Eco-Epidemiological Evidence of the Transmission of Avian and Human Influenza A Viruses in Wild Pigs in Campeche, Mexico
Abstract
1. Introduction
2. Material and Methods
2.1. Area Under Study
2.2. Ethical Considerations
2.3. Sample Categorization
2.4. Sample Collection, Transport, and Storage
2.5. Hemagglutination Inhibition
2.6. Extraction and Molecular Detection of Viral RNA
2.7. Virus Isolation
2.8. Sequencing
2.9. Phylogenetic Analysis
2.10. Categorizing the Degree of Landscape Anthropization
2.11. Statistical Analysis
3. Results
3.1. Categorizing Sampled Animals
3.2. Hemagglutination Inhibition
3.3. qRT-PCR of Influenza A Virus M Gene
3.4. Virus Isolation
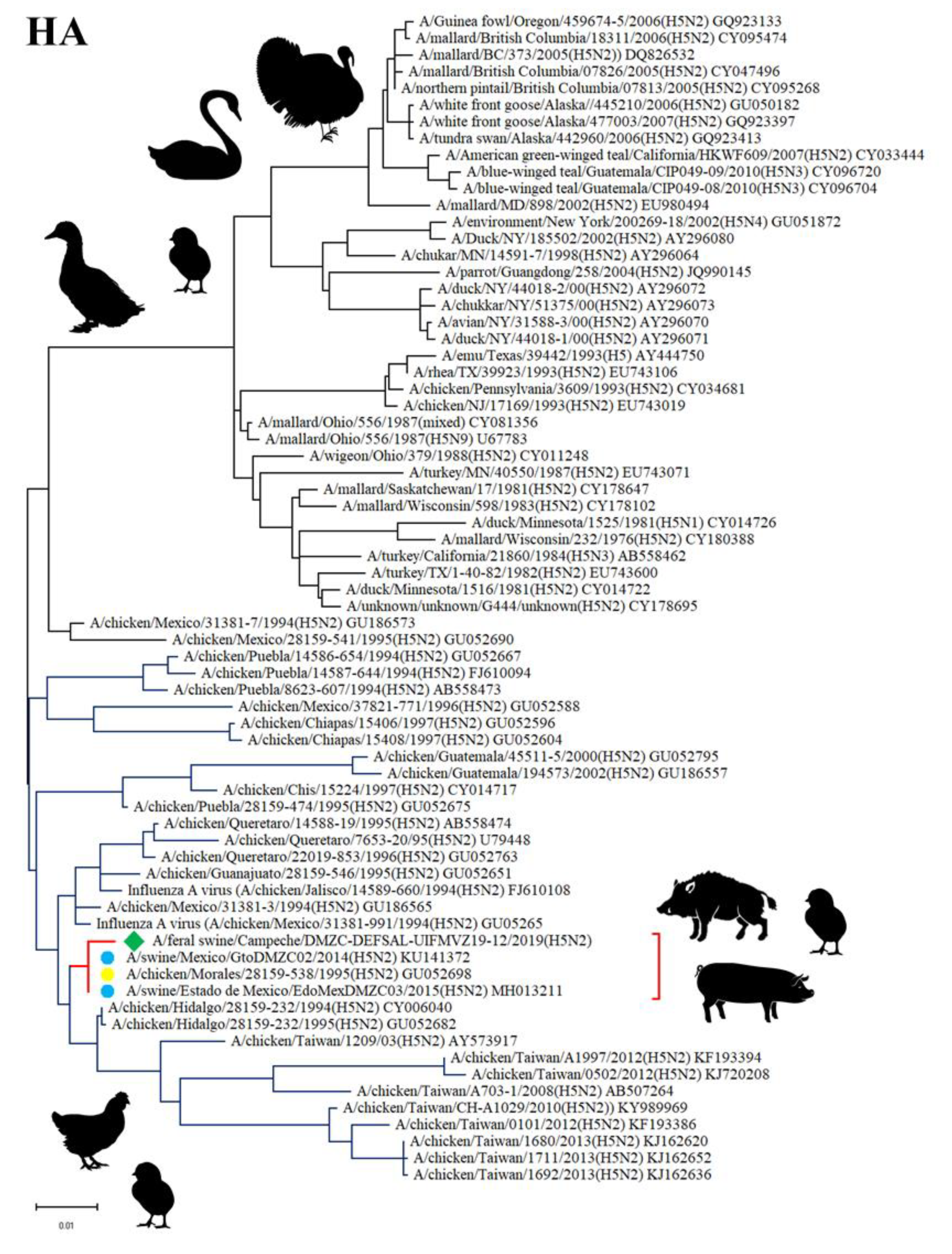
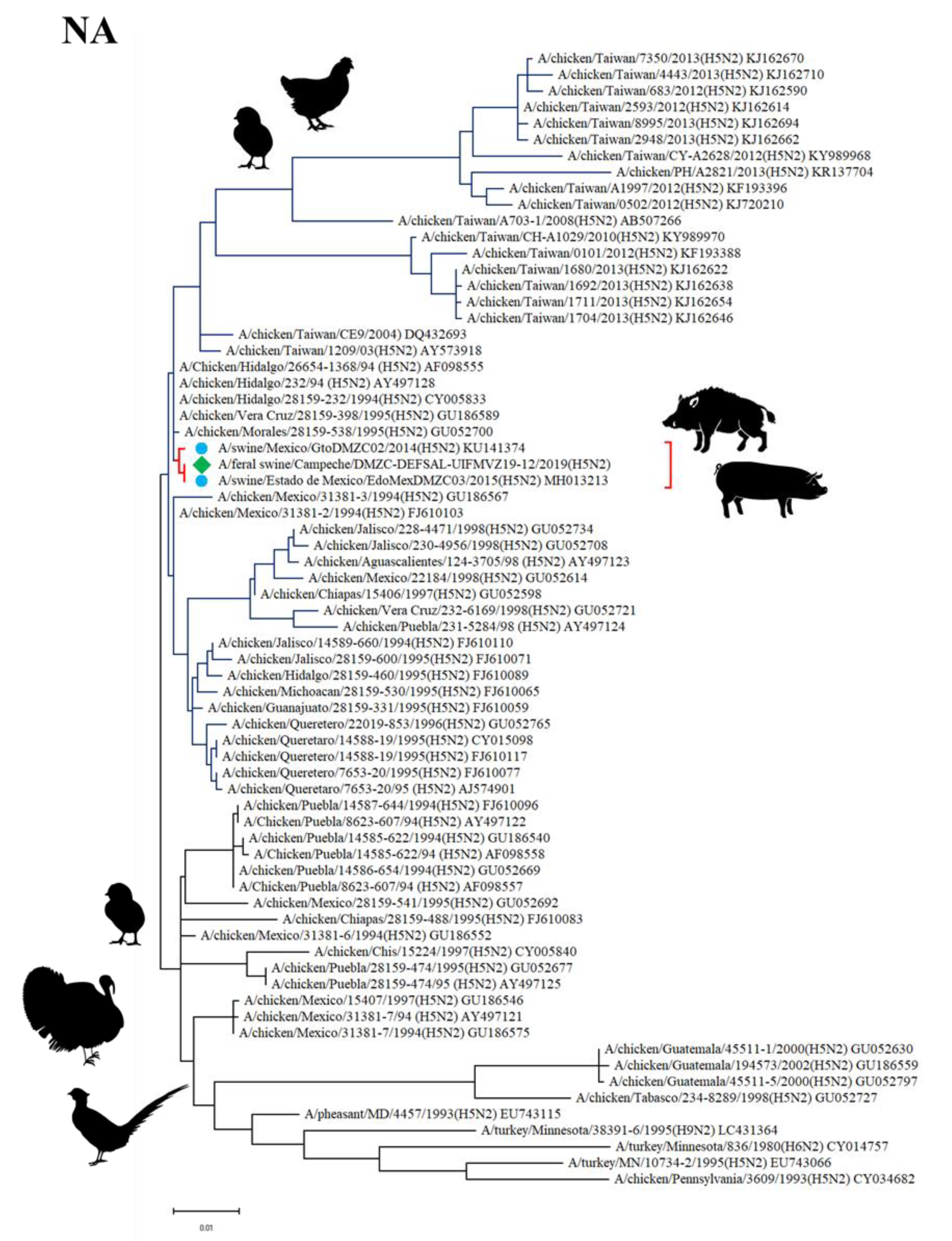
3.5. Sequencing and Phylogenetic Analysis
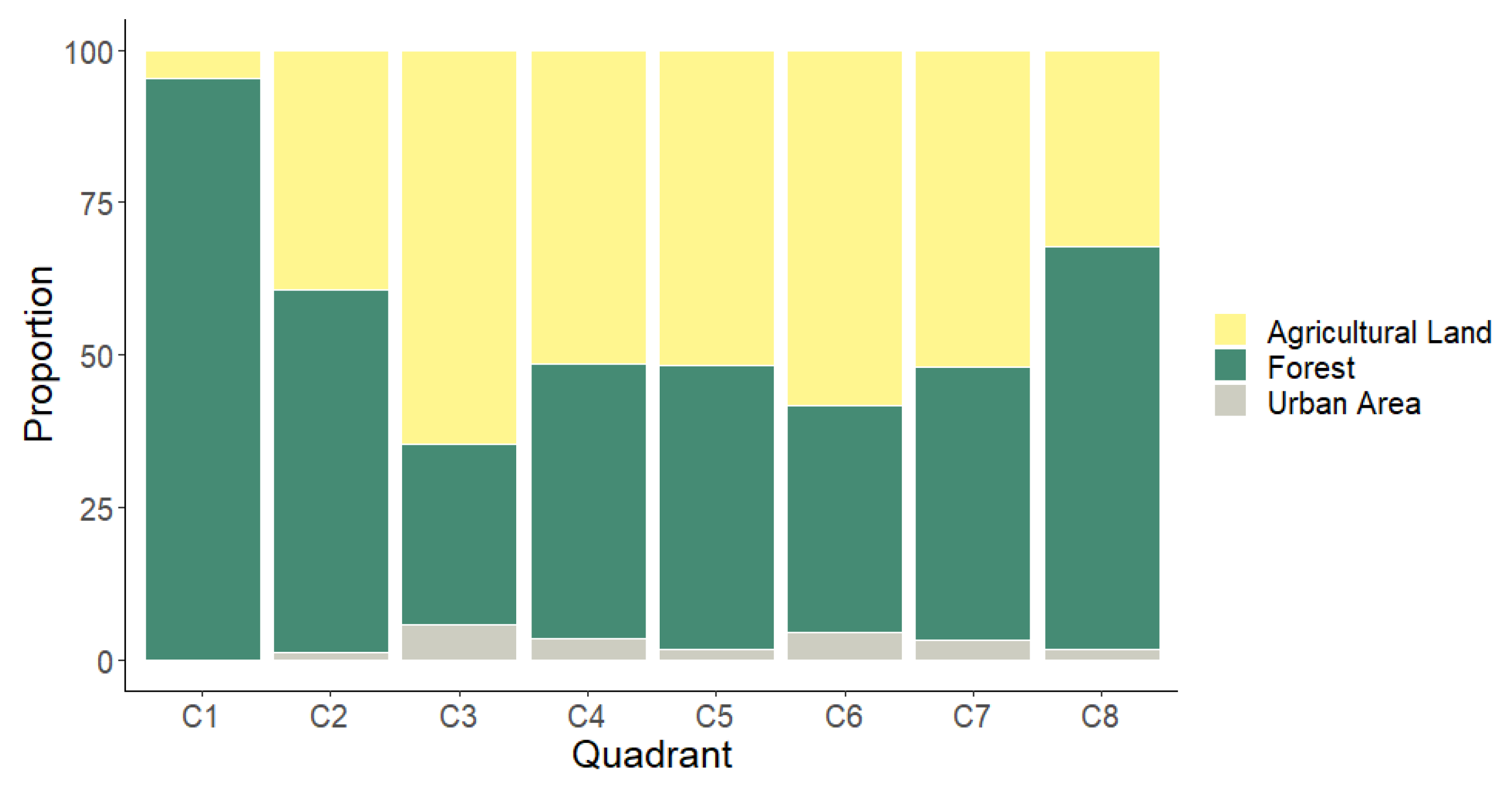
3.6. Categorizing the Degree of Landscape Anthropization
3.7. Statistical Analysis
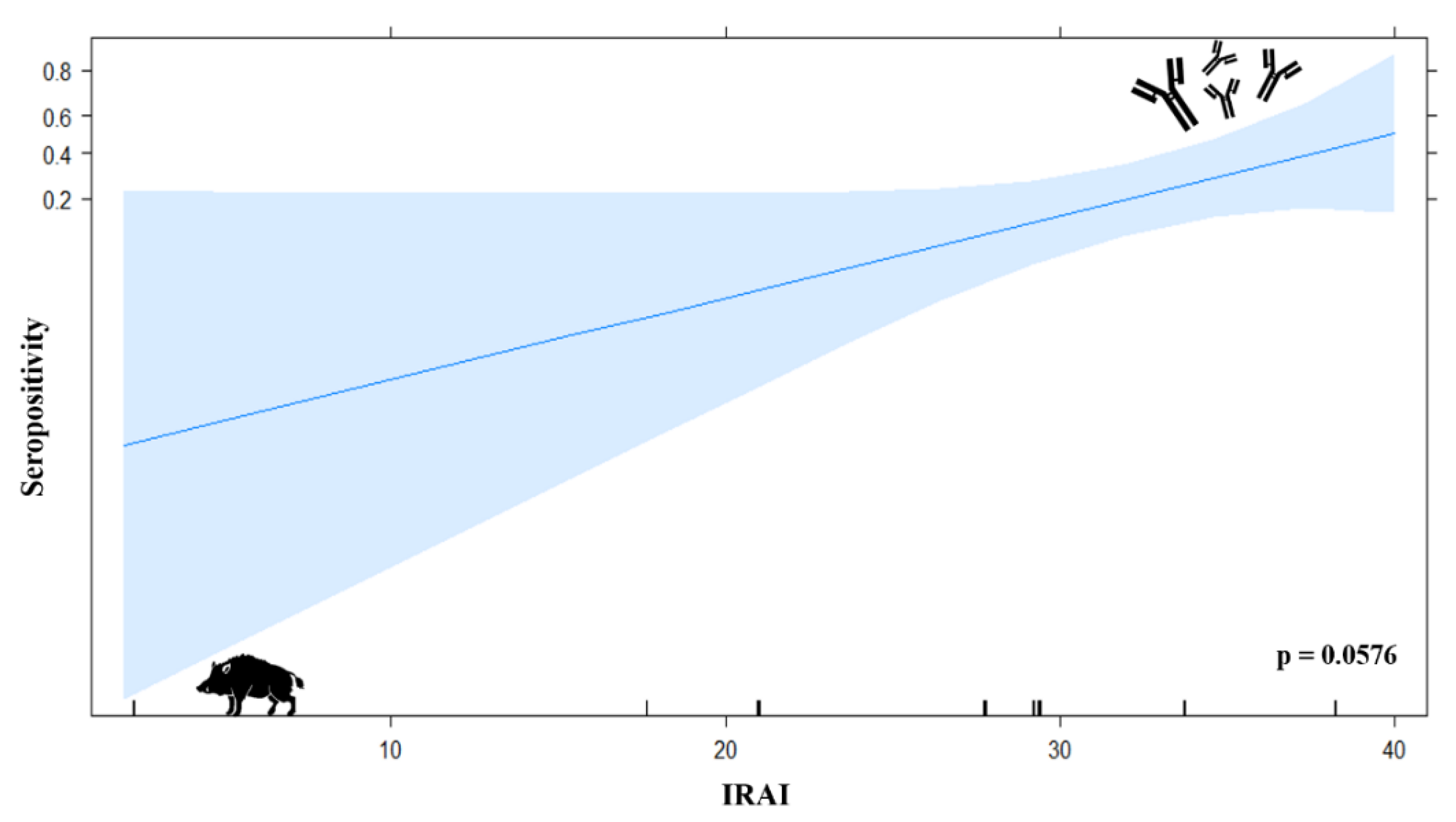
3.7.1. Logistic Regression Model
