Innate Type 2 Responses to Respiratory Syncytial Virus Infection
Abstract
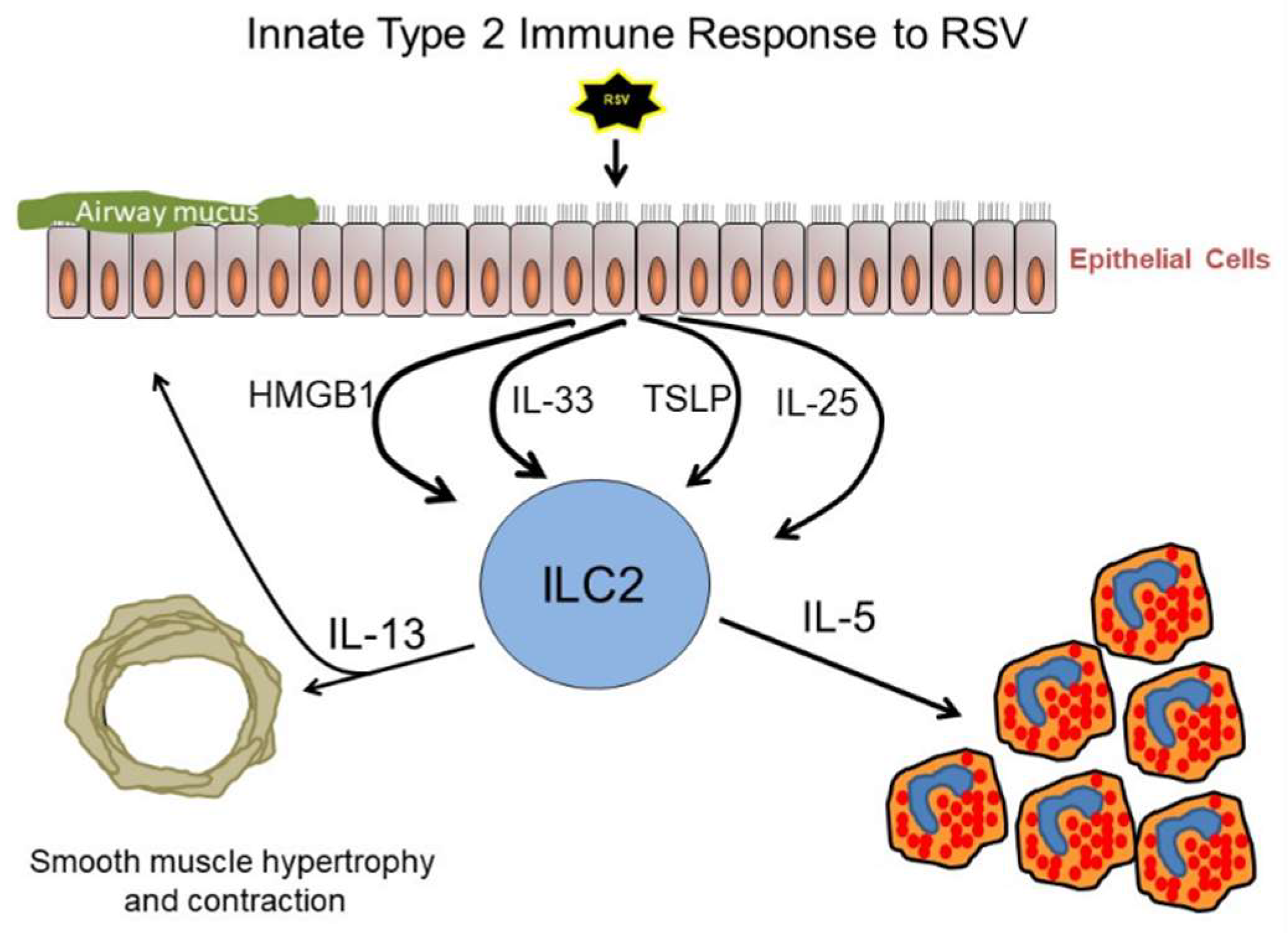
1. Introduction
2. Group 2 Innate Lymphoid Cells (ILC2)
3. Thymic Stromal Lymphopoietin (TSLP)
4. Interleukin-33 (IL-33)
5. High Mobility Group Box 1 (HMGB1)
6. Interleukin-25 (IL-25)
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leader, S.; Kohlhase, K. Respiratory syncytial virus-coded pediatric hospitalizations. 1997 to 1999. Pediatr. Infect. Dis. J. 2002, 21, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, G.; Perez, M.K. Respiratory syncytial virus infection and bronchiolitis. Pediatr. Rev. 2014, 35, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Plesca, D.A.; Cora, F.; Buzoianu, E.; Moiceanu, M.; Hurduc, V. Risk factors for severe bronchiolitis—A retrospective study. Eur. Respir. J. 2012, 40, 4660. [Google Scholar]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory syncytial virus—A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef]
- Hashem, M.; Hall, C.B. Respiratory syncytial virus in healthy adults: The cost of a cold. J. Clin. Virol. 2003, 27, 14–21. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Falsey, A.R.; Walsh, E.E. Respiratory syncytial virus infection in elderly adults. Drugs Aging 2005, 22, 577–587. [Google Scholar] [CrossRef]
- Talbot, H.K.; Belongia, E.A.; Walsh, E.E.; Schaffner, W. Respiratory Syncytial Virus in Older Adults: A Hidden Annual Epidemic. Infect. Dis. Clin. Pract. 2016, 24, 295–302. [Google Scholar] [CrossRef]
- Smith, D.K.; Seales, S.; Budzik, C. Respiratory syncytial virus bronchiolitis in children. Am. Fam. Physician 2017, 95, 94–99. [Google Scholar]
- Walsh, E.E. Respiratory Syncytial Virus Infection: An Illness for All Ages. Clin. Chest Med. 2017, 38, 29–36. [Google Scholar] [CrossRef]
- Johnson, J.E.; Gonzales, R.A.; Olson, S.J.; Wright, P.F.; Graham, B.S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 2007, 20, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Proesmans, M. New therapies for acute RSV infections: Where are we? Eur. J. Pediatr. 2019, 178, 131–138. [Google Scholar] [CrossRef] [PubMed]
- DeVincenzo, J.P. Therapy of respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2000, 19, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Zhurilo, N.I.; Chudinov, M.V.; Matveev, A.V.; Smirnova, O.S.; Konstantinova, I.D.; Miroshnikov, A.I.; Prutkov, A.N.; Grebenkina, L.E.; Pulkova, N.V.; Shvets, V.I. Isosteric ribavirin analogues: Synthesis and antiviral activities. Bioorg. Med. Chem. Lett. 2018, 28, 11–14. [Google Scholar] [CrossRef]
- Kim, J.A.; Seong, R.K.; Kumar, M.; Shin, O.S. Favipiravir and ribavirin inhibit replication of Asian and African strains of zika virus in different cell models. Viruses 2018, 10, 72. [Google Scholar] [CrossRef]
- Turner, T.L.; Kopp, B.T.; Paul, G.; Landgrave, L.C.; Hayes, D.; Thompson, R. Respiratory syncytial virus: Current and emerging treatment options. Clin. Outcomes Res. 2014, 6, 217–225. [Google Scholar] [CrossRef]
- Sun, Z.; Pan, Y.; Jiang, S.; Lu, L. Respiratory syncytial virus entry inhibitors targeting the F protein. Viruses 2013, 5, 211–225. [Google Scholar] [CrossRef]
- Ventre, K.; Randolph, A.G. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst. Rev. 2007, 24, CD000181. [Google Scholar]
- Schickli, J.H.; Whitacre, D.C.; Tang, R.S.; Kaur, J.; Lawlor, H.; Peters, C.J.; Jones, J.E.; Peterson, D.L.; McCarthy, M.P.; van Nest, G.; et al. Palivizumab epitope-displaying virus-like particles protect rodents from RSV challenge. J. Clin. Investig. 2015, 125, 1637–1647. [Google Scholar] [CrossRef]
