Role of MicroRNAs in Host Defense against Infectious Bursal Disease Virus (IBDV) Infection: A Hidden Front Line
Abstract
1. Introduction
2. Virus Characteristics
3. The Battle between IBDV and Host
4. MiRNA Biogenesis and Nomenclature
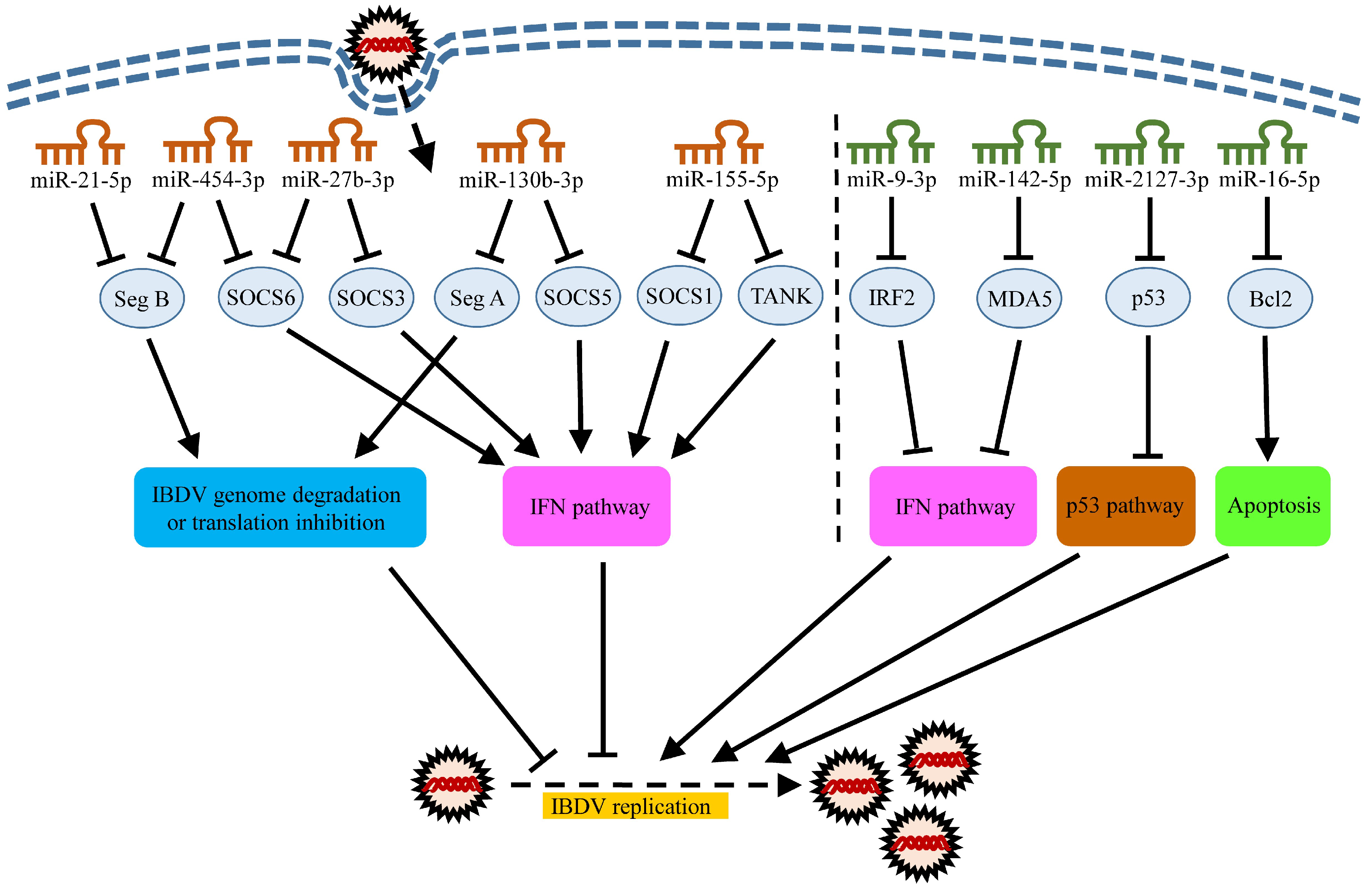
5. Roles of MiRNAs in Host Response to IBDV Infection
5.1. MiRNAs Inhibiting IBDV Infection
5.1.1. gga-miR-130b-3p
5.1.2. gga-miR-454-3p
5.1.3. gga-miR-155-5p
5.1.4. gga-miR-21-5p
5.1.5. gga-miR-27b-3p
5.2. miRNAs Promoting IBDV Replication
5.2.1. gga-miR-2127-3p
5.2.2. gga-miR-142-5p
5.2.3. gga-miR-9-3p
5.2.4. gga-miR-16-5p
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cosgrove, A.S. An Apparently New Disease of Chickens: Avian Nephrosis. Avian Dis. 1962, 6, 385. [Google Scholar] [CrossRef]
- Müller, H.; Islam; Raue, R. Research on infectious bursal disease -The past, the present and the future. Vet. Microbiol. 2003, 97, 153–165. [Google Scholar] [CrossRef]
- Khan, R.S.A.; Sajid, S.; Habib, M.; Ali, W.; Shah, M.S.-U.-D.; Sarfraz, M. History of Gumboro (infectious bursal disease) in Pakistan. Saudi Pharm. J. 2017, 25, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Stoute, S.T.; Jackwood, D.J.; Crossley, B.M.; Michel, L.; Blakey, J.R. Molecular epidemiology of endemic and very virulent infectious bursal disease virus genogroups in backyard chickens in California, 2009–2017. J. Vet. Diagn. Investig. 2019, 31, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J. Infectious bursal disease virus of chickens: Pathogenesis and immunosuppression. Dev. Comp. Immunol. 2000, 24, 223–235. [Google Scholar] [CrossRef]
- Burkhardt, E.; Müller, H. Susceptibility of chicken blood lymphoblasts and monocytes to infectious bursal disease virus (IBDV). Arch. Virol. 1987, 94, 297–303. [Google Scholar] [CrossRef]
- Ingrao, F.; Rauw, F.; Lambrecht, B.; Berg, T.V.D. Infectious Bursal Disease: A complex host–pathogen interaction. Dev. Comp. Immunol. 2013, 41, 429–438. [Google Scholar] [CrossRef]
- Ye, C.; Jia, L.; Sun, Y.; Hu, B.; Wang, L.; Lu, X.; Zhou, J. Inhibition of Antiviral Innate Immunity by Birnavirus VP3 Protein via Blockage of Viral Double-Stranded RNA Binding to the Host Cytoplasmic RNA Detector MDA5. J. Virol. 2014, 88, 11154–11165. [Google Scholar] [CrossRef]
- El-Aried, T.A.; Mansour, S.M.G.; ElBakrey, R.M.; Ismail, A.E.-S.N.; Eid, A.A.M. Infectious Bursal Disease Virus: Molecular Epidemiologic Perspectives and Impact on Vaccine Efficacy Against Avian Influenza and Newcastle Disease Viruses. Avian Dis. 2019, 63, 606–618. [Google Scholar] [CrossRef]
- Winterfield, R.W.; Hoerr, F.J.; Fadly, A.M. Vaccination Against Infectious Bronchitis and the Immunosuppressive Effects of Infectious Bursal Disease. Poult. Sci. 1978, 57, 386–391. [Google Scholar] [CrossRef]
- Chen, F.; Liu, J.; Yan, Z.; Liu, D.; Ji, J.; Qin, J.; Li, H.; Ma, J.; Bi, Y.; Xie, Q. Complete Genome Sequence Analysis of a Natural Reassortant Infectious Bursal Disease Virus in China. J. Virol. 2012, 86, 11942–11943. [Google Scholar] [CrossRef]
- Fan, L.; Wu, T.; Hussain, A.; Gao, Y.; Zeng, X.; Wang, Y.; Gao, L.; Li, K.; Wang, Y.; Liu, C.; et al. Novel variant strains of infectious bursal disease virus isolated in China. Vet. Microbiol. 2019, 230, 212–220. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Lau, N.C. An Abundant Class of Tiny RNAs with Probable Regulatory Roles in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Ambros, V. An extensive class of small RNAs in Caenorhabditis elegans. Science 2001, 294, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhu, N.; Hao, F.; Song, Y.; Wang, Z.; Ni, Y.; Ding, L. The Regulatory Role of Non-coding RNAs on Programmed Cell Death Four in Inflammation and Cancer. Front. Oncol. 2019, 9, 919. [Google Scholar] [CrossRef] [PubMed]