3.7.2. Chi-Squared (χ2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajao, D.S.; Vincent, A.L. Swine as a Model for Influenza A Virus Infection and Immunity. ILAR J. 2015, 56, 44–52. [Google Scholar] [CrossRef]
- Short, K.R.; Richard, M.; Verhagen, J.H.; Riel, D.; Schrauwen, E.J.A.; Brand, J.M.A.; Van den Brand, J.M.; Herfst, S. One health, multiple challenges: The inter-species transmission of influenza a virus. One Health 2015, 1, 1–13. [Google Scholar] [CrossRef]
- Stephenson, I.; Nicholson, K.G. Influenza: Vaccination and treatment. Eur. Respir. 2001, 3, 1282–1293. [Google Scholar] [CrossRef]
- Arbeláez, G.; Calderón, D.; Rincón, M.; Lora, Á.; Mercado, M. Improvement of two diagnostics methods for detection of influenza swine virus. Univ. Sci. 2008, 13, 65–74. [Google Scholar]
- Ong, A.K.; Chen, M.I.; Lin, L.; Tan, A.S.; Nwe, N.W.; Barkham, T.; Tay, S.Y.; Leo, Y.S. Improving the Clinical Diagnosis of Influenza—A Comparative Analysis of New Influenza A (H1N1) Cases. PLoS ONE 2009, 4, e8453. [Google Scholar] [CrossRef]
