari, M.; McCarthy, F.; Nanduri, B.; Pinchuk, G.; Pinchuk, L. Understanding the Pathogenesis of Cytopathic and Noncytopathic Bovine Viral Diarrhea Virus Infection Using Proteomics. In Proteomic Applications in Biology; IntechOpen: London, UK, 2012. [Google Scholar]
- Bittar, J.H.; Palomares, R.A.; Hurley, D.J.; Hoyos-Jaramillo, A.; Rodriguez, A.; Stoskute, A.; Hamrick, B.; Norton, N.; Adkins, M.; Saliki, J.T.; et al. Immune response and onset of protection from Bovine viral diarrhea virus 2 infection induced by modified-live virus vaccination concurrent with injectable trace minerals administration in newly received beef calves. Vet. Immunol. Immunopathol. 2020, 225, 110055. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, S.M.; Johnson, C.; Bauermann, F.V.; McGill, J.; Palmer, M.V.; Sacco, R.E.; Ridpath, J.F. Changes observed in the thymus and lymph nodes 14 days after exposure to BVDV field strains of enhanced or typical virulence in neonatal calves. Vet. Immunol. Immunopathol. 2014, 160, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Liebler-Tenorio, E.M.; Ridpath, J.F.; Neill, J.D. Distribution of viral antigen and development of lesions after experimental infection of calves with a BVDV 2 strain of low virulence. J. Vet. Diagn. Investig. 2003, 15, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, L.N. Immunology of bovine viral diarrhea virus. Vet. Clin. N. Am. Food Anim. Pract. 1995, 11, 501–520. [Google Scholar] [CrossRef]
- Sopp, P.; Hooper, L.B.; Clarke, M.C.; Howard, C.J.; Brownlie, J. Detection of bovine viral diarrhoea virus p80 protein in subpopulations of bovine leukocytes. J. Gen. Virol. 1994, 75, 1189–1194. [Google Scholar] [CrossRef]
- Roth, J.A.; Kaeberle, M.L. Isolation of neutrophils and eosinophils from the peripheral blood of cattle and comparison of their functional activities. J. Immunol. Methods 1981, 45, 153–164. [Google Scholar] [CrossRef]
- Welsh, M.D.; Adair, B.M.; Foster, J.C. Effect of BVD virus infection on alveolar macrophage functions. Vet. Immunol. Immunopathol. 1995, 46, 195–210. [Google Scholar] [CrossRef]
- Lambot, M.; Hanon, E.; Lecomte, C.; Hamers, C.; Letesson, J.-J.; Pastoret, P.-P. Bovine viral diarrhoea virus induces apoptosis in blood mononuclear cells by a mechanism largely dependent on monocytes. J. Gen. Virol. 1998, 79, 1745–1749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, G.; Aldridge, S.; Clarke, M.C.; McCauley, J.W. Cell death induced by cytopathic bovine viral diarrhoea virus is mediated by apoptosis. J. Gen. Virol. 1996, 77, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Risalde, M.A.; Romero-Palomo, F.; Lecchi, C.; Ceciliani, F.; Bazzocchi, C.; Comazzi, S.; Besozzi, M.; Gómez-Villamandos, J.C.; Luzzago, C. BVDV permissiveness and lack of expression of co-stimulatory molecules on PBMCs from calves pre-infected with BVDV. Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101388. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.C.; Thakur, N.; Darweesh, M.F.; Morarie-Kane, S.E.; Rajput, M.K. Immune response to bovine viral diarrhea virus—looking at newly defined targets. Anim. Health Res. Rev. 2015, 16, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Pedrera, M.; Gomez-Villamandos, J.C.; Romero-Trevejo, J.L.; Risalde, M.A.; Molina, V.; Sanchez-Cordon, P.J. Apoptosis in lymphoid tissues of calves inoculated with non-cytopathic bovine viral diarrhea virus genotype 1: Activation of effector caspase-3 and role of macrophages. J. Gen. Virol. 