- Utikal, J.; Abba, M.; Novak, D.; Moniuszko, M.; Allgayer, H. Function and significance of MicroRNAs in benign and malignant human stem cells. Semin. Cancer Biol. 2015, 35, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Wang, B.; Chen, X.; He, Z.; Wang, Y.; Li, X.; Cao, H.; Zheng, S.J. MicroRNA gga-miR-130b Suppresses Infectious Bursal Disease Virus Replication via Targeting of the Viral Genome and Cellular Suppressors of Cytokine Signaling 5. J. Virol. 2017, 92, e01646-17. [Google Scholar] [CrossRef]
- Fu, M.; Wang, B.; Chen, X.; He, Z.; Wang, Y.; Li, X.; Cao, H.; Zheng, S.J. gga-miR-454 suppresses infectious bursal disease virus (IBDV) replication via directly targeting IBDV genomic segment B and cellular Suppressors of Cytokine Signaling 6 (SOCS6). Virus Res. 2018, 252, 29–40. [Google Scholar] [CrossRef]
- Wang, B.; Fu, M.; Liu, Y.; Wang, Y.; Li, X.; Cao, H.; Zheng, S.J. gga-miR-155 Enhances Type I Interferon Expression and Suppresses Infectious Burse Disease Virus Replication via Targeting SOCS1 and TANK. Front. Microbiol. 2018, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhao, M.; Li, X.; Gao, L.; Cao, H.; Wang, Y.; Zheng, S.J. gga-miR-27b-3p enhances type I interferon expression and suppresses infectious bursal disease virus replication via targeting cellular suppressors of cytokine signaling 3 and 6 (SOCS3 and 6). Virus Res. 2020, 281, 197910. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Wang, Y.-S.; Du, X.-N.; Liu, H.-J.; Zhang, H.-B. gga-miR-9* inhibits IFN production in antiviral innate immunity by targeting interferon regulatory factor 2 to promote IBDV replication. Vet. Microbiol. 2015, 178, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Wang, Y.-S.; Meng, K.; Pan, Q.-X.; Wang, X.-L.; Xia, X.-X.; Zhu, Y.-M.; Bi, Z.-W.; Zhang, H.-B.; Luo, K. gga-miR-2127 downregulates the translation of chicken p53 and attenuates chp53-mediated innate immune response against IBDV infection. Vet. Microbiol. 2017, 198, 34–42. [Google Scholar] [CrossRef]
- Ouyang, W.; Qian, J.; Pan, Q.-X.; Wang, J.; Xia, X.-X.; Wang, X.-L.; Zhu, Y.-M.; Wang, Y.-S. gga-miR-142-5p attenuates IRF7 signaling and promotes replication of IBDV by directly targeting the chMDA5′s 3′ untranslated region. Vet. Microbiol. 2018, 221, 74–80. [Google Scholar] [CrossRef]
- Duan, X.; Zhao, M.; Wang, Y.; Li, X.; Cao, H.; Zheng, S.J. Epigenetic Upregulation of Chicken MicroRNA-16-5p Expression in DF-1 Cells following Infection with Infectious Bursal Disease Virus (IBDV) Enhances IBDV-Induced Apoptosis and Viral Replication. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Dobos, P.; Hill, B.J.; Hallett, R.; Kells, D.T.; Becht, H.; Teninges, D. Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. J. Virol. 1979, 32, 593–605. [Google Scholar] [CrossRef]
- Müller, H.; Scholtissek, C.; Becht, H. The genome of infectious bursal disease virus consists of two segments of double-stranded RNA. J. Virol. 1979, 31, 584–589. [Google Scholar] [CrossRef]
- Lombardo, E.; Maraver, A.; Espinosa, I.; Fernandez-Arias, A.; Rodriguez, J.F. VP5, the Nonstructural Polypeptide of Infectious Bursal Disease Virus, Accumulates within the Host Plasma Membrane and Induces Cell Lysis. Virology 2000, 277, 345–357. [Google Scholar] [CrossRef]
- Irigoyen, N.; Caston, J.R.; Rodriguez, J.F. Host Proteolytic Activity Is Necessary for Infectious Bursal Disease Virus Capsid Protein Assembly*. J. Biol. Chem. 2012, 287, 24473–24482. [Google Scholar] [CrossRef]
- Lejal, N.; Da Costa, B.; Delmas, B.; Huet, J.-C. Role of Ser-652 and Lys-692 in the protease activity of infectious bursal disease virus VP4 and identification of its substrate cleavage sites. J. Gen. Virol. 2000, 81, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Kibenge, F.S.B.; Jackwood, D.J.; Mercado, C.C. Nucleotide sequence analysis of genome segment A of infectious bursal disease virus. J. Gen. Virol. 1990, 71, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, N.; Garriga, D.; Navarro, A.; Verdaguer, N.; Rodriguez, J.F.; Caston, J.R. Autoproteolytic Activity Derived from the Infectious Bursal Disease Virus Capsid Protein. J. Biol. Chem. 2009, 284, 8064–8072. [Google Scholar] [CrossRef] [PubMed]