- Connor, E.M. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in hieh-risk infants. the IMpact-RSV study group. Radiology 1999, 210, 295–296. [Google Scholar]
- Feltes, T.F.; Cabalka, A.K.; Meissner, H.C.; Piazza, F.M.; Carlin, D.A.; Top, F.H.; Connor, E.M.; Sondheimer, H.M. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J. Pediatr. 2003, 143, 532–540. [Google Scholar] [CrossRef]
- Behzadi, M.A.; Leyva-Grado, V.H. Overview of current therapeutics and novel candidates against influenza, respiratory syncytial virus, and Middle East respiratory syndrome coronavirus infections. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, A.; Peneva, D.; Incerti, D.; McLaurin, K.; Stevens, W. Assessing Variation in the Cost of Palivizumab for Respiratory Syncytial Virus Prevention in Preterm Infants. Pharm. Open 2018, 2, 53–61. [Google Scholar] [CrossRef]
- Wegzyn, C.; Toh, L.K.; Notario, G.; Biguenet, S.; Unnebrink, K.; Park, C.; Makari, D.; Norton, M. Safety and Effectiveness of Palivizumab in Children at High Risk of Serious Disease Due to Respiratory Syncytial Virus Infection: A Systematic Review. Infect. Dis. Ther. 2014, 3, 133–158. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, N.M.; Gentile, A.; Lucion, F.; Nokes, D.J.; Munywoki, P.K.; Madhi, S.A.; Groome, M.J.; Cohen, C.; Moyes, J.; Thorburn, K.; et al. Global respiratory syncytial virus-associated mortality in young children, (RSV GOLD): A retrospective case series. Lancet Glob. Health 2017, 5, e984–e991. [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef]
- Russell, C.D.; Unger, S.A.; Walton, M.; Schwarze, J. The human immune response to respiratory syncytial virus infection. Clin. Microbiol. Rev. 2017, 30, 481–502. [Google Scholar] [CrossRef]
- Johnson, T.R.; Hong, S.; van Kaer, L.; Koezuka, Y.; Graham, B.S. NK T Cells Contribute to Expansion of CD8+ T Cells and Amplification of Antiviral Immune Responses to Respiratory Syncytial Virus. J. Virol. 2002, 76, 4294–4303. [Google Scholar] [CrossRef]
- Kaiko, G.E.; Phipps, S.; Angkasekwinai, P.; Dong, C.; Foster, P.S. NK Cell Deficiency Predisposes to Viral-Induced Th2-Type Allergic Inflammation via Epithelial-Derived IL-25. J. Immunol. 2010, 185, 4681–4690. [Google Scholar] [CrossRef]
- Openshaw, P.J.M.; Dean, G.S.; Culley, F.J. Links between respiratory syncytial virus bronchiolitis and childhood asthma: Clinical and research approaches. Pediatr. Infect. Dis. J. 2003, 22, S58–S65. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, G. Respiratory syncytial virus and asthma: Speed-dating or long-term relationship? Curr. Opin. Pediatr. 2013, 25, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Sigurs, N.; Aljassim, F.; Kjellman, B.; Robinson, P.D.; Sigurbergsson, F.; Bjarnason, R.; Gustafsson, P.M. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010. [Google Scholar] [CrossRef] [PubMed]
- Tse, S.M.; Coull, B.A.; Sordillo, J.E.; Datta, S.; Gold, D.R. Gender- and age-specific risk factors for wheeze from birth through adolescence. Pediatr. Pulmonol. 2015, 50, 955–962. [Google Scholar] [CrossRef]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef]
- Osman, M.; Tagiyeva, N.; Wassall, H.J.; Ninan, T.K.; Devenny, A.M.; McNeill, G.; Helms, P.J.; Russell, G. Changing trends in sex specific prevalence rates for childhood asthma, eczema, and hay fever. Pediatr. Pulmonol. 2007, 42, 60–65. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Gourgiotis, D.; Javadyan, A.; Bossios, A.; Kallergi, K.; Psarras, S.; Tsolia, M.N.; Kafetzis, D. Does respiratory syncytial virus subtype influences the severity of acute bronchiolitis in hospitalized infants? Respir. Med. 2004, 98, 879–882. [Google Scholar] [CrossRef]