- Rejmanek, D.; Hosseini, P.R.; Mazet, J.A.K.; Daszak, P. Evolutionary Dynamics and Global Diversity of Influenza a Virus. J. Virol. 2015, 89, 10993–11001. [Google Scholar] [CrossRef]
- Pérez-Rivera, C.M.; López, M.S.; Arnaud, F.G.; Carreón, N.R. Detection of antibodies against pathogens in feral and domestic pigs (Sus scrofa) at the Sierra La Laguna Biosphere Reserve, Mexico. Vet. México 2017, 4, 1–11. [Google Scholar]
- Linnaeus, C. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. JB Delamolliere 1758, 10, 1–824. [Google Scholar]
- Wehr, N.; Hessand, S.; Litton, C. Biology and Impacts of Pacific Island Invasive Species. 14. Sus scrofa, the Feral pig (Artiodactyla: Suidae). Pac. Sci. 2017, 72, 1–41. [Google Scholar] [CrossRef]
- Mayer, J.J.; Brisbin, L. Wild Pigs: Biology, Damage, Control Techniques and Management; Savannah River National Laboratory Aiken: Jackson, SC, USA, 2000; pp. 5–205. [Google Scholar]
- Adeola, A.C.; Oluwole, O.O.; Oladele, B.M.; Olorungbounmi, T.O.; Boladuro, B.; Olaogun, S.C.; Peng, M.S. Analysis of the genetic variation in mitochondrial DNA, Y—Chromosome sequences, and MC1R sheds light on the ancestry of Nigerian indigenous pigs. Genet. Sel. Evol. 2017, 49, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Garcia, M.N.; Ballari, S.A. Impact of wild boar (Sus scrofa) in its introduced and native range: A review. Biol. Invasions 2012, 14, 2283–2300. [Google Scholar] [CrossRef]
- Ditchkoff, S.S.; West, B.C. Ecology and management of feral pigs. Hum. Wildl. Confl. 2007, 1, 149–151. [Google Scholar]
- Hidalgo-Mihart, M.G.; Pérez-Hernández, D.; Pérez-Solano, L.A.; Contreras-Moreno, F.; Angulo-Morales, J.; Hernández-Nava, J. Primer registro de una población de cerdos asilvestrados en el área de la Laguna de Términos, Campeche, México. Rev. Mex. De Biodivers. 2014, 85, 990–994. [Google Scholar] [CrossRef]
- Álvarez-Romero, J.G.; Medellín, R.A.; Oliveras, I.A.; Gómez de Silva, H.; Sánchez, O. Animales Exóticos en México: Una Amenaza Para la Biodiversidad, 1st ed.; Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad, Instituto de Ecología, UNAM; Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2008; p. 518. [Google Scholar]
- West, B.C.; Cooper, A.L.; Armstrong, J.B. Managing wild pigs: A technical guide. Hum. -Wildl. Interact. Monogr. 2009, 1, 1–55. [Google Scholar]
- Solís-Cámara, A.B.; Arnaud-Franco, G.; Álvarez, S.; Galina-Tessaro, P.; José, J. Evaluación de la población de cerdos asilvestrados (Sus scrofa) y su impacto en la Reserva de la Biosfera Sierra La Laguna, Baja California Sur, México. Trop. Conserv. Sci. 2009, 2, 173–188. [Google Scholar] [CrossRef]
- Tisdell, C.A.; Oxford, S.; York, N.; Paris, T. Wild Pigs: Environmental Pest or Economic Resource? Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species. A Selection from the Global Invasive Species Databas; Invasive Species Specialist Group: Auckland, New Zealand, 2000; Volume 12, p. 3. [Google Scholar]
- OIE Manual de las Pruebas de Diagnóstico y de las Vacunas para los Animales Terrestres 2017. Available online: https://www.oie.int/es/normas/manual-terrestre/ (accessed on 20 December 2019).