- Von Einem, U.I.; Gorbalenya, A.E.; Schirrmeier, H.; Behrens, S.-E.; Letzel, T.; Mundt, E. VP1 of infectious bursal disease virus is an RNA-dependent RNA polymerase. J. Gen. Virol. 2004, 85, 2221–2229. [Google Scholar] [CrossRef]
- Lombardo, E.; Maraver, A.; Caston, J.R.; Rivera-Torres, J.; Fernández-Arias, A.; Serrano, A.; Carrascosa, J.L.; Rodriguez, J.F. VP1, the Putative RNA-Dependent RNA Polymerase of Infectious Bursal Disease Virus, Forms Complexes with the Capsid Protein VP3, Leading to Efficient Encapsidation into Virus-Like Particles. J. Virol. 1999, 73, 6973–6983. [Google Scholar] [CrossRef]
- Qin, Y.; Zheng, S.J. Infectious Bursal Disease Virus-Host Interactions: Multifunctional Viral Proteins that Perform Multiple and Differing Jobs. Int. J. Mol. Sci. 2017, 18, 161. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Lam, K. Apoptosis in chicken embryos induced by the infectious bursal disease virus. J. Comp. Pathol. 1995, 112, 327–338. [Google Scholar] [CrossRef]
- Vasconcelos, A.C.; Lam, K.M. Apoptosis induced by infectious bursal disease virus. J. Gen. Virol. 1994, 75, 1803–1806. [Google Scholar] [CrossRef]
- Kim, I.-J.; You, S.K.; Kim, H.; Yeh, H.-Y.; Sharma, J.M. Characteristics of Bursal T Lymphocytes Induced by Infectious Bursal Disease Virus. J. Virol. 2000, 74, 8884–8892. [Google Scholar] [CrossRef]
- Wei, L.; Hou, L.; Zhu, S.; Wang, J.; Zhou, J.; Liu, J. Infectious bursal disease virus activates the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway by interaction of VP5 protein with the p85alpha subunit of PI3K. Virology 2011, 417, 211–220. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Xue, Y.; Li, X.; Cao, H.; Zheng, S.J. Critical Role for Voltage-Dependent Anion Channel 2 in Infectious Bursal Disease Virus-Induced Apoptosis in Host Cells via Interaction with VP5. J. Virol. 2011, 86, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, Z.; Xu, Z.; Wang, B.; Li, X.; Cao, H.; Wang, Y.; Zheng, S.J. The Association of Receptor of Activated Protein Kinase C 1(RACK1) with Infectious Bursal Disease Virus Viral Protein VP5 and Voltage-dependent Anion Channel 2 (VDAC2) Inhibits Apoptosis and Enhances Viral Replication. J. Biol. Chem. 2015, 290, 8500–8510. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xu, Z.; Wang, Y.; Li, X.; Cao, H.; Zheng, S.J. VP2 of Infectious Bursal Disease Virus Induces Apoptosis via Triggering Oral Cancer Overexpressed 1 (ORAOV1) Protein Degradation. Front. Microbiol. 2017, 8, 1351. [Google Scholar] [CrossRef] [PubMed]
- Liniger, M.; Summerfield, A.; Zimmer, G.; McCullough, K.C.; Ruggli, N. Chicken Cells Sense Influenza A Virus Infection through MDA5 and CARDIF Signaling Involving LGP2. J. Virol. 2011, 86, 705–717. [Google Scholar] [CrossRef]
- Ye, C.; Yu, Z.; Xiong, Y.; Wang, Y.; Ruan, Y.; Guo, Y.; Chen, M.; Luan, S.; Zhang, E.; Liu, H. STAU1 binds to IBDV genomic double-stranded RNA and promotes viral replication via attenuation of MDA5-dependent β interferon induction. FASEB J. 2018, 33, 286–300. [Google Scholar] [CrossRef]
- Wang, B.; Duan, X.; Fu, M.; Liu, Y.; Wang, Y.; Li, X.; Cao, H.; Zheng, S.J. The association of ribosomal protein L18 (RPL18) with infectious bursal disease virus viral protein VP3 enhances viral replication. Virus Res. 2018, 245, 69–79. [Google Scholar] [CrossRef]
- Yang, B.; Yan, N.; Liu, A.; Li, Y.; Chen, Z.; Gao, L.; Qi, X.; Gao, Y.; Liu, C.; Zhang, Y.; et al. Chicken eEF1α is a Critical Factor for the Polymerase Complex Activity of Very Virulent Infectious Bursal Disease Virus. Viruses 2020, 12, 249. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Li, X.; Li, X.; Cao, H.; Zheng, S.J. Critical Roles of Glucocorticoid-Induced Leucine Zipper in Infectious Bursal Disease Virus (IBDV)-Induced Suppression of Type I Interferon Expression and Enhancement of IBDV Growth in Host Cells via Interaction with VP4. J. Virol. 2012, 87, 1221–1231. [Google Scholar] [CrossRef]