- Klassen, T.P.; Sutcliffe, T.; Watters, L.K.; Wells, G.A.; Allen, U.D.; Li, M.M. Dexamethasone in salbutamol-treated inpatients with acute bronchiolitis: A randomized, controlled trial. J. Pediatr. 1997, 130, 191–196. [Google Scholar] [CrossRef]
- Plint, A.C.; Johnson, D.W.; Patel, H.; Wiebe, N.; Correll, R.; Brant, R.; Mitton, C.; Gouin, S.; Bhatt, M.; Joubert, G.; et al. Epinephrine and dexamethasone in children with bronchiolitis. N. Engl. J. Med. 2009, 360, 2079–2089. [Google Scholar] [CrossRef]
- Corneli, H.M.; Zorc, J.J.; Majahan, P.; Shaw, K.N.; Holubkov, R.; Reeves, S.D.; Ruddy, R.M.; Malik, B.; Nelson, K.A.; Bregstein, J.S.; et al. A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. N. Engl. J. Med. 2007, 357, 331–339. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, J.; Du, L.; Tang, Y. Association of cytokine responses with disease severity in infants with respiratory syncytial virus infection. Acta Paediatr. 2007, 91, 914–922. [Google Scholar] [CrossRef]
- Mukherjee, S.; Lukacs, N.W. Association of IL-13 in respiratory syncytial virus-induced pulmonary disease: Still a promising target. Expert Rev. Anti-Infect. Ther. 2010, 8, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, N.W.; Moore, M.L.; Rudd, B.D.; Berlin, A.A.; Collins, R.D.; Olson, S.J.; Ho, S.B.; Peebles, R.S. Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am. J. Pathol. 2006, 169, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.L.; Chi, M.H.; Luongo, C.; Lukacs, N.W.; Polosukhin, V.V.; Huckabee, M.M.; Newcomb, D.C.; Buchholz, U.J.; Crowe, J.E.; Goleniewska, K.; et al. A Chimeric A2 Strain of Respiratory Syncytial Virus, RSV) with the Fusion Protein of RSV Strain Line 19 Exhibits Enhanced Viral Load, Mucus, and Airway Dysfunction. J. Virol. 2009, 83, 4185–4194. [Google Scholar] [CrossRef]
- Stokes, K.L.; Chi, M.H.; Sakamoto, K.; Newcomb, D.C.; Currier, M.G.; Huckabee, M.M.; Lee, S.; Goleniewska, K.; Pretto, C.; Williams, J.V.; et al. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J. Virol. 2011, 85, 5782–5793. [Google Scholar] [CrossRef]
- Chapman, D.G.; Irvin, C.G. Mechanisms of airway hyper-responsiveness in asthma: The past, present and yet to come. Clin. Exp. Allergy 2015, 45, 706–719. [Google Scholar] [CrossRef]
- Zhu, Z.; Homer, R.J.; Wang, Z.; Chen, Q.; Geba, G.P.; Wang, J.; Zhang, Y.; Elias, J.A. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 1999, 103, 779–788. [Google Scholar] [CrossRef]
- Greenfeder, S.; Umland, S.P.; Cuss, F.M.; Chapman, R.W.; Egan, R.W. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir. Res. 2001, 2, 71–79. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Dyer, K.D.; Domachowske, J.B. Respiratory viruses and eosinophils: Exploring the connections. Antiviral Res. 2009, 83, 1–9. [Google Scholar] [CrossRef]
- Callaway, Z.; Kim, C.-K. Respiratory Viruses, Eosinophilia and Their Roles in Childhood Asthma. Int. Arch. Allergy Immunol. 2011, 155, 1–11. [Google Scholar] [CrossRef]
