Modelling Lyssavirus Infections in Human Stem Cell-Derived Neural Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cell Culture
2.2.1. Generation of Human Neural Precursor Cells (hNPCs)
2.2.2. Differentiation of hNPCs
2.3. Calcium Imaging
2.4. Virus Stock Amplification and Quantification
2.5. Viral Infection of Human ESC- and iPSC-Derived Neural Cultures
2.6. Generation of Trans-Synaptic Human Neural Cultures Using Microfluidics
2.7. Immunocytochemistry
2.8. Apoptosis (TUNEL) Assay
2.9. Confocal Imaging
2.10. RNA Extraction and cDNA Generation
2.11. qPCR for Chemokine and Cytokine Gene Expression Analysis
2.12. qPCR for Apoptosis and Necrosis Markers
2.13. qPCR Assays for Detection of Lyssavirus
2.14. Statistical Analysis
3. Results
3.1. Generation and Characterization of Human ESC- and iPSC-Derived Neural Cultures
3.2. Lyssavirus Infection of Human Stem Cell-Derived Neural Cultures
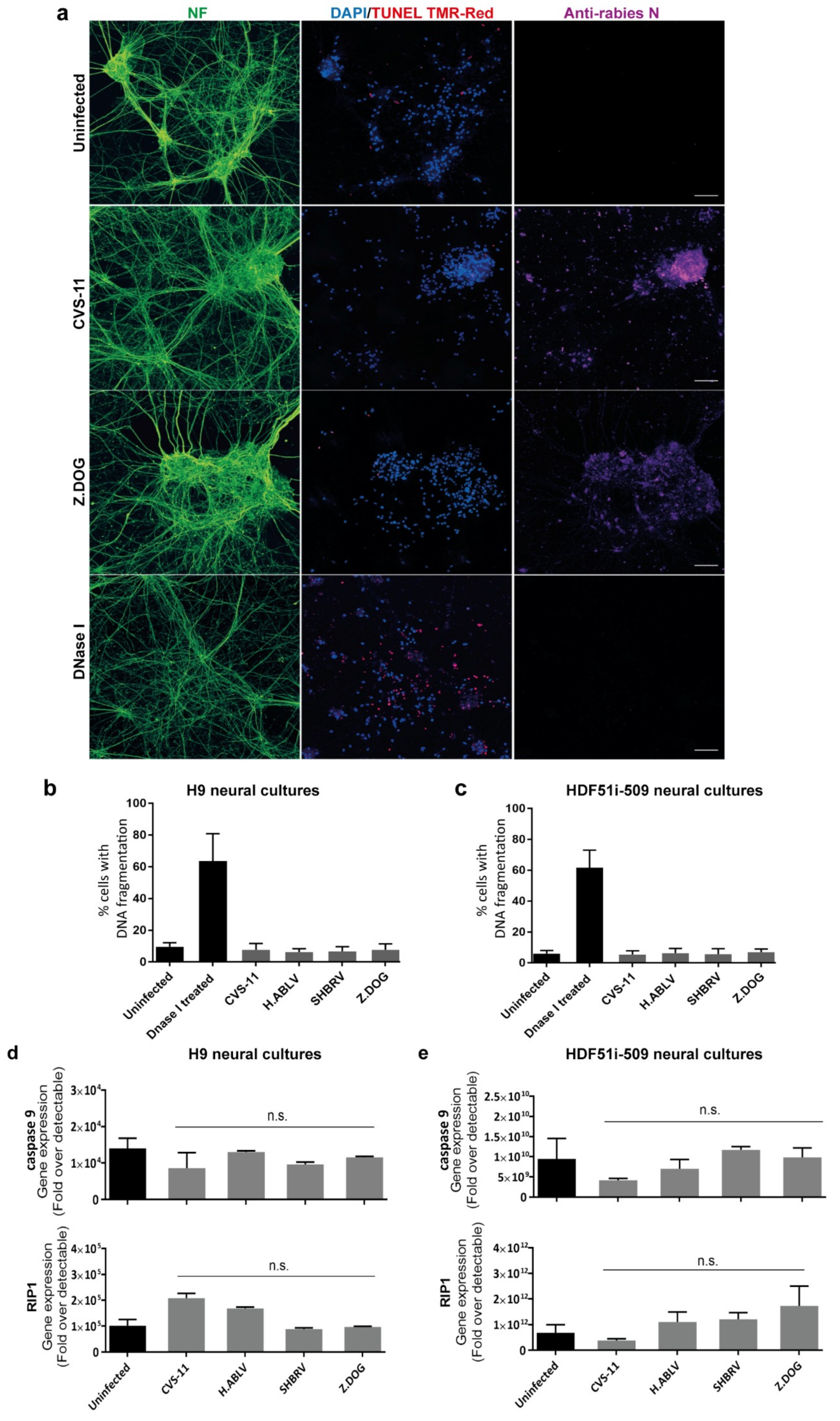
3.3. Lack of Apoptosis in Human Stem Cell-Derived Neural Cultures Infected with Lyssavirus
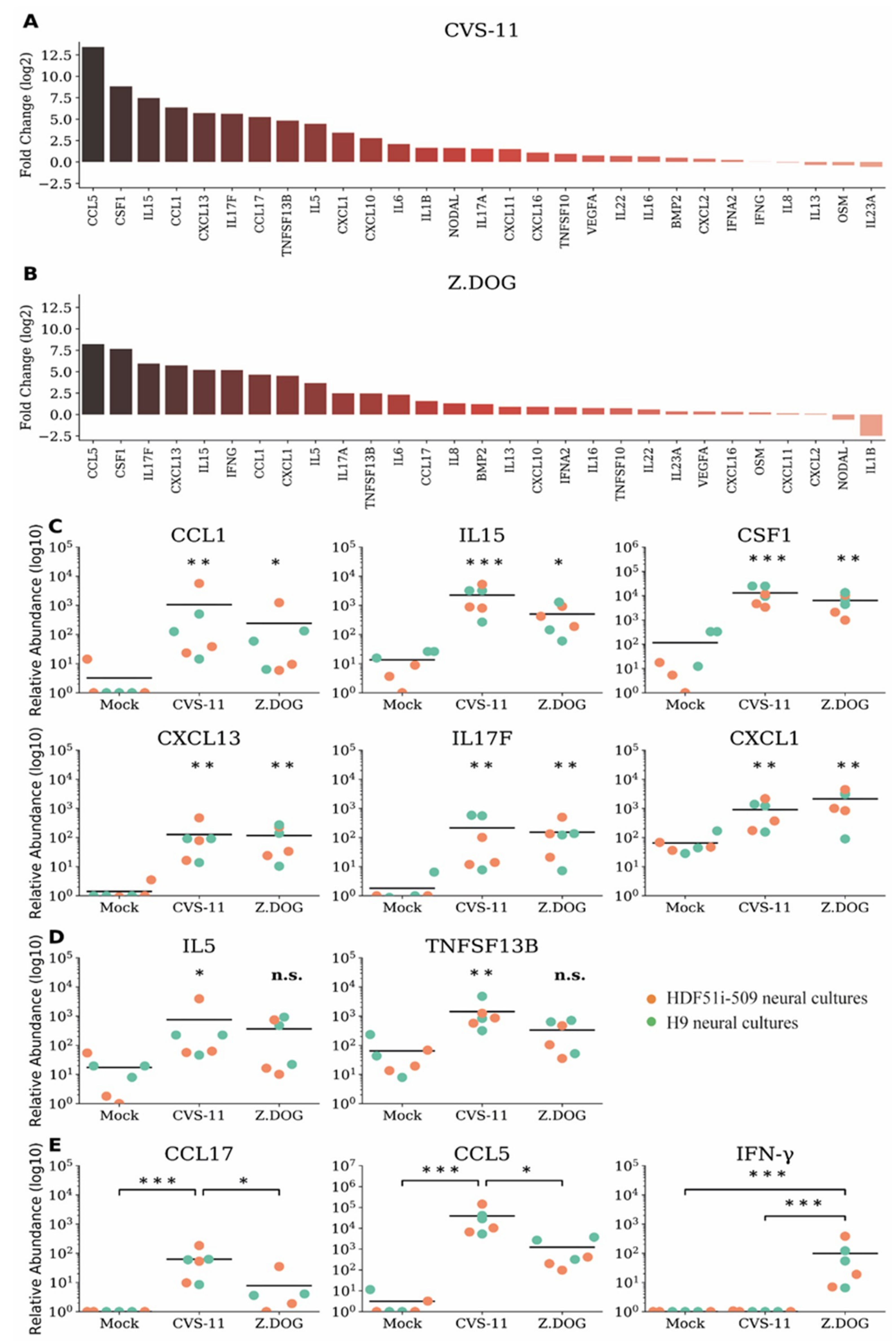
3.4. Upregulation of Distinct Pro-Inflammatory Chemokine and Cytokines in Human Stem Cell-Derived Neural Cultures during Infection with Rabies Virus
3.5. Stem Cell-Derived Ex-Vivo Models of Human Neuronal Network Reveals Differential Dynamics in the Axonal Transmission of Rabies Virus Strains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Delmas, O.; Holmes, E.C.; Talbi, C.; Larrous, F.; Dacheux, L.; Bouchier, C.; Bourhy, H. Genomic diversity and evolution of the lyssaviruses. Plos One 2008, 3, e2057. [Google Scholar] [CrossRef] [PubMed]
- Troupin, C.; Dacheux, L.; Tanguy, M.; Sabeta, C.; Blanc, H.; Bouchier, C.; Vignuzzi, M.; Duchene, S.; Holmes, E.C.; Bourhy, H. Large-scale phylogenomic analysis reveals the complex evolutionary history of rabies virus in multiple carnivore hosts. Plos Pathog. 2016, 12, e1006041. [Google Scholar] [CrossRef] [PubMed]
- Shipley, R.; Wright, E.; Selden, D.; Wu, G.; Aegerter, J.; Fooks, A.R.; Banyard, A.C. Bats and viruses: Emergence of novel lyssaviruses and association of bats with viral zoonoses in the eu. Trop. Med. Infect. Dis. 2019, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, C.; Kuzmin, I.; Meslin, F. Lyssaviruses and rabies: Current conundrums, concerns, contradictions and controversies. F1000Research 2017, 6, 184. [Google Scholar] [CrossRef] [PubMed]
- Schnell, M.J.; McGettigan, J.P.; Wirblich, C.; Papaneri, A. The cell biology of rabies virus: Using stealth to reach the brain. Nat. Rev. Microbiol. 2010, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Knobel, D.L.; Cleaveland, S.; Coleman, P.G.; Fèvre, E.M.; Meltzer, M.I.; Miranda, M.E.G.; Shaw, A.; Zinsstag, J.; Meslin, F.-X. Re-evaluating the burden of rabies in africa and asia. Bull. World Health Organ. 2005, 83, 360–368. [Google Scholar]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S. Estimating the global burden of endemic canine rabies. Plos Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar]