- He, Z.; Chen, X.; Fu, M.; Tang, J.; Li, X.; Cao, H.; Wang, Y.; Zheng, S.J. Infectious bursal disease virus protein VP4 suppresses type I interferon expression via inhibiting K48-linked ubiquitylation of glucocorticoid-induced leucine zipper (GILZ). Immunobiology 2018, 223, 374–382. [Google Scholar] [CrossRef]
- Gao, L.; Li, K.; Zhong, L.; Zhang, L.; Qi, X.; Wang, Y.; Gao, Y.; Wang, X. Eukaryotic translational initiation factor 4AII reduces the replication of infectious bursal disease virus by inhibiting VP1 polymerase activity. Antivir. Res. 2017, 139, 102–111. [Google Scholar] [CrossRef]
- Han, C.; Zeng, X.; Yao, S.; Gao, L.; Zhang, L.; Qi, X.; Duan, Y.; Yang, B.; Gao, Y.; Liu, C.; et al. Voltage-Dependent Anion Channel 1 Interacts with Ribonucleoprotein Complexes To Enhance Infectious Bursal Disease Virus Polymerase Activity. J. Virol. 2017, 91, e00584-17. [Google Scholar] [CrossRef]
- Ye, C.; Wang, Y.; Zhang, E.; Han, X.; Yu, Z.; Liu, H. VP1 and VP3 Are Required and Sufficient for Translation Initiation of Uncapped Infectious Bursal Disease Virus Genomic Double-Stranded RNA. J. Virol. 2018, 92, e01345-17. [Google Scholar] [CrossRef]
- Mata, C.P.; Mertens, J.; Fontana, J.; Luque, D.; Allende-Ballestero, C.; Reguera, D.; Trus, B.L.; Steven, A.C.; Carrascosa, J.L.; Caston, J.R. The RNA-Binding Protein of a Double-Stranded RNA Virus Acts like a Scaffold Protein. J. Virol. 2018, 92, JVI.00968–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shi, L.; Zhang, Y.; Peng, X.; Zheng, T.; Li, Y.; Hu, B.; Zheng, X.; Zhou, J.-Y. Ubiquitination Is Essential for Avibirnavirus Replication by Supporting VP1 Polymerase Activity. J. Virol. 2019, 93, e01899-18. [Google Scholar] [CrossRef]
- Wu, H.; Yang, H.; Ji, G.; Zheng, T.; Zhang, Y.; Deng, T.; Zheng, X.; Zhou, J.; Hu, B. SUMO1 Modification Facilitates Avibirnavirus Replication by Stabilizing Polymerase VP1. J. Virol. 2019, 93, e02227-18. [Google Scholar] [CrossRef] [PubMed]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, I.; Hansen, T.B. Biogenesis and Function of Ago-Associated RNAs. Trends Genet. 2017, 33, 208–219. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2018, 20, 5–20. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2013, 42, D68–D73. [Google Scholar] [CrossRef]
- Pfeffer, S.; Zavolan, M.; Grässer, F.A.; Chien, M.; Russo, J.J.; Ju, J.; John, B.; Enright, A.; Marks, D.; Sander, C.; et al. Identification of Virus-Encoded MicroRNAs. Science 2004, 304, 734–736. [Google Scholar] [CrossRef]
- Yao, Y.; Nair, V. Role of Virus-Encoded microRNAs in Avian Viral Diseases. Viruses 2014, 6, 1379–1394. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xia, J.; Zhang, K.; Yang, Q. Genome-wide profiling of chicken dendritic cell response to infectious bursal disease. BMC Genom. 2016, 17, 878. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, O.A.; Goltsov, A.Y.; Abramova, L.I.; Kaleda, V.G.; Orlova, V.A.; Rogaev, E. MicroRNA in schizophrenia: Genetic and expression analysis of miR-130b (22q11). Biochemistry (Moscow) 2007, 72, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ma, Y.; Peng, H.; Gong, L.; Xiao, M.; Xiang, L.; He, D.; Cao, K. MiR-130b promotes the progression of oesophageal squamous cell carcinoma by targeting SASH1. J. Cell. Mol. Med. 2018, 23, 93–103. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, C.; Yang, Z.; Liu, W.; Yuan, Y.; Li, K.; Zhang, Y.; Wang, Y.; Shi, Y.; Qiu, Y.; et al. Dysregulated Sp1/miR-130b-3p/HOXA5 axis contributes to tumor angiogenesis and progression of hepatocellular carcinoma. Theranostics 2020, 10, 5209–5224. [Google Scholar] [CrossRef]
- Mu, H.Q.; He, Y.H.; Wang, S.B.; Yang, S.; Wang, Y.J.; Nan, C.J.; Bao, Y.F.; Xie, Q.P.; Chen, Y. MiR-130b/TNF-α/NF-κB/VEGFA loop inhibits prostate cancer angiogenesis. Clin. Transl. Oncol. 2019, 22, 111–121. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, W.-D.; Peng, H.; Pan, H.-F.; Ye, D.-Q. SOCS signaling in autoimmune diseases: Molecular mechanisms and therapeutic implications. Eur. J. Immunol. 2014, 44, 1265–1275. [Google Scholar] [CrossRef]
- Kubo, M.; Hanada, T.; Yoshimura, A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003, 4, 1169–1176. [Google Scholar] [CrossRef]