2009, 90, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- Romero--Palomo, F.; Risalde, M.A.; Gómez--Villamandos, J.C. Immunopathologic Changes in the Thymus of Calves Pre--infected with BVDV and Challenged with BHV--1. Transbound. Emerg. Dis. 2017, 64, 574–584. [Google Scholar] [CrossRef]
- Roth, J.A.; Bolin, S.R.; Frank, D.E. Lymphocyte blastogenesis and neutrophil function in cattle persistently infected with bovine viral diarrhea virus. Am. J. Vet. Res. 1986, 47, 1139–1141. [Google Scholar]
- Ridpath, J.F.; Bendfeldt, S.; Neill, J.D.; Liebler-Tenorio, E. Lymphocytopathogenic activity in vitro correlates with high virulence in vivo for BVDV type 2 strains: Criteria for a third biotype of BVDV. Virus Res. 2006, 118, 62–69. [Google Scholar] [CrossRef]
- Adler, B.; Adler, H.; Pfister, H.; Jungi, T.W.; Peterhans, E. Macrophages infected with cytopathic bovine viral diarrhoea virus release a factor(s) capable of priming uninfected macrophages for activation-induced apoptosis. J. Virol. 1997, 71, 3255–3258. [Google Scholar] [CrossRef]
- Schweizer, M.; Peterhans, E. Oxidative stress in cells infected with bovine viral diarrhoea virus: A crucial step in the induction of apoptosis. J. Gen. Virol. 1999, 80, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Bruschke, C.J.M.; Hulst, M.M.; Moormann, R.J.M.; Rijn, P.A.V.; Oirschot, J.T.V. Glycoprotein Erns of Pestiviruses Induces Apoptosis in Lymphocytes of Several Species. J. Virol. 1997, 71, 6692–6696. [Google Scholar] [CrossRef] [PubMed]
- Fetzer, C.; Tews, B.A.; Meyers, G. The carboxy-terminal sequence of the pestivirus glycoprotein Erns represents an unusual type of membrane anchor. J. Virol. 2005, 79, 11901–11913. [Google Scholar] [CrossRef]
- Summerfield, A.; Zingle, K.; Inumaru, S.; McCullough, C. Induction of apoptosis in bone marrow neutrophil-lineage by classical swine fever. J. Gen. Virol. 2001, 82, 1309–1318. [Google Scholar] [CrossRef]
- Hoff, H.S.; Donis, R.O. Induction of apoptosis and cleavage of poly (ADP-ribose) polymerase by cytopathic bovine viral diarrhea virus infection. Virus Res. 1997, 49, 101–113. [Google Scholar] [CrossRef]
- Schweizer, M.; Peterhans, E. Noncytopathic bovine viral diarrhea virus inhibits double-stranded RNA-induced apoptosis and interferon synthesis. J. Virol. 2001, 75, 4692–4698. [Google Scholar] [CrossRef] [PubMed]
- Mangan, D.F.; Robertson, B.; Wahl, S.M. IL-4 enhances programmed cell death (apoptosis) in stimulated human monocytes. J. Immunol. 1992, 148, 1812–1816. [Google Scholar] [PubMed]
- Adler, H.; Jungi, T.W.; Pfister, H.; Strasser, M.; Sileghem, M.; Peterhans, E. Cytokine regulation by virus infection: Bovine viral diarrhea virus, a flavivirus, downregulates production of tumor necrosis factor alpha in macrophages in vitro. J. Virol. 1996, 70, 2650–2653. [Google Scholar] [CrossRef]
- Choi, K.S. The effect of bovine viral diarrhea virus on bovine monocyte phenotype. Iran. J. Vet. Res. 2017, 18, 13. [Google Scholar]