- Kessels, J.A.; Recuenco, S.; Navarro-Vela, A.M.; Deray, R.; Vigilato, M.; Ertl, H.; Durrheim, D.; Rees, H.; Nel, L.H.; Abela-Ridder, B. Pre-exposure rabies prophylaxis: A systematic review. Bull. World Health Organ. 2017, 95, 210. [Google Scholar] [CrossRef]
- Dietzschold, B.; Schnell, M.; Koprowski, H. Pathogenesis of rabies. In The World of Rhabdoviruses; Springer: New York, NY, USA, 2005; pp. 45–56. [Google Scholar]
- Gluska, S.; Zahavi, E.E.; Chein, M.; Gradus, T.; Bauer, A.; Finke, S.; Perlson, E. Rabies virus hijacks and accelerates the p75ntr retrograde axonal transport machinery. Plos Pathog. 2014, 10, e1004348. [Google Scholar] [CrossRef]
- Hosking, M.P.; Lane, T.E. The role of chemokines during viral infection of the cns. Plos Pathog. 2010, 6, e1000937. [Google Scholar] [CrossRef]
- Veiga-Fernandes, H.; Artis, D. Neuronal–immune system cross-talk in homeostasis. Science 2018, 359, 1465–1466. [Google Scholar] [CrossRef]
- Arima, Y.; Kamimura, D.; Sabharwal, L.; Yamada, M.; Bando, H.; Ogura, H.; Atsumi, T.; Murakami, M. Regulation of immune cell infiltration into the cns by regional neural inputs explained by the gate theory. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.S.; Lin, E.; Zhang, B.; Luster, A.D.; Tollett, J.; Samuel, M.A.; Engle, M.; Diamond, M.S. Neuronal cxcl10 directs cd8+ t-cell recruitment and control of west nile virus encephalitis. J. Virol. 2005, 79, 11457–11466. [Google Scholar] [CrossRef]
- Aravalli, R.N.; Hu, S.; Rowen, T.N.; Palmquist, J.M.; Lokensgard, J.R. Cutting edge: Tlr2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J. Immunol. 2005, 175, 4189–4193. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Becher, B.; Prat, A.; Antel, J.P. Brain-immune connection: Immuno-regulatory properties of cns-resident cells. Glia 2000, 29, 293–304. [Google Scholar] [CrossRef]
- Lafon, M. Evasive strategies in rabies virus infection. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2011; Volume 79, pp. 33–53. [Google Scholar]
- Brzózka, K.; Finke, S.; Conzelmann, K.-K. Inhibition of interferon signaling by rabies virus phosphoprotein p: Activation-dependent binding of stat1 and stat2. J. Virol. 2006, 80, 2675–2683. [Google Scholar] [CrossRef]
- Wiltzer, L.; Okada, K.; Yamaoka, S.; Larrous, F.; Kuusisto, H.V.; Sugiyama, M.; Blondel, D.; Bourhy, H.; Jans, D.A.; Ito, N. Interaction of rabies virus p-protein with stat proteins is critical to lethal rabies disease. J. Infect. Dis. 2013, 209, 1744–1753. [Google Scholar] [CrossRef]