- Cohn, L.; Homer, R.J.; Marinov, A.; Rankin, J.; Bottomly, K. Induction of airway mucus production by T helper 2 (Th2) cells: A critical role for interleukin 4 in cell recruitment but not mucus production. J. Exp. Med. 1997, 186, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Steinke, J.W.; Borish, L. Th2 cytokines and asthma. Interleukin-4: Its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir. Res. 2001, 2, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.L.; Bellosi, A.; Hardman, C.S.; Drynan, L.F.; Wong, S.H.; Cruickshank, J.P.; McKenzie, A.N.J. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 2012, 129, 191–198. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Krauß, R.H.; Sun, A.C.; Takei, F. Lung Natural Helper Cells Are a Critical Source of Th2 Cell-Type Cytokines in Protease Allergen-Induced Airway Inflammation. Immunity 2012, 36, 451–463. [Google Scholar] [CrossRef]
- Chang, Y.J.; Kim, H.Y.; Albacker, L.A.; Baumgarth, N.; McKenzie, A.N.J.; Smith, D.E.; Dekruyff, R.H.; Umetsu, D.T. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011, 12, 631–638. [Google Scholar] [CrossRef]
- Hong, J.Y.; Bentley, J.K.; Chung, Y.; Lei, J.; Steenrod, J.M.; Chen, Q.; Sajjan, U.S.; Hershenson, M.B. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J. Allergy Clin. Immunol. 2014, 134. [Google Scholar] [CrossRef]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.A.; Bucks, C.; Kane, C.M.; Fallon, P.G.; Pannell, R.; et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464, 1367–1370. [Google Scholar] [CrossRef]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.I.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate production of TH 2 cytokines by adipose tissue-associated c-Kit+ Sca-1+ lymphoid cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef]
- Price, A.E.; Liang, H.E.; Sullivan, B.M.; Reinhardt, R.L.; Eisley, C.J.; Erle, D.J.; Locksley, R.M. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 11489–11494. [Google Scholar] [CrossRef]
- Camelo, A.; Rosignoli, G.; Ohne, Y.; Stewart, R.A.; Overed-Sayer, C.; Sleeman, M.A.; May, R.D. IL-33, IL-25, and TSLP induce a distinct phenotypic and activation profile in human type 2 innate lymphoid cells. Blood Adv. 2017, 1, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Hinde, H.L.; Wu, Q.; Bentley, J.K.; Han, M.B.M.; Rajput, C.; Hong, J.Y.; Lei, J. The Innate Cytokines IL-25, IL-33, and TSLP Cooperate in the Induction of Type 2 Innate Lymphoid Cell Expansion and Mucous Metaplasia in Rhinovirus-Infected Immature Mice. J. Immunol. 2017, 199, 1308–1318. [Google Scholar] [CrossRef]
- Zhang, K.; Jin, Y.; Lai, D.; Wang, J.; Wang, Y.; Wu, X.; Scott, M.; Li, Y.; Hou, J.; Billiar, T.; et al. RAGE-induced ilc2 expansion in acute lung injury due to haemorrhagic shock. Thorax 2020, 75, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Dupilumab: First Global Approval. Drugs 2017, 77, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in Persistent Asthma with Elevated Eosinophil Levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef]
- Efficacy and Safety Study of GSK3772847 in Subjects with Moderately Severe Asthma. Available online: https://clinicaltrials.gov/ct2/show/NCT03207243 (accessed on 30 April 2020).
- Repeat Dose Study of GSK3772847 in Participants with Moderate to Severe Asthma with Allergic Fungal Airway Disease (AFAD). Available online: https://clinicaltrials.gov/ct2/show/NCT03393806 (accessed on 30 April 2020).
- Evaluation of SAR440340 and as Combination Therapy with Dupilumab in Moderate-To-Severe Asthma Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT03387852 (accessed on 30 April 2020).
- Proof of Concept Study to Investigate ANB020 Activity in Adult Patients with Severe Eosinophilic Asthma. Available online: https://clinicaltrials.gov/ct2/show/NCT03469934?term=ANB020&draw=2&rank=1 (accessed on 30 April 2020).
- Etokimab—AnaptysBio. Available online: https://www.anaptysbio.com/pipeline/etokimab/ (accessed on 30 April 2020).