- Lopez, B.I.; Santiago, K.G.; Lee, D.; Ha, S.; Seo, K. RNA Sequencing (RNA-Seq) Based Transcriptome Analysis in Immune Response of Holstein Cattle to Killed Vaccine against Bovine Viral Diarrhea Virus Type, I. Animals 2020, 10, 344. [Google Scholar] [CrossRef]
- Aldo, M.A.; Yahel, L.R.; Montserrat, R.H.; Elena, S.S.; Lourdes, A.P.; Alejandro, B.G. The NADL strain of bovine viral diarrhea virus induces the secretion of IL-1β through caspase 1 in bovine macrophages. Res. Vet. Sci. 2020. [Google Scholar] [CrossRef]
- Elmowalid, G. Unmasking the Effect of Bovine Viral Diarrhea Virus on Macrophage Inflammatory Functions. Ph.D. Thesis, South Dakota State University, Brookings, SD, USA, 2003. [Google Scholar]
- Schaut, R.G.; Ridpath, J.F.; Sacco, R.E. Bovine Viral Diarrhea Virus Type 2 Impairs Macrophage Responsiveness to Toll-Like Receptor Ligation with the Exception of Toll-Like Receptor 7. PLoS ONE 2016, 11, e0159491. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Mwangi, W.; Brown, W.C.; Splitter, G.A.; Zhuang, Y.; Kegerreis, K.; Palmer, G.H. Enhancement of antigen acquisition by dendritic cells and MHC class II-restricted epitope presentation to CD4+ T cells using VP22 DNA vaccine vectors that promote intercellular spreading. J. Leukoc. Biol. 2005, 78, 401–411. [Google Scholar] [CrossRef]
- Rajput, M.K.; Darweesh, M.F.; Park, K.; Braun, L.J.; Mwangi, W.; Young, A.J.; Chase, C.C. The effect of bovine viral diarrhea virus (BVDV) strains on bovine monocyte-derived dendritic cells (Mo-DC) phenotype and capacity to produce BVDV. Virol. J. 2014, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001, 111, A3-B1–A3-B3. [Google Scholar]
- Perler, L.; Schweizer, M.; Jungi, T.W.; Peterhans, E. Bovine viral diarrhoea virus and bovine herpesvirus-1 prime uninfected macrophages forlipopolysaccharide-triggered apoptosis by interferon-dependent and-independent pathways. J. Gen. Virol. 2000, 81, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Hwang, K.K.; Chae, C. Classical swine fever virus induces tumor necrosis factor-alpha and lymphocyte apoptosis. Arch. Virol. 2004, 149, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F.; Driskell, E.; Chase, C.C.; Neill, J.D. Characterization and detection of BVDV related reproductive disease in white tail deer. In Proceedings of the 13th International World Association of Veterinary Laboratory Diagnosticians Symposium, Melbourne, Australia, 11–14 November 2007; p. 34. [Google Scholar]
- Gels, T.; Schagger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar]
- Konnai, S.; Usui, T.; Ohashi, K.; Onuma, M. The rapid quantitative analysis of bovine cytokine genes by real-time RT-PCR. Vet. Microbiol. 2003, 94, 283–294. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Hou, L.; Wilkerson, M.; Kapil, S.; Mosie, R.D.; Shuman, W.; Reddy, J.R.; Loughin, T.; Minocha, H.C. The effect of different bovine viral diarrhea virus genotypes and biotypes on the metabolic activity and activation status of bovine peripheral blood mononuclear cells. Viral Immunol. 1998, 11, 233–244. [Google Scholar] [CrossRef]
- Ketelsen, A.T.; Johnson, D.W.; Muscoplat, C.C. Depression of bovine monocyte chemotactic responses by bovine viral diarrhea virus. Infect. Immun. 1979, 25, 565–568. [Google Scholar] [CrossRef]