- Ito, N.; Moseley, G.W.; Blondel, D.; Shimizu, K.; Rowe, C.L.; Ito, Y.; Masatani, T.; Nakagawa, K.; Jans, D.A.; Sugiyama, M. Role of interferon antagonist activity of rabies virus phosphoprotein in viral pathogenicity. J. Virol. 2010, 84, 6699–6710. [Google Scholar] [CrossRef]
- Fernandes, E.R.; de Andrade Jr, H.F.; Lancellotti, C.L.P.; Quaresma, J.A.S.; Demachki, S.; da Costa Vasconcelos, P.F.; Duarte, M.I.S. In situ apoptosis of adaptive immune cells and the cellular escape of rabies virus in cns from patients with human rabies transmitted by desmodus rotundus. Virus Res. 2011, 156, 121–126. [Google Scholar] [CrossRef]
- Baloul, L.; Lafon, M. Apoptosis and rabies virus neuroinvasion. Biochimie 2003, 85, 777–788. [Google Scholar] [CrossRef]
- Scott, T.P.; Nel, L.H. Subversion of the immune response by rabies virus. Viruses 2016, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiao, S.; Tao, X.; Tang, Q.; Jiao, W.; Xiao, J.; Xu, X.; Zhang, Y.; Liang, G.; Wang, H. Met-ccl5 represents an immunotherapy strategy to ameliorate rabies virus infection. J. Neuroinflammation 2014, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Mansfield, K.; Hicks, D.; Nunez, A.; Healy, D.; Brookes, S.; McKimmie, C.; Fazakerley, J.; Fooks, A. Inflammatory responses in the nervous system of mice infected with a street isolate of rabies virus. Dev. Biol. 2008, 131, 65–72. [Google Scholar]
- Chai, Q.; He, W.Q.; Zhou, M.; Lu, H.; Fu, Z.F. Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J. Virol. 2014, 88, 4698–4710. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; She, R.; Huang, Y.; Fu, Z.F. Expression of neuronal cxcl10 induced by rabies virus infection initiates infiltration of inflammatory cells, production of chemokines and cytokines, and enhancement of blood-brain barrier permeability. J. Virol. 2015, 89, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.C.; Randle, E.; Lawrance, G.; Rossiter, J.P. Neuronal apoptosis does not play an important role in human rabies encephalitis. J. Neurovirology 2008, 14, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Suja, M.; Mahadevan, A.; Madhusudana, S.; Shankar, S. Role of apoptosis in rabies viral encephalitis: A comparative study in mice, canine, and human brain with a review of literature. Pathol. Res. Int. 2011, 2011. [Google Scholar] [CrossRef]
- Préhaud, C.; Lay, S.; Dietzschold, B.; Lafon, M. Glycoprotein of nonpathogenic rabies viruses is a key determinant of human cell apoptosis. J. Virol. 2003, 77, 10537–10547. [Google Scholar] [CrossRef]
- Hemachudha, T.; Ugolini, G.; Wacharapluesadee, S.; Sungkarat, W.; Shuangshoti, S.; Laothamatas, J. Human rabies: Neuropathogenesis, diagnosis, and management. Lancet Neurol. 2013, 12, 498–513. [Google Scholar] [CrossRef]