- Klose, C.S.N.; Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016, 17, 765–774. [Google Scholar] [CrossRef]
- Kabata, H.; Moro, K.; Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol. Rev. 2018, 286, 37–52. [Google Scholar] [CrossRef]
- Ishii, T.; Muroi, M.; Horiguchi, K.; Tanamoto, K.; Nagase, T.; Yamashita, N. Activation through toll-like receptor 2 on group 2 innate lymphoid cells can induce asthmatic characteristics. Clin. Exp. Allergy 2019, 49, 1624–1632. [Google Scholar] [CrossRef]
- Maggi, L.; Montaini, G.; Mazzoni, A.; Rossettini, B.; Capone, M.; Rossi, M.C.; Santarlasci, V.; Liotta, F.; Rossi, O.; Gallo, O.; et al. Human circulating group 2 innate lymphoid cells can express CD154 and promote IgE production. J. Allergy Clin. Immunol. 2017, 139, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.D.; Siefker, D.; Jones, T.L.; You, D.; Taylor, R.; DeVincenzo, J.; Cormier, S.A. Elevated levels of type 2 respiratory innate lymphoid cells in human infants with severe respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 2019, 200, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Stier, M.T.; Goleniewska, K.; Cephus, J.Y.; Newcomb, D.C.; Sherrill, T.P.; Boyd, K.L.; Bloodworth, M.H.; Moore, M.L.; Chen, K.; Kolls, J.K.; et al. STAT1 Represses Cytokine-Producing Group 2 and Group 3 Innate Lymphoid Cells during Viral Infection. J. Immunol. 2017, 199, 510–519. [Google Scholar] [CrossRef]
- De Weerd, N.A.; Nguyen, T. The interferons and their receptors-distribution and regulation. Immunol. Cell Biol. 2012, 90, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Parronchi, P.; De Carli, M.; Manetti, R.; Simonelli, C.; Sampognaro, S.; Piccinni, M.P.; Macchia, D.; Maggi, E.; del Prete, G.; Romagnani, S. IL-4 and IFN (alpha and gamma) exert opposite regulatory effects on the development of cytolytic potential by Th1 or Th2 human T cell clones. J. Immunol. 1992, 149, 2977–2983. [Google Scholar]
- Thwaites, R.S.; Coates, M.; Ito, K.; Ghazaly, M.; Feather, C.; Abdulla, F.; Tunstall, T.; Jain, P.; Cass, L.; Rapeport, G.; et al. Reduced nasal viral load and IFN responses in infants with respiratory syncytial virus bronchiolitis and respiratory failure. Am. J. Respir. Crit. Care Med. 2018, 198, 1074–1084. [Google Scholar] [CrossRef]
- Jenkins, S.J.; Perona-Wright, G.; Worsley, A.G.F.; Ishii, N.; MacDonald, A.S. Dendritic Cell Expression of OX40 Ligand Acts as a Costimulatory, Not Polarizing, Signal for Optimal Th2 Priming and Memory Induction In Vivo. J. Immunol. 2007, 179, 3515–3523. [Google Scholar] [CrossRef]
- Wu, J.; Cui, Y.; Zhu, W.; Bai, S.; Zhao, N.; Liu, B. Critical role of OX40/OX40L in ILC2-mediated activation of CD4+T cells during respiratory syncytial virus infection in mice. Int. Immunopharmacol. 2019, 76, 105784. [Google Scholar] [CrossRef]
- Han, X.; Bai, S.; Cui, Y.; Zhu, W.; Zhao, N.; Liu, B. Essential role of CD4+ T cells for the activation of group 2 innate lymphoid cells during respiratory syncytial virus infection in mice. Immunotherapy 2019, 11, 1303–1313. [Google Scholar] [CrossRef]
- Ziegler, S.F.; Artis, D. Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 2010, 11, 289–293. [Google Scholar] [CrossRef]
- He, R.; Geha, R.S. Thymic stromal lymphopoietin. Ann. N. Y. Acad. Sci. 2010, 1183, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F.; Roan, F.; Bell, B.D.; Stoklasek, T.A.; Kitajima, M.; Han, H. Advances in Pharmacology; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 66, pp. 129–155. [Google Scholar]
- Tsilingiri, K.; Fornasa, G.; Rescigno, M. Thymic Stromal Lymphopoietin: To Cut a Long Story Short. CMGH 2017, 3, 174–182. [Google Scholar] [CrossRef]
- Ying, S.; O’Connor, B.; Ratoff, J.; Meng, Q.; Mallett, K.; Cousins, D.; Robinson, D.; Zhang, G.; Zhao, J.; Lee, T.H.; et al. Thymic Stromal Lymphopoietin Expression Is Increased in Asthmatic Airways and Correlates with Expression of Th2-Attracting Chemokines and Disease Severity. J. Immunol. 2005, 174, 8183–8190. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Lv, Z.; Li, Y.; Chen, Y.; Huang, K.; Corrigan, C.J.; Ying, S. Elevated Expression of IL-33 and TSLP in the Airways of Human Asthmatics In Vivo: A Potential Biomarker of Severe Refractory Disease. J. Immunol. 2018, 200, 2253–2262. [Google Scholar] [CrossRef]