- Kazi, J.U.; Kabir, N.N.; Flores-Morales, A.; Rönnstrand, L. SOCS proteins in regulation of receptor tyrosine kinase signaling. Cell. Mol. Life Sci. 2014, 71, 3297–3310. [Google Scholar] [CrossRef]
- Li, L.; Gao, F.; Jiang, Y.; Yu, L.; Zhou, Y.; Zheng, H.; Tong, W.; Yang, S.; Xia, T.; Qu, Z.; et al. Cellular miR-130b inhibits replication of porcine reproductive and respiratory syndrome virus in vitro and in vivo. Sci. Rep. 2015, 5, 17010. [Google Scholar] [CrossRef]
- Singaravelu, R.; O’Hara, S.; Jones, D.M.; Chen, R.; Taylor, N.G.; Srinivasan, P.; Quan, C.; Roy, M.G.; Steenbergen, R.H.; Kumar, A.; et al. MicroRNAs regulate the immunometabolic response to viral infection in the liver. Nat. Methods 2015, 11, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, X.; Han, B.; Qu, L.; Liu, C.; Song, J.; Lian, L.; Yang, N. Gga-miR-130b-3p inhibits MSB1 cell proliferation, migration, invasion, and its downregulation in MD tumor is attributed to hypermethylation. Oncotarget 2018, 9, 24187–24198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lei, Z.; Tang, X.; Si, A.; Yang, P.; Wang, L.; Luo, T.; Guo, G.; Zhang, Q.; Cheng, Z. microRNA-454 promotes liver tumor-initiating cell expansion by regulating SOCS6. Exp. Cell Res. 2020, 390, 111955. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Yu, H.; Xie, P.; Liu, W.; Wang, K.; Ni, H. miR-454-3p exerts tumor-suppressive functions by down-regulation of NFATc2 in glioblastoma. Gene 2019, 710, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Y.; Lv, B.; Jin, H. miR-454 performs tumor-promoting effects in oral squamous cell carcinoma via reducing NR3C2. J. Oral Pathol. Med. 2020, 49, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Gulei, D.; Raduly, L.; Broseghini, E.; Ferracin, M.; Berindan-Neagoe, I. The extensive role of miR-155 in malignant and non-malignant diseases. Mol. Asp. Med. 2019, 70, 33–56. [Google Scholar] [CrossRef]
- Poles, W.A.; Nishi, E.E.; De Oliveira, M.B.; Eugênio, A.I.P.; De Andrade, T.A.; Campos, A.H.F.M.; De Campos, R.R.; Vassallo, J.; Alves, A.C.; Neto, C.S.; et al. Targeting the polarization of tumor-associated macrophages and modulating mir-155 expression might be a new approach to treat diffuse large B-cell lymphoma of the elderly. Cancer Immunol. Immunother. 2018, 68, 269–282. [Google Scholar] [CrossRef]
- Wu, M.; Duan, Q.; Liu, X.; Zhang, P.; Fu, Y.; Zhang, Z.; Liu, L.; Cheng, J.; Jiang, H. MiR-155-5p promotes oral cancer progression by targeting chromatin remodeling gene ARID2. Biomed. Pharmacother. 2019, 122, 109696. [Google Scholar] [CrossRef]
- Bondada, M.S.; Yao, Y.; Nair, V. Multifunctional miR-155 Pathway in Avian Oncogenic Virus-Induced Neoplastic Diseases. Non-Coding RNA 2019, 5, 24. [Google Scholar] [CrossRef]
- Yao, R.; Ma, Y.-L.; Liang, W.; Li, H.-H.; Ma, Z.-J.; Yu, X.; Liao, Y.-H. MicroRNA-155 Modulates Treg and Th17 Cells Differentiation and Th17 Cell Function by Targeting SOCS1. PLoS ONE 2012, 7, e46082. [Google Scholar] [CrossRef]
- Jablonski, K.A.; Gaudet, A.; Amici, S.A.; Popovich, P.G.; Guerau-De-Arellano, M. Control of the Inflammatory Macrophage Transcriptional Signature by miR-155. PLoS ONE 2016, 11, e0159724. [Google Scholar] [CrossRef]
- Banerjee, A.; Schambach, F.; DeJong, C.S.; Hammond, S.M.; Reiner, S.L. Micro-RNA-155 inhibits IFN-γ signaling in CD4+ T cells. Eur. J. Immunol. 2010, 40, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Fioravanti, J.; Zhu, W.; Wang, H.; Wu, T.; Hu, J.; Lacey, N.E.; Gautam, S.; Le Gall, J.B.; Yang, X.; et al. miR-155 harnesses Phf19 to potentiate cancer immunotherapy through epigenetic reprogramming of CD8+ T cell fate. Nat. Commun. 2019, 10, 2157. [Google Scholar] [CrossRef] [PubMed]