- Mitrabhakdi, E.; Shuangshoti, S.; Wannakrairot, P.; Lewis, R.A.; Susuki, K.; Laothamatas, J.; Hemachudha, T. Difference in neuropathogenetic mechanisms in human furious and paralytic rabies. J. Neurol. Sci. 2005, 238, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Warrell, M.J.; Warrell, D.A. Rabies: The clinical features, management and prevention of the classic zoonosis. Clin. Med. 2015, 15, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Piccinotti, S.; Whelan, S.P. Rabies internalizes into primary peripheral neurons via clathrin coated pits and requires fusion at the cell body. Plos Pathog. 2016, 12, e1005753. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Nolden, T.; Schröter, J.; Römer-Oberdörfer, A.; Gluska, S.; Perlson, E.; Finke, S. Anterograde glycoprotein-dependent transport of newly generated rabies virus in dorsal root ganglion neurons. J. Virol. 2014, 88, 14172–14183. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, V.; Green, D.; Locke, K.; O’Brien, C.M.; Dearnley, M.; Bingham, J. Novel role of sarm1 mediated axonal degeneration in the pathogenesis of rabies. Plos Pathog. 2020, 16, e1008343. [Google Scholar] [CrossRef] [PubMed]
- Préhaud, C.; Mégret, F.; Lafage, M.; Lafon, M. Virus infection switches tlr-3-positive human neurons to become strong producers of beta interferon. J. Virol. 2005, 79, 12893–12904. [Google Scholar] [CrossRef]
- Mégret, F.; Prehaud, C.; Lafage, M.; Moreau, P.; Rouas-Freiss, N.; Carosella, E.D.; Lafon, M. Modulation of hla-g and hla-e expression in human neuronal cells after rabies virus or herpes virus simplex type 1 infections. Hum. Immunol. 2007, 68, 294–302. [Google Scholar] [CrossRef]
- D’Aiuto, L.; Bloom, D.C.; Naciri, J.N.; Smith, A.; Edwards, T.G.; McClain, L.; Callio, J.A.; Jessup, M.; Wood, J.; Chowdari, K. Modeling herpes simplex virus 1 infections in human central nervous system neuronal cells using two-and three-dimensional cultures derived from induced pluripotent stem cells. J. Virol. 2019, 93, e00111–e00119. [Google Scholar] [CrossRef]
- Sadaoka, T.; Schwartz, C.L.; Rajbhandari, L.; Venkatesan, A.; Cohen, J.I. Human embryonic stem cell-derived neurons are highly permissive for varicella-zoster virus lytic infection. J. Virol. 2018, 92, e01108–e01117. [Google Scholar] [CrossRef]
- Dawes, B.E.; Gao, J.; Atkins, C.; Nelson, J.T.; Johnson, K.; Wu, P.; Freiberg, A.N. Human neural stem cell-derived neuron/astrocyte co-cultures respond to la crosse virus infection with proinflammatory cytokines and chemokines. J. Neuroinflammation 2018, 15, 315. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C. Brain-region-specific organoids using mini-bioreactors for modeling zikv exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Garcez, P.P.; Loiola, E.C.; da Costa, R.M.; Higa, L.M.; Trindade, P.; Delvecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Desole, G.; Sinigaglia, A.; Riccetti, S.; Masi, G.; Pacenti, M.; Trevisan, M.; Barzon, L. Modelling neurotropic flavivirus infection in human induced pluripotent stem cell-derived systems. Int. J. Mol. Sci. 2019, 20, 5404. [Google Scholar] [CrossRef] [PubMed]