- Toki, S.; Goleniewska, K.; Zhang, J.; Zhou, W.; Newcomb, D.C.; Zhou, B.; Kita, H.; Boyd, K.L.; Peebles, R.S. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy 2020. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, M.L.; Calvo, C.; Moreira, A.; Canas, J.A.; Pozo, F.; Sastre, B.; Quevedo, S.; Casas, I.; del Pozo, V. Thymic stromal lymphopoietin, IL-33, and periostin in hospitalized infants with viral bronchiolitis. Medicine 2017, 96, e6787. [Google Scholar] [CrossRef] [PubMed]
- Izuhara, K.; Ohta, S.; Ono, J. Using periostin as a biomarker in the treatment of asthma. Allergy Asthma Immunol. Res. 2016, 8, 491–498. [Google Scholar] [CrossRef]
- Lee, H.-C.; Headley, M.B.; Loo, Y.-M.; Berlin, A.; Gale, M.; Debley, J.S.; Lukacs, N.W.; Ziegler, S.F. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J. Allergy Clin. Immunol. 2012, 130, 1187–1196. [Google Scholar] [CrossRef]
- Miazgowicz, M.M.; Elliott, M.S.; Debley, J.S.; Ziegler, S.F. Respiratory syncytial virus induces functional thymic stromal lymphopoietin receptor in airway epithelial cells. J. Inflamm. Res. 2013, 6, 53–61. [Google Scholar]
- Qiao, J.; Li, A.; Jin, X. TSLP from RSV-stimulated rat airway epithelial cells activates myeloid dendritic cells. Immunol. Cell Biol. 2011, 89, 231–238. [Google Scholar] [CrossRef]
- Han, J.; Dakhama, A.; Jia, Y.; Wang, M.; Zeng, W.; Takeda, K.; Shiraishi, Y.; Okamoto, M.; Ziegler, S.F.; Gelfand, E.W. Responsiveness to respiratory syncytial virus in neonates is mediated through thymic stromal lymphopoietin and OX40 ligand. J. Allergy Clin. Immunol. 2012, 130, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Stier, M.T.; Bloodworth, M.H.; Toki, S.; Newcomb, D.C.; Goleniewska, K.; Boyd, K.L.; Quitalig, M.; Hotard, A.L.; Moore, M.L.; Hartert, T.V.; et al. Respiratory syncytial virus infection activates IL-13–producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J. Allergy Clin. Immunol. 2016, 138, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Malinczak, C.A.; Fonseca, W.; Rasky, A.J.; Ptaschinski, C.; Morris, S.; Ziegler, S.F.; Lukacs, N.W. Sex-associated TSLP-induced immune alterations following early-life RSV infection leads to enhanced allergic disease. Mucosal Immunol. 2019, 12, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Girard, J.P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef]
- Qi, F.; Bai, S.; Wang, D.; Xu, L.; Hu, H.; Zeng, S.; Chai, R.; Liu, B. Macrophages produce IL-33 by activating MAPK signaling pathway during RSV infection. Mol. Immunol. 2017, 87, 284–292. [Google Scholar] [CrossRef]
- Qi, F.; Wang, D.; Liu, J.; Zeng, S.; Xu, L.; Hu, H.; Liu, B. Respiratory macrophages and dendritic cells mediate respiratory syncytial virus-induced IL-33 production in TLR3- or TLR7-dependent manner. Int. Immunopharmacol. 2015, 29, 408–415. [Google Scholar] [CrossRef]
- Ding, W.; Zou, G.L.; Zhang, W.; Lai, X.N.; Chen, H.W.; Xiong, L.X. Interleukin-33: Its emerging role in allergic diseases. Molecules 2018, 23, 1665. [Google Scholar] [CrossRef]
- Kearley, J.; Barker, J.E.; Robinson, D.S.; Lloyd, C.M. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 2005, 202, 1539–1547. [Google Scholar] [CrossRef]
- Saravia, J.; You, D.; Shrestha, B.; Jaligama, S.; Siefker, D.; Lee, G.I.; Harding, J.N.; Jones, T.L.; Rovnaghi, C.; Bagga, B.; et al. Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33. PLoS Pathog. 2015, 11, e1005217. [Google Scholar] [CrossRef]
- Bloodworth, M.H.; Rusznak, M.; Pfister, C.C.; Zhang, J.; Bastarache, L.; Calvillo, S.A.; Chappell, J.D.; Boyd, K.L.; Toki, S.; Newcomb, D.C.; et al. Glucagon-like peptide 1 receptor signaling attenuates respiratory syncytial virus–induced type 2 responses and immunopathology. J. Allergy Clin. Immunol. 2018, 142, 683–687. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Qi, F.; Zeng, S.; Xu, L.; Hu, H.; Wang, D.; Liu, B. Natural helper cells contribute to pulmonary eosinophilia by producing IL-13 via IL-33/ST2 pathway in a murine model of respiratory syncytial virus infection. Int. Immunopharmacol. 2015, 28, 337–343. [Google Scholar] [CrossRef]