- Goncalves-Alves, E.; Saferding, V.; Schliehe, C.; Benson, R.; Kurowska-Stolarska, M.; Brunner, J.S.; Puchner, A.; Podesser, B.K.; Smolen, J.S.; Redlich, K.; et al. MicroRNA-155 Controls T Helper Cell Activation During Viral Infection. Front. Immunol. 2019, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- Dudda, J.C.; Salaun, B.; Ji, Y.; Palmer, D.C.; Monnot, G.C.; Merck, E.; Boudousquie, C.; Utzschneider, D.T.; Escobar, T.M.; Perret, R.; et al. MicroRNA-155 is required for effector CD8+ T cell responses to virus infection and cancer. Immunity 2013, 38, 742–753. [Google Scholar] [CrossRef]
- Liau, N.P.D.; Laktyushin, A.; Lucet, I.S.; Murphy, J.M.; Yao, S.; Whitlock, E.; Callaghan, K.; Nicola, N.A.; Kershaw, N.J.; Babon, J.J. The molecular basis of JAK/STAT inhibition by SOCS1. Nat. Commun. 2018, 9, 1558. [Google Scholar] [CrossRef]
- Villalobos-Hernandez, A.; Bobbala, D.; Kandhi, R.; Khan, G.M.; Mayhue, M.; Dubois, C.M.; Ferbeyre, G.; Saucier, C.; Ramanathan, S.; Ilangumaran, S. SOCS1 inhibits migration and invasion of prostate cancer cells, attenuates tumor growth and modulates the tumor stroma. Prostate Cancer Prostatic Dis. 2016, 20, 36–47. [Google Scholar] [CrossRef]
- Pomerantz, J.L.; Baltimore, D. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 1999, 18, 6694–6704. [Google Scholar] [CrossRef]
- Cheng, G.; Baltimore, D. TANK, a co-inducer with TRAF2 of TNF- and CD 40L-mediated NF-kappaB activation. Genes Dev. 1996, 10, 963–973. [Google Scholar] [CrossRef]
- Wang, W.; Huang, X.; Xin, H.-B.; Fu, M.; Xue, A.; Wu, Z.-H. TRAF Family Member-associated NF-κB Activator (TANK) Inhibits Genotoxic Nuclear Factor κB Activation by Facilitating Deubiquitinase USP10-dependent Deubiquitination of TRAF6 Ligase*. J. Biol. Chem. 2015, 290, 13372–13385. [Google Scholar] [CrossRef]
- Ye, J.; Guo, R.; Shi, Y.; Qi, F.; Guo, C.; Yang, L. miR-155 Regulated Inflammation Response by the SOCS1-STAT3-PDCD4 Axis in Atherogenesis. Mediat. Inflamm. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Grillo, A.R.; Scarpa, M.; Brun, P.; D’Inca, R.; Nai, L.; Banerjee, A.; Cavallo, D.; Barzon, L.; Palù, G.; et al. MiR-155 modulates the inflammatory phenotype of intestinal myofibroblasts by targeting SOCS1 in ulcerative colitis. Exp. Mol. Med. 2015, 47, e164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, Y.; Zhang, L.; Zhao, H. MicroRNA-155 Participates in Smoke-Inhalation-Induced Acute Lung Injury through Inhibition of SOCS-1. Molecules (Basel, Switzerland) 2020, 25, 1022. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, M.T.; Dy, G.; Tam, W.; Beemon, K.L. Reticuloendotheliosis Virus Strain T Induces miR-155, Which Targets JARID2 and Promotes Cell Survival. J. Virol. 2009, 83, 12009–12017. [Google Scholar] [CrossRef]
- Gao, C.; Dang, S.; Zhai, J.; Zheng, S. Regulatory mechanism of microRNA-155 in chicken embryo fibroblasts in response to reticuloendotheliosis virus infection. Vet. Microbiol. 2020, 242, 108610. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.; Gao, H.; Chen, B.; Si, C.; Wang, S. Downregulation of miR-155-5p facilitates enterovirus 71 replication through suppression of type I IFN response by targeting FOXO3/IRF7 pathway. Cell Cycle 2019, 19, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ming, X.; Li, W.; Bi, M.; Yan, B.; Wang, X.; Yang, P.; Yang, B. The microRNA-155 mediates hepatitis B virus replication by reinforcing SOCS1 signalling-induced autophagy. Cell Biochem. Funct. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Yu, Z.-H.; Yu, C.; Jia, Y.-Y.; He, L.; Liao, C.-S.; Li, J.; Zhang, C.-J.; Li, Y.-J.; Wu, T.-C.; et al. Effect of gga-miR-155 on cell proliferation, apoptosis and invasion of Marek’s disease virus (MDV) transformed cell line MSB1 by targeting RORA. BMC Vet. Res. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Stik, G.; Dambrine, G.; Pfeffer, S.; Rasschaert, D. The Oncogenic MicroRNA OncomiR-21 Overexpressed during Marek’s Disease Lymphomagenesis Is Transactivated by the Viral Oncoprotein Meq. J. Virol. 2012, 87, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Asangani, I.A.; Rasheed, S.A.K.; Nikolova, D.A.; Leupold, J.H.; Colburn, N.H.; Post, S.; Allgayer, H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2007, 27, 2128–2136. [Google Scholar] [CrossRef]