- Muffat, J.; Li, Y.; Omer, A.; Durbin, A.; Bosch, I.; Bakiasi, G.; Richards, E.; Meyer, A.; Gehrke, L.; Jaenisch, R. Human induced pluripotent stem cell-derived glial cells and neural progenitors display divergent responses to zika and dengue infections. Proc. Natl. Acad. Sci. 2018, 115, 7117–7122. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-C.; Shen, C.-I.; Lin, H.; Chen, C.-J.; Chang, C.-Y.; Chen, S.-M.; Lee, H.-C.; Lai, P.-S.; Su, H.-L. Susceptibility of human embryonic stem cell-derived neural cells to japanese encephalitis virus infection. Plos One 2014, 9, e114990. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, S.-C. Neural subtype specification from human pluripotent stem cells. Cell Stem Cell 2016, 19, 573–586. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183. [Google Scholar] [CrossRef]
- Avior, Y.; Sagi, I.; Benvenisty, N. Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 2016, 17, 170. [Google Scholar] [CrossRef]
- Kim, K.; Zhao, R.; Doi, A.; Ng, K.; Unternaehrer, J.; Cahan, P.; Hongguang, H.; Loh, Y.-H.; Aryee, M.J.; Lensch, M.W. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 1117. [Google Scholar] [CrossRef]
- Ohi, Y.; Qin, H.; Hong, C.; Blouin, L.; Polo, J.M.; Guo, T.; Qi, Z.; Downey, S.L.; Manos, P.D.; Rossi, D.J. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human ips cells. Nat. Cell Biol. 2011, 13, 541. [Google Scholar] [CrossRef]
- Bar-Nur, O.; Russ, H.A.; Efrat, S.; Benvenisty, N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 2011, 9, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Weissbein, U.; Benvenisty, N.; Ben-David, U. Genome maintenance in pluripotent stem cells. J Cell Biol 2014, 204, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.; Pelizzola, M.; Kida, Y.S.; Hawkins, R.D.; Nery, J.R.; Hon, G.; Antosiewicz-Bourget, J.; O’Malley, R.; Castanon, R.; Klugman, S. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 2011, 471, 68. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Diep, D.; Gore, A.; Panopoulos, A.D.; Montserrat, N.; Plongthongkum, N.; Kumar, S.; Fung, H.-L.; Giorgetti, A.; Bilic, J. Identification of a specific reprogramming-associated epigenetic signature in human induced pluripotent stem cells. Proc. Natl. Acad. Sci. 2012, 109, 16196–16201. [Google Scholar] [CrossRef] [PubMed]