- Saluzzo, S.; Gorki, A.D.; Rana, B.M.J.; Martins, R.; Scanlon, S.; Starkl, P.; Lakovits, K.; Hladik, A.; Korosec, A.; Sharif, O.; et al. First-Breath-Induced Type 2 Pathways Shape the Lung Immune Environment. Cell Rep. 2017, 18, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Steer, C.A.; Shim, B.; Mathae, L.; Takei, F. Activation of neonatal mouse lung ILC2s by endogenous IL-33 impacts type 2 responses later in life. J. Immunol. 2018, 200, 44. [Google Scholar]
- De Kleer, I.M.; Kool, M.; de Bruijn, M.J.W.; Willart, M.; van Moorleghem, J.; Schuijs, M.J.; Plantinga, M.; Beyaert, R.; Hams, E.; Fallon, P.G.; et al. Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung. Immunity 2016, 45, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Lai, A.C.Y.; Chi, P.Y.; Thio, C.L.P.; Chen, W.Y.; Tsai, C.H.; Lee, Y.L.; Lukacs, N.W.; Chang, Y.J. Pulmonary IL-33 orchestrates innate immune cells to mediate RSV-evoked airway hyperreactivity and eosinophilia. Allergy Eur. J. Allergy Clin. Immunol. 2019. [Google Scholar] [CrossRef]
- Fonseca, W.; Malinczak, C.-A.; Schuler, C.F.; Best, S.K.K.; Rasky, A.J.; Morris, S.B.; Cui, T.X.; Popova, A.P.; Lukacs, N.W. Uric acid pathway activation during respiratory virus infection promotes Th2 immune response via innate cytokine production and ILC2 accumulation. Mucosal Immunol. 2020. [Google Scholar] [CrossRef]
- Ives, A.; Nomura, J.; Martinon, F.; Roger, T.; LeRoy, D.; Miner, J.N.; Simon, G.; Busso, N.; So, A. Xanthine oxidoreductase regulates macrophage IL1β secretion upon NLRP3 inflammasome activation. Nat. Commun. 2015, 6, 6555. [Google Scholar] [CrossRef]
- Han, M.; Ishikawa, T.; Bermick, J.R.; Rajput, C.; Lei, J.; Goldsmith, A.M.; Jarman, C.R.; Lee, J.; Bentley, J.K.; Hershenson, M.B. IL-1β prevents ILC2 expansion, type 2 cytokine secretion, and mucus metaplasia in response to early-life rhinovirus infection in mice. Allergy 2020. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, Z.; Xie, T.; Ji, J.; Xu, J.; Lin, L.; Yan, J.; Kang, A.; Dai, Q.; Dong, Y.; et al. Rhein suppresses lung inflammatory injury induced by human respiratory syncytial virus through inhibiting NLRP3 inflammasome activation via NF-κB pathway in mice. Front. Pharmacol. 2020, 10. [Google Scholar] [CrossRef]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef]
- Lee, S.A.; Kwak, M.S.; Kim, S.; Shin, J.S. The role of high mobility group box 1 in innate immunity. Yonsei Med. J. 2014, 55, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Livesey, K.M.; Cheh, C.W.; Farkas, A.; Loughran, P.; Hoppe, G.; Bianchi, M.E.; Tracey, K.J.; Zeh, H.J.; et al. Endogenous HMGB1 regulates autophagy. J. Cell Biol. 2010, 190, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Shim, E.J.; Chun, E.; Lee, H.S.; Bang, B.R.; Kim, T.W.; Cho, S.H.; Min, K.U.; Park, H.W. The role of high-mobility group box-1 (HMGB1) in the pathogenesis of asthma. Clin. Exp. Allergy 2012, 42, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Cuppari, C.; Manti, S.; Chirico, V.; Caruso, R.; Salpietro, V.; Giacchi, V.; Laganà, F.; Arrigo, T.; Salpietro, C.; Leonardi, S. Sputum high mobility group box-1 in asthmatic children: A noninvasive sensitive biomarker reflecting disease status. Ann. Allergy Asthma Immunol. 2015, 115, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yu, G.; Xie, J.; Tang, W.; Gao, L.; Long, X.; Ren, L.; Xie, X.; Deng, Y.; Fu, Z.; et al. High-mobility group box-1 protein from CC10 + club cells promotes type 2 response in the later stage of respiratory syncytial virus infection. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L280–L290. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Kong, J.; Liang, Y.; Huang, H.; Wen, H.; Zheng, X.; Wu, L.; Chen, Y. HMGB1 contributes to allergen-induced airway remodeling in a murine model of chronic asthma by modulating airway inflammation and activating lung fibroblasts. Cell. Mol. Immunol. 2015, 12, 409–423. [Google Scholar] [CrossRef]
- Ma, L.; Zeng, J.; Mo, B.; Wang, C.; Huang, J.; Sun, Y.; Yu, Y.; Liu, S. High mobility group box 1: A novel mediator of Th2-type responseinduced airway inflammation of acute allergic asthma. J. Thorac. Dis. 2015, 7, 1732–1741. [Google Scholar]
- Hou, C.C.; Zhao, H.J.; Cai, S.X.; Li, W.J.; Tong, W.C.; Liu, L.Y. Respiratory syncytial virus increases the expression and release of high mobility group Box-1 protein in the lung tissue of mice. J. South. Med. Univ. 2010, 30, 700–703. [Google Scholar]