- Del Castillo, C.E.; Gómez, A.F. Texto y Cuaderno de Trabajo de Laboratorio de Virología; FMVZ-UNAM: Mexico City, Mexico, 2010. [Google Scholar]
- Saavedra-Montañez, M.; Vaca, L.; Ramírez-Mendoza, H.; Gaitán-Peredo, C.; Bautista-Martínez, R.; Segura-Velázquez, R.; Sánchez-Betancourt, J.I. Identification and genomic characterization of influenza viruses with different origin in Mexican pigs. Transbound. Emerg. Dis. 2018, 2019, 186–194. [Google Scholar] [CrossRef]
- Martínez, W. Estudio integrado del grado de antropización (INRA) a escala de paisaje: Propuesta metodológica y evaluación. INTROPICA 2010, 5, 45–54. [Google Scholar]
- Mcilroy, J.C. New techniques for an old problem—Recent advances in feral pig control in Australia. Manag. Commun. 1990, 3, 241–244. [Google Scholar]
- Hampton, J.; Pluske, J.; Spencer, P. A preliminary genetic study of the social biology of feral pigs in south-western Australia and the implications for management. Wildl. Res. 2004, 31, 375–381. [Google Scholar] [CrossRef]
- Salbosa, L.L.; Lepczyk, C. Movement in a Hawaiian Forest Ecosystem Using GPS Satellite Collars. Nat. Preced. 2009, 3903, 1–16. [Google Scholar]
- Martínez-Dueñas, W.A. Diversidad y distribución horizontal de Calliphoridae (Insecta: Diptera) en un valle interandino con diferentes grados de antropización (Popayán—Colombia). In Tesis de Pregrado, Departamento de Biología, Facultad de Ciencias Naturales, Exactas y de la Educación; Universidad del Cauca: Popayán, Colombia, 2003; p. 142. [Google Scholar]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 14: Logistic regression. Crit. Care 2005, 9, 11–118. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.E.; Sun, H.; Carrel, M.; Cunningham, F.L.; Baroch, J.A.; Hanson-Dorr, K.C.; Smith, D.R. Feral Swine in the United States Have Been Exposed to both Avian and Swine Influenza A Viruses. Appl. Environ. Microbiol. 2017, 83, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Bauer, N.E.; Rodgers, S.; Bazan, L.R.; Mesenbrink, B.T.; Gidlewski, T. Antibodies to various zoonotic pathogens detected in feral swine (Sus scrofa) at abattoirs in Texas, USA. J. Food Prot. 2017, 80, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.K.W.; Poon, L.E.O.L.M. Biology of Influenza A Virus. Ann. N. Y. Acad. Sci. 2007, 25, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.I.; Gramer, M.R.; Vincent, A.L.; Holmes, E.C. Global transmission of influenza viruses from humans to swine. J. Gen. Virol. 2012, 93, 2195–2203. [Google Scholar] [CrossRef]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and Disease Emergence: Dynamics at the Wildlife–Livestock–Human Interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef]
- Nelson, M.I.; Vincent, A.L. Reverse zoonosis of influenza to swine: New perspectives on the human-animal interface. Trends Microbiol. 2015, 23, 142–153. [Google Scholar] [CrossRef]
- Pedersen, K.; Turnage, C.T.; Gaston, W.D.; Arruda, P.; Alls, S.A.; Gidlewski, T. Pseudorabies detected in hunting dogs in Alabama and Arkansas after close contact with feral swine (Sus scrofa). BMC Vet. Res. 2018, 14, 1–7. [Google Scholar] [CrossRef]