- Dukhovny, A.; Sloutskin, A.; Markus, A.; Yee, M.B.; Kinchington, P.R.; Goldstein, R.S. Varicella-zoster virus infects human embryonic stem cell-derived neurons and neurospheres but not pluripotent embryonic stem cells or early progenitors. J. Virol. 2012, 86, 3211–3218. [Google Scholar] [CrossRef]
- Jones, J.C.; Sabatini, K.; Liao, X.; Tran, H.T.; Lynch, C.L.; Morey, R.E.; Glenn-Pratola, V.; Boscolo, F.S.; Yang, Q.; Parast, M.M. Melanocytes derived from transgene-free human induced pluripotent stem cells. J. Investig. Dermatol. 2013, 133, 2104. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Murphy, A.R.; Ghobrial, I.; Jamshidi, P.; Laslett, A.; O’Brien, C.M.; Cameron, N.R. Tailored emulsion-templated porous polymer scaffolds for ipsc-derived human neural precursor cell culture. Polym. Chem. 2017, 8, 6617–6627. [Google Scholar] [CrossRef]
- Beers, J.; Gulbranson, D.R.; George, N.; Siniscalchi, L.I.; Jones, J.; Thomson, J.A.; Chen, G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat. Protoc. 2012, 7, 2029. [Google Scholar] [CrossRef]
- Itskovitz-Eldor, J.; Schuldiner, M.; Karsenti, D.; Eden, A.; Yanuka, O.; Amit, M.; Soreq, H.; Benvenisty, N. Differentiation of human embryonic stem cells into embryoid bodies comprising the three embryonic germ layers. Mol. Med. 2000, 6, 88. [Google Scholar] [CrossRef]
- Pera, M.F.; Andrade, J.; Houssami, S.; Reubinoff, B.; Trounson, A.; Stanley, E.G.; Ward-van Oostwaard, D.; Mummery, C. Regulation of human embryonic stem cell differentiation by bmp-2 and its antagonist noggin. J Cell Sci 2004, 117, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Itsykson, P.; Ilouz, N.; Turetsky, T.; Goldstein, R.S.; Pera, M.F.; Fishbein, I.; Segal, M.; Reubinoff, B.E. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol. Cell. Neurosci. 2005, 30, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, L.; Rodgers, L.; Cui, W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells 2005, 23, 1234–1241. [Google Scholar] [CrossRef]
- Sonntag, K.C.; Pruszak, J.; Yoshizaki, T.; Van Arensbergen, J.; Sanchez-Pernaute, R.; Isacson, O. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells 2007, 25, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Pollard, S.M.; Gorba, T.; Reitano, E.; Toselli, M.; Biella, G.; Sun, Y.; Sanzone, S.; Ying, Q.-L.; Cattaneo, E. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. Plos Biol. 2005, 3, e283. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Pollard, S.; Conti, L.; Toselli, M.; Biella, G.; Parkin, G.; Willatt, L.; Falk, A.; Cattaneo, E.; Smith, A. Long-term tripotent differentiation capacity of human neural stem (ns) cells in adherent culture. Mol. Cell. Neurosci. 2008, 38, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.H.; Martin, J.; Elia, J.; Flippin, J.; Paramban, R.I.; Hefferan, M.P.; Vidal, J.G.; Mu, Y.; Killian, R.L.; Israel, M.A. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. Plos One 2011, 6, e17540. [Google Scholar] [CrossRef]