- Manti, S.; Harford, T.J.; Salpietro, C.; Rezaee, F.; Piedimonte, G. Induction of high-mobility group Box-1 in vitro and in vivo by respiratory syncytial virus. Pediatr. Res. 2018, 83, 1049–1056. [Google Scholar] [CrossRef]
- Hosakote, Y.M.; Brasier, A.R.; Casola, A.; Garofalo, R.P.; Kurosky, A. Respiratory Syncytial Virus Infection Triggers Epithelial HMGB1 Release as a Damage-Associated Molecular Pattern Promoting a Monocytic Inflammatory Response. J. Virol. 2016, 90, 9618–9631. [Google Scholar] [CrossRef]
- Rayavara, K.; Kurosky, A.; Stafford, S.J.; Garg, N.J.; Brasier, A.R.; Garofalo, R.P.; Hosakote, Y.M. Proinflammatory Effects of Respiratory Syncytial Virus–Induced Epithelial HMGB1 on Human Innate Immune Cell Activation. J. Immunol. 2018, 201, 2753–2766. [Google Scholar] [CrossRef] [PubMed]
- Arikkatt, J.; Ullah, M.A.; Short, K.R.; Zhang, V.; Gan, W.J.; Loh, Z.; Werder, R.B.; Simpson, J.; Sly, P.D.; Mazzone, S.B.; et al. RAGE deficiency predisposes mice to virus-induced paucigranulocytic asthma. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.; Lynch, J.P.; Loh, Z.; Zhang, V.; Werder, R.B.; Spann, K.; Phipps, S. The Absence of Interferon-β Promotor Stimulator-1 (IPS-1) Predisposes to Bronchiolitis and Asthma-like Pathology in Response to Pneumoviral Infection in Mice. Sci. Rep. 2017, 7, 2353. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.; Loh, Z.; Ullah, M.A.; Lynch, J.P.; Werder, R.B.; Collinson, N.; Zhang, V.; Dondelinger, Y.; Bertrand, M.J.M.; Everard, M.L.; et al. RSV Infection Promotes Necroptosis and HMGB1 Release by Airway Epithelial Cells. Am. J. Respir. Crit. Care Med. 2020. [Google Scholar] [CrossRef]
- Chang, S.H.; Dong, C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell. Signal. 2011, 23, 1069–1075. [Google Scholar] [CrossRef]
- Xu, M.; Dong, C. IL-25 in allergic inflammation. Immunol. Rev. 2017, 278, 185–191. [Google Scholar] [CrossRef]
- Mindt, B.C.; Fritz, J.H.; Duerr, C.U. Group 2 innate lymphoid cells in pulmonary immunity and tissue homeostasis. Front. Immunol. 2018, 9, 840. [Google Scholar] [CrossRef]
- Barlow, J.L.; McKenzie, A.N.J. IL-25: A key requirement for the regulation of type-2 immunity. BioFactors 2009, 35, 178–182. [Google Scholar] [CrossRef]
- Yao, X.; Sun, Y.; Wang, W.; Sun, Y. Interleukin (IL)-25: Pleiotropic roles in asthma. Respirology 2016, 21, 638–647. [Google Scholar] [CrossRef]
- Petersen, B.C.; Dolgachev, V.; Rasky, A.; Lukacs, N.W. IL-17E (IL-25) and IL-17RB promote respiratory syncytial virus-induced pulmonary disease. J. Leukoc. Biol. 2014, 95, 809–815. [Google Scholar] [CrossRef]
- Siegle, J.S.; Hansbro, N.; Herbert, C.; Rosenberg, H.F.; Domachowske, J.B.; Asquith, K.L.; Foster, P.S.; Kumar, R.K. Early-life viral infection and allergen exposure interact to induce an asthmatic phenotype in mice. Respir. Res. 2010, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Huang, L.; Ji, X.; Yao, S.; Abdelaziz, M.H.; Cai, W.; Wang, H.; Cheng, J.; Dineshkumar, K.; Aparna, V.; et al. HMGB1-induced ILC2s activate dendritic cells by producing IL-9 in asthmatic mouse model. Cell. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bai, S.; Cui, Y.; Zhao, N.; Qi, F.; Liu, J.; Zeng, S.; Xu, L.; Hu, H.; Liu, B. Respiratory syncytial virus prevents the subsequent development of ovalbumin-induced allergic responses by inhibiting ILC2 via the IL-33/ST2 pathway. Immunotherapy 2018, 10, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norlander, A.E.; Peebles, R.S., Jr. Innate Type 2 Responses to Respiratory Syncytial Virus Infection. Viruses 2020, 12, 521. https://doi.org/10.3390/v12050521
Norlander AE, Peebles RS Jr. Innate Type 2 Responses to Respiratory Syncytial Virus Infection. Viruses. 2020; 12(5):521. https://doi.org/10.3390/v12050521
Chicago/Turabian StyleNorlander, Allison E., and R. Stokes Peebles, Jr. 2020. "Innate Type 2 Responses to Respiratory Syncytial Virus Infection" Viruses 12, no. 5: 521. https://doi.org/10.3390/v12050521
APA StyleNorlander, A. E., & Peebles, R. S., Jr. (2020). Innate Type 2 Responses to Respiratory Syncytial Virus Infection. Viruses, 12(5), 521. https://doi.org/10.3390/v12050521