- Meng, F.; Henson, R.; Wehbe–Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 Regulates Expression of the PTEN Tumor Suppressor Gene in Human Hepatocellular Cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef]
- Ouyang, W.; Wang, Y.; Wang, Y.; Wang, X.; Zhu, X.; Bi, Z.; Fan, H. Profiles of host-cellular microRNAs induced by infectious bursal disease virus infection. Chin. J. Vet. Sci. 2012, 32, 329–336. [Google Scholar]
- Wang, Y.-S.; Ouyang, W.; Pan, Q.-X.; Wang, X.-L.; Xia, X.-X.; Bi, Z.; Wang, Y.-Q.; Wang, X.-M. Overexpression of microRNA gga-miR-21 in chicken fibroblasts suppresses replication of infectious bursal disease virus through inhibiting VP1 translation. Antivir. Res. 2013, 100, 196–201. [Google Scholar] [CrossRef]
- Huang, J.; Ma, G.; Fu, L.; Jia, H.; Zhu, M.; Li, X.; Zhao, S. Pseudorabies viral replication is inhibited by a novel target of miR-21. Virology 2014, 456, 319–328. [Google Scholar] [CrossRef]
- Kanokudom, S.; Vilaivan, T.; Wikan, N.; Thepparit, C.; Smith, D.R.; Assavalapsakul, W. miR-21 promotes dengue virus serotype 2 replication in HepG2 cells. Antivir. Res. 2017, 142, 169–177. [Google Scholar] [CrossRef]
- Xia, B.; Lu, J.; Wang, R.; Yang, Z.; Zhou, X.; Huang, P.-T. miR-21-3p Regulates Influenza A Virus Replication by Targeting Histone Deacetylase-8. Front. Microbiol. 2018, 8, 175. [Google Scholar] [CrossRef]
- Hannafon, B.N.; Cai, A.; Calloway, C.L.; Xu, Y.-F.; Zhang, R.; Fung, K.-M.; Ding, W. miR-23b and miR-27b are oncogenic microRNAs in breast cancer: Evidence from a CRISPR/Cas9 deletion study. BMC Cancer 2019, 19, 642. [Google Scholar] [CrossRef]
- Feng, Q.; Wu, X.; Li, F.; Ning, B.; Lu, X.; Zhang, Y.; Pan, Y.; Wenxian, G. miR-27b inhibits gastric cancer metastasis by targeting NR2F2. Protein Cell 2016, 8, 114–122. [Google Scholar] [CrossRef]
- Wu, J.; Ji, Z.; Qiao, M.; Peng, X.; Wu, H.; Song, Z.; Zhao, H.; Liu, G.; Li, F.; Mei, S. MicroRNA transcriptome analysis of poly I:C-stimulated and PRRSV-infected porcine alveolar macrophages. J. Appl. Genet. 2019, 60, 375–383. [Google Scholar] [CrossRef]
- Buck, A.H.; Perot, J.; Chisholm, M.A.; Kumar, D.S.; Tuddenham, L.; Cognat, V.; Marcinowski, L.; Dölken, L.; Pfeffer, S. Post-transcriptional regulation of miR-27 in murine cytomegalovirus infection. RNA 2010, 16, 307–315. [Google Scholar] [CrossRef]
- Zhao, X.; Song, X.; Bai, X.; Fei, N.; Huang, Y.; Zhao, Z.; Du, Q.; Zhang, H.; Zhang, L.; Tong, D. miR-27b attenuates apoptosis induced by transmissible gastroenteritis virus (TGEV) infection via targeting runt-related transcription factor 1 (RUNX1). PeerJ 2016, 4, e1635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, X.; Yang, J.; Meng, K. Molecular Mechanism of Host miRNA-2127 Targeting p53 Promoting H9N2 Subtype of Avian Influenza Virus Replication in vitro. Shandong Agric. Sci. 2019, 51, 91–95. [Google Scholar]
- Ji, J.; Xu, X.; Wang, X.; Yao, L.; Shang, H.; Li, H.; Ma, J.; Bi, Y.; Xie, Q. Expression of dysregulated miRNA in vivo in DF-1 cells during the course of subgroup J avian leukosis virus infection. Microb. Pathog. 2019, 126, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, J.; Xie, Q.; Shang, H.; Zhang, H.; Xin, X.; Chen, F.; Sun, B.; Xue, C.; Ma, J.; et al. Aberrant expression of liver microRNA in chickens infected with subgroup J avian leukosis virus. Virus Res. 2012, 169, 268–271. [Google Scholar] [CrossRef]
- Magor, K.E.; Navarro, D.M.; Barber, M.R.; Petkau, K.; Fleming-Canepa, X.; Blyth, G.; Blaine, A.H. Defense genes missing from the flight division. Dev. Comp. Immunol. 2013, 41, 377–388. [Google Scholar] [CrossRef]
- Li, Z.; Lan, Y.; Zhao, K.; Lv, X.; Ding, N.; Lu, H.; Zhang, J.; Yue, H.; Shi, J.; Song, D.; et al. miR-142-5p Disrupts Neuronal Morphogenesis Underlying Porcine Hemagglutinating Encephalomyelitis Virus Infection by Targeting Ulk1. Front. Microbiol. 2017, 7, 350. [Google Scholar] [CrossRef]