- Spencer, P.B.S.; Hampton, J.O. Illegal translocation and genetic structure of feral pigs in Western Australia. J. Wildl. Manag. 2005, 69, 377–384. [Google Scholar] [CrossRef]
- Goedbloed, D.J.; Hooft, P.; Van, Megens, H.; Langenbeck, K.; Lutz, W.; Crooijmans, R.P.M.A.; Prins, H.H.T. Reintroductions and genetic introgression from domestic pigs have shaped the genetic population structure of Northwest European wild boar. BMC Genet. 2013, 14, 1–10. [Google Scholar] [CrossRef]
- Hernández, F.A.; Parker, B.M.; Pylant, C.L.; Smyser, T.J.; Piaggio, A.J.; Lance, S.L.; Milleson, M.P.; Austin, J.D.; Wisely, S.M. Invasion ecology of wild pigs (Sus scrofa) in Florida, USA: The role of humans in the expansion and colonization of an invasive wild ungulate. Biol. Invasions 2018, 20, 1865–1880. [Google Scholar] [CrossRef]
- Tabak, M.A.; Piaggio, A.J.; Miller, R.S.; Swetizer, R.A.; Ernest, H.B. Anthropogenic factors predict movement of an invasive species. Ecosphere 2017, 8, e01844. [Google Scholar] [CrossRef]
- Sun, H.; Cunningham, F.L.; Harris, J.; Xu, Y.; Long, L.P.; Hanson-Dorr, K.; Wan, X.F. Dynamics of virus shedding and antibody responses in influenza A virus-infected feral swine. J. Gen. Virol. 2015, 96, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.M.; Pedersen, K.; Wan, X.F.; Cunningham, F.L.; Webb, C.T.; Wilber, M.Q. Individual-Level Antibody Dynamics Reveal Potential Drivers of Influenza A Seasonality in Wild Pig Populations. Integr. Comp. Biol. 2019, 59, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, A.E.; Peck, H.A.; Hurt, A.C.; Cooke, J.; Cassey, P. Short Communication Proposed Surveillance for Influenza A in Feral pigs. EcoHealth 2016, 13, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Graaf, M.D.; Fouchier, R.A.M. Role of receptor binding specificity in influenza a virus transmission and pathogenesis. EMBO J. 2014, 33, 823–841. [Google Scholar] [CrossRef] [PubMed]
- Rossi Eraud, C.; Gilot, E.; Rossi, S. Innate Immunity Correlates with Host Fitness in Wild Boar (Sus scrofa) Exposed to Classical Swine Fever. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef]
- Song, Q.Q.; Zhang, F.X.; Liu, J.J.; Ling, Z.S.; Zhu, Y.L.; Jiang, S.J.; Xie, Z.J. Dog to dog transmission of a novel influenza virus (H5N2) isolated from a canine. Vet. Microbiol. 2013, 161, 331–333. [Google Scholar] [CrossRef]
- Lee, J.H.; Pascua, P.N.Q.; Song, M.; Baek, Y.H.; Kim, C.; Choi, H.; Choi, Y.K. Isolation and Genetic Characterization of H5N2 Influenza Viruses from Pigs in Korea. J. Virol. 2009, 83, 4205–4215. [Google Scholar] [CrossRef]
- Villarreal Chávez, C.; Rivera Cruz, E. An Update on Avian Influenza in Mexico. Avian Dis. 2003, 47, 1002–1005. [Google Scholar] [CrossRef]
- INEGI Censo Agrícola, Ganadero y Forestal 2007. Available online: http://www.inegi.org.mx/est/contenidos/proyectos/agro/ca2007/resultados_agricola//resultados_agricola/ (accessed on 20 August 2019).