- Potgieter, L.N.; McCracken, M.D.; Hopkins, F.M.; Walker, R.D.; Guy, J.S. Experimental production of bovine respiratory tract disease with bovine viral diarrhea virus. Am. J. Vet. Res. 1984, 45, 1582–1585. [Google Scholar]
- Blond, D.; Raoul, H.; Le Grand, R.; Dormont, D. Nitric oxide synthesis enhances human immunodeficiency virus replication in primary human macrophages. J. Virol. 2000, 74, 8904–8912. [Google Scholar] [CrossRef]
- Lasarte, J.J.; Sarobe, P.; Boya, P.; Casares, N.; Arribillaga, L.; de Cerio, A.L.D.; Gorraiz, M.; Borrás-Cuesta, F.; Prieto, J. A recombinant adenovirus encoding hepatitis C virus core and E1 proteins protects mice against cytokine--induced liver damage. Hepatology 2003, 37, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Charleston, B.; Hope, J.C.; Carr, B.V.; Howard, C.J. Masking of two in vitro immunological assays for Mycobacterium bovis (BCG) in calves acutely infected with non-cytopathic bovine viral diarrhoea virus. Vet. Rec. 2001, 149, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Archambault, D.; Béliveau, C.; Couture, Y.; Carman, S. Clinical response and immunomodulation following experimental challenge of calves with type 2 noncytopathogenic bovine viral diarrhea virus. Vet. Res. 2000, 31, 215–227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.R.; Nanduri, B.; Pharr, G.T.; Stokes, J.V.; Pinchuk, L.M. Bovine viral diarrhea virus infection affects the expression of proteins related to professional antigen presentation in bovine monocytes. Biochim. Et Biophys. Acta (Bba)-Proteins Proteom. 2009, 1794, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Jirillo, E.; Greco, B.; Caradonna, L.; Satalino, R.; Pugliese, V.; Cozzolongo, R.; Cuppone, R.; Manghisi, O.G. Evaluation of cellular immune responses and soluble mediators in patients with chronic hepatitis C virus (cHCV) infection. Immunopharmacol. Immunotoxicol. 1995, 17, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Wang, S.Y.; King, C.C. Bacterial lipopolysaccharide inhibits dengue virus infection of primary human monocytes/macrophages by blockade of virus entry via a CD14-dependent mechanism. J. Virol. 1999, 73, 2650–2657. [Google Scholar] [CrossRef]
- Jensen, J.; Schultz, R.D. Effect of infection by bovine viral diarrhea virus (BVDV) in vitro on interleukin-1 activity of bovine monocytes. Vet. Immunol. Immunopathol. 1991, 29, 251–265. [Google Scholar] [CrossRef]
- Markham, R.J.; Ramnaraine, M.L. Release of immunosuppressive substances from tissue culture cells infected with bovine viral diarrhea virus. Am. J. Vet. Res. 1985, 46, 879–883. [Google Scholar]
- Van Reeth, K.; Adair, B. Macrophages and respiratory viruses. Pathol.-Biol. 1997, 45, 184–192. [Google Scholar]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. The Complement System and Innate Immunity; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Darweesh, M.F.; Rajput, M.K.; Braun, L.J.; Rohila, J.S.; Chase, C.C. BVDV Npro protein mediates the BVDV induced immunosuppression through interaction with cellular S100A9 protein. Microb. Pathog. 