- Chanda, S.; Nandi, S.; Chawla-Sarkar, M. Rotavirus-induced miR-142-5p elicits proviral milieu by targeting non-canonical transforming growth factor beta signalling and apoptosis in cells. Cell. Microbiol. 2015, 18, 733–747. [Google Scholar] [CrossRef]
- Yin, H.; He, H.; Shen, X.; Zhao, J.; Cao, X.; Han, S.; Cui, C.; Chen, Y.; Wei, Y.; Xia, L.; et al. miR-9-5p Inhibits Skeletal Muscle Satellite Cell Proliferation and Differentiation by Targeting IGF2BP3 through the IGF2-PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 1655. [Google Scholar] [CrossRef]
- Davila, J.L.; Goff, L.A.; Ricupero, C.L.; Camarillo, C.; Oni, E.N.; Swerdel, M.R.; Toro-Ramos, A.J.; Li, J.; Hart, R.P. A Positive Feedback Mechanism That Regulates Expression of miR-9 during Neurogenesis. PLoS ONE 2014, 9, e94348. [Google Scholar] [CrossRef]
- Dong, C.; Sun, X.; Guan, Z.; Zhang, M.; Duan, M. Modulation of influenza A virus replication by microRNA-9 through targeting MCPIP1. J. Med Virol. 2016, 89, 41–48. [Google Scholar] [CrossRef]
- Lai, F.W.; Stephenson, K.B.; Mahony, J.; Lichty, B.D. Human Coronavirus OC43 Nucleocapsid Protein Binds MicroRNA 9 and Potentiates NF- B Activation. J. Virol. 2013, 88, 54–65. [Google Scholar] [CrossRef]
- Hoseinbeyki, M.; Taha, M.; Javeri, A. miR-16 enhances miR-302/367-induced reprogramming and tumor suppression in breast cancer cells. IUBMB Life 2020. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, F.; Yu, X.; Wang, B.; Yang, Y.; Zhou, X.; Cheng, R.; Xia, S.; Zhou, X. miR-16 inhibits NLRP3 inflammasome activation by directly targeting TLR4 in acute lung injury. Biomed. Pharmacother. 2019, 112, 108664. [Google Scholar] [CrossRef]
- Jia, X.; Ouyang, H.; Abdalla, B.A.; Xu, H.; Nie, Q.; Zhang, X. miR-16 controls myoblast proliferation and apoptosis through directly suppressing Bcl2 and FOXO1 activities. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2017, 1860, 674–684. [Google Scholar] [CrossRef]
- An, F.; Gong, B.; Wang, H.; Yu, N.; Zhao, G.; Lin, L.; Tang, W.; Yu, H.; Bao, S.; Xie, Q. miR-15b and miR-16 regulate TNF mediated hepatocyte apoptosis via BCL2 in acute liver failure. Apoptosis 2012, 17, 702–716. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, Z.; Sun, J.; Zhang, Y.; Wei, C.; Ke, X.; Liu, Y.; Deng, L.; Wang, H. MiR-16-5p mediates a positive feedback loop in EV71-induced apoptosis and suppresses virus replication. Sci. Rep. 2017, 7, 16422. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Y.; Zhao, Y.; Sun, Y.; Zou, M.; Fu, Y.; Peng, X. Upregulated gga-miR-16-5p Inhibits the Proliferation Cycle and Promotes the Apoptosis of MG-Infected DF-1 Cells by Repressing PIK3R1-Mediated the PI3K/Akt/NF-κB Pathway to Exert Anti-Inflammatory Effect. Int. J. Mol. Sci. 2019, 20, 1036. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Shen, P.; Zhang, X.; Xia, X.; Xia, B. Effective inhibition of replication of infectious bursal disease virus by miRNAs delivered by vectors and targeting the VP2 gene. J. Virol. Methods 2010, 165, 127–132. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Shen, P.; Zhang, X.; Xia, X. Effective inhibition of infectious bursal disease virus replication by recombinant avian adeno-associated virus-delivered microRNAs. J. Gen. Virol. 2009, 90, 1417–1422. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zheng, S.J. Role of MicroRNAs in Host Defense against Infectious Bursal Disease Virus (IBDV) Infection: A Hidden Front Line. Viruses 2020, 12, 543. https://doi.org/10.3390/v12050543
Li J, Zheng SJ. Role of MicroRNAs in Host Defense against Infectious Bursal Disease Virus (IBDV) Infection: A Hidden Front Line. Viruses. 2020; 12(5):543. https://doi.org/10.3390/v12050543
Chicago/Turabian StyleLi, Jiaxin, and Shijun J. Zheng. 2020. "Role of MicroRNAs in Host Defense against Infectious Bursal Disease Virus (IBDV) Infection: A Hidden Front Line" Viruses 12, no. 5: 543. https://doi.org/10.3390/v12050543
APA StyleLi, J., & Zheng, S. J. (2020). Role of MicroRNAs in Host Defense against Infectious Bursal Disease Virus (IBDV) Infection: A Hidden Front Line. Viruses, 12(5), 543. https://doi.org/10.3390/v12050543