- Guang-jian, Z.; Zong-shuai, L.; Yan-li, Z.; Shi-jin, J.; Zhi-jing, X. Genetic characterization of a novel influenza A virus H5N2 isolated from a dog in China. Vet. Microbiol. 2012, 155, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Novelo, E.; Ruíz-Piña, H.; Escobedo-Ortegón, J.; Rodríguez-Vivas, I.; Bolio González, M.; Polanco-Rodríguez, A.; Manrique-Saide, P. Situación actual y perspectivas para el estudio de las enfermedades zoonóticas emergentes, reemergentes y olvidadas en la Península de Yucatán, México. Trop. Subtrop. Agroecosystems 2011, 14, 35–54. [Google Scholar]
- Rulli, M.C.; Santini, M.; Hayman, D.T.S.; Odorico, P.D. The nexus between forest fragmentation in Africa and Ebola virus disease outbreaks. Nat. Publ. Group 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.; França, F.; Gardner, T.A.; Hicks, C.C.; Lennox, G.D.; Berenguer, E.; Castello, L.; Economo, E.P.; Ferreira, J.; Guénard, B. The future of hyperdiverse tropical ecosystems. Nat. Res. 2018, 559, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Merenlender, A.M. Landscapes that work for biodiversity and people. Science 2018, 362, 1–9. [Google Scholar] [CrossRef]

| Variable | n | H1N1hu * | HI1N1sw * | H1N2sw * | H3N2sw * | H5N2av * |
|---|---|---|---|---|---|---|
| Total | 61 | |||||
| Seropositivity (Frequency % [CI: 5.85–23.65]) | 9 (14.75) | 7 (11.47) | 5 (8.19) | 0 (0) | 0 (0) | 1 (1.63) |
| Ab titer (mean) Range of observations | 595 80–2560 | 144 80–320 | 0 10 | 0 10 | 80 80 | |
| Sex | ||||||
| Female | 4 | 4 | 2 | 0 | 0 | 0 |
| Male | 5 | 3 | 3 | 0 | 0 | 1 |
| Age | ||||||
| Piglet | 4 | 4 | 3 | 0 | 0 | 0 |
| Juvenile | 2 | 2 | 0 | 0 | 0 | 0 |
| Adult | 3 | 1 | 2 | 0 | 0 | 1 |
| Variable | n | Nasal Swab | Trachea | Lung |
|---|---|---|---|---|
| Total | 61 | |||
| Positive (Frequency % [CI: 9.70–29.65]) | 12 (19.67) | 8 (13.11) | 3 (4.91) | 1 (1.63) |
| Ct value (mean) Range of observations | 32.94 28.43–34.36 | 32.43 31.20–34.39 | 34.19 34.19 | |
| Sex | ||||
| Female | 6 | 4 | 1 | 1 |
| Male | 6 | 4 | 2 | 0 |
| Age | ||||
| Piglet | 3 | 3 | 0 | 0 |
| Juvenile | 2 | 2 | 0 | 0 |
| Adult | 7 | 3 | 3 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maya-Badillo, B.A.; Ojeda-Flores, R.; Chaves, A.; Reveles-Félix, S.; Orta-Pineda, G.; Martínez-Mercado, M.J.; Saavedra-Montañez, M.; Segura-Velázquez, R.; Sanvicente, M.; Sánchez-Betancourt, J.I. Eco-Epidemiological Evidence of the Transmission of Avian and Human Influenza A Viruses in Wild Pigs in Campeche, Mexico. Viruses 2020, 12, 528. https://doi.org/10.3390/v12050528
Maya-Badillo BA, Ojeda-Flores R, Chaves A, Reveles-Félix S, Orta-Pineda G, Martínez-Mercado MJ, Saavedra-Montañez M, Segura-Velázquez R, Sanvicente M, Sánchez-Betancourt JI. Eco-Epidemiological Evidence of the Transmission of Avian and Human Influenza A Viruses in Wild Pigs in Campeche, Mexico. Viruses. 2020; 12(5):528. https://doi.org/10.3390/v12050528
Chicago/Turabian StyleMaya-Badillo, Brenda Aline, Rafael Ojeda-Flores, Andrea Chaves, Saul Reveles-Félix, Guillermo Orta-Pineda, María José Martínez-Mercado, Manuel Saavedra-Montañez, René Segura-Velázquez, Mauro Sanvicente, and José Iván Sánchez-Betancourt. 2020. "Eco-Epidemiological Evidence of the Transmission of Avian and Human Influenza A Viruses in Wild Pigs in Campeche, Mexico" Viruses 12, no. 5: 528. https://doi.org/10.3390/v12050528
APA StyleMaya-Badillo, B. A., Ojeda-Flores, R., Chaves, A., Reveles-Félix, S., Orta-Pineda, G., Martínez-Mercado, M. J., Saavedra-Montañez, M., Segura-Velázquez, R., Sanvicente, M., & Sánchez-Betancourt, J. I. (2020). Eco-Epidemiological Evidence of the Transmission of Avian and Human Influenza A Viruses in Wild Pigs in Campeche, Mexico. Viruses, 12(5), 528. https://doi.org/10.3390/v12050528