2018, 121, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Liebler-Tenorio, E.M.; Ridpath, J.F.; Neill, J.D. Distribution of viral antigen and development of lesions after experimental infection with highly virulent bovine viral diarrhea virus type 2 in calves. Am. J. Vet. Res. 2002, 63, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Jungmann, A.; Nieper, H.; Müller, H. Apoptosis is induced by infectious bursal disease virus replication in productively infected cells as well as in antigen-negative cells in their vicinity. J. Gen. Virol. 2001, 82, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.; He, B.; Wang, T.; Zhao, S.; Wu, C.; Yue, S.; Zhang, S.; He, M.; Wang, L.; et al. PD-1 blockade inhibits lymphocyte apoptosis and restores proliferation and anti-viral immune functions of lymphocyte after CP and NCP BVDV infection in vitro. Vet. Microbiol. 2018, 226, 74–80. [Google Scholar] [CrossRef]
- Shi, H.; Ni, W.; Sheng, J.; Chen, C. Lentivirus-mediated bta-miR-193a Overexpression Promotes Apoptosis of MDBK Cells by Targeting BAX and Inhibits BVDV Replication. Kafkas Üniversitesi Vet. Fakültesi Derg. 2017, 23, 587–593. [Google Scholar]



| Cytokine | Forward Primer | Reverse Primer | Annealing Temp °C |
|---|---|---|---|
| TNF-α | AGACCCCAGCACCCAGGACTCG | GGAGATGCCATCTGTGTGAGTG | 55 |
| IL-1α | GATGCCTGAGACACCCAA | GAAAGTCAGTGATCGAGGG | 53 |
| IL-1β | CAAGGAGAGGAAAGAGACA | TGAGAAGTGCTGATGTACCA | 53 |
| IL-6 | TCCAGAACGAGTATGAGG | CATCCGAATAGCTCTCAG | 52 |
| β-actin | CGCACCACTGGCATTGTC | TCCAAGGCGACGTAGCAG | 55 |
| IFN-α | ACACACACCTGGT | GATGACAGCAGAAATGA | 52 |
| IFN-β | RTCTGSAGCCAAT | CAGGCACACCTGT | 52 |
| IFN-γ | ATAACCAGGTCATTCAAAGG | ATTCTGACTTCTCTTCCGCT | 55 |
| IL-8 | TGGGCCACACTGTGAAAAT | TCATGGATCTTGCTTCTCAGC | 53 |
| IL-10 | TGCTGGATGACTTTAAGGG | AGGGCAGAAAGCGATGACA | 53 |
| IL-12 | GAGGCCTGTTTACCACTGGA | CTCATAGATACTTCTAAGGCACAG | 58 |
| IL-4 | TGCATTGTTAGCGTCTCCTG | AGGTCTTTCAGCGTACTTGT | 56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelsalam, K.; Rajput, M.; Elmowalid, G.; Sobraske, J.; Thakur, N.; Abdallah, H.; Ali, A.A.H.; Chase, C.C.L. The Effect of Bovine Viral Diarrhea Virus (BVDV) Strains and the Corresponding Infected-Macrophages’ Supernatant on Macrophage Inflammatory Function and Lymphocyte Apoptosis. Viruses 2020, 12, 701. https://doi.org/10.3390/v12070701
Abdelsalam K, Rajput M, Elmowalid G, Sobraske J, Thakur N, Abdallah H, Ali AAH, Chase CCL. The Effect of Bovine Viral Diarrhea Virus (BVDV) Strains and the Corresponding Infected-Macrophages’ Supernatant on Macrophage Inflammatory Function and Lymphocyte Apoptosis. Viruses. 2020; 12(7):701. https://doi.org/10.3390/v12070701
Chicago/Turabian StyleAbdelsalam, Karim, Mrigendra Rajput, Gamal Elmowalid, Jacob Sobraske, Neelu Thakur, Hossam Abdallah, Ahmed A. H. Ali, and Christopher C. L. Chase. 2020. "The Effect of Bovine Viral Diarrhea Virus (BVDV) Strains and the Corresponding Infected-Macrophages’ Supernatant on Macrophage Inflammatory Function and Lymphocyte Apoptosis" Viruses 12, no. 7: 701. https://doi.org/10.3390/v12070701
APA StyleAbdelsalam, K., Rajput, M., Elmowalid, G., Sobraske, J., Thakur, N., Abdallah, H., Ali, A. A. H., & Chase, C. C. L. (2020). The Effect of Bovine Viral Diarrhea Virus (BVDV) Strains and the Corresponding Infected-Macrophages’ Supernatant on Macrophage Inflammatory Function and Lymphocyte Apoptosis. Viruses, 12(7), 701. https://doi.org/10.3390/v12070701