- Bardy, C.; Van Den Hurk, M.; Eames, T.; Marchand, C.; Hernandez, R.V.; Kellogg, M.; Gorris, M.; Galet, B.; Palomares, V.; Brown, J. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc. Natl. Acad. Sci. 2015, 112, E2725–E2734. [Google Scholar] [CrossRef]
- Watmuff, B.; Hartley, B.J.; Hunt, C.P.; Fabb, S.A.; Pouton, C.W.; Haynes, J.M. Human pluripotent stem cell derived midbrain pitx3egfp/w neurons: A versatile tool for pharmacological screening and neurodegenerative modeling. Front. Cell. Neurosci. 2015, 9, 104. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Rahmadane, I.; Certoma, A.F.; Peck, G.R.; Fitria, Y.; Payne, J.; Colling, A.; Shiell, B.J.; Beddome, G.; Wilson, S.; Yu, M. Development and validation of an immunoperoxidase antigen detection test for improved diagnosis of rabies in indonesia. Plos Negl. Trop. Dis. 2017, 11, e0006079. [Google Scholar] [CrossRef] [PubMed]
- Hardikar, A.A.; Farr, R.J.; Joglekar, M.V. Circulating micrornas: Understanding the limits for quantitative measurement by real-time pcr. J. Am. Heart Assoc. 2014, 3, e000792. [Google Scholar] [CrossRef] [PubMed]
- Nadin-Davis, S.A.; Sheen, M.; Wandeler, A.I. Development of real-time reverse transcriptase polymerase chain reaction methods for human rabies diagnosis. J. Med Virol. 2009, 81, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Foord, A.; Heine, H.; Pritchard, L.; Lunt, R.; Newberry, K.; Rootes, C.; Boyle, D. Molecular diagnosis of lyssaviruses and sequence comparison of australian bat lyssavirus samples. Aust. Vet. J. 2006, 84, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Krieger, A.M.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J. Scipy 1.0--fundamental algorithms for scientific computing in python. arXiv 2019, arXiv:1907.10121. [Google Scholar] [CrossRef]
- Terpilowski, M. Scikit-posthocs: Pairwise multiple comparison tests in python. J. Open Source Softw. 2019, 4, 1169. [Google Scholar] [CrossRef]
- Xiang, J.; Wan, C.; Guo, R.; Guo, D. Is hydrogen peroxide a suitable apoptosis inducer for all cell types? Biomed Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Yan, X.; Prosniak, M.; Curtis, M.T.; Weiss, M.L.; Faber, M.; Dietzschold, B.; Fu, Z.F. Silver-haired bat rabies virus variant does not induce apoptosis in the brain of experimentally infected mice. J. Neurovirology 2001, 7, 518–527. [Google Scholar]
- Morimoto, K.; Hooper, D.C.; Spitsin, S.; Koprowski, H.; Dietzschold, B. Pathogenicity of different rabies virus variants inversely correlates with apoptosis and rabies virus glycoprotein expression in infected primary neuron cultures. J. Virol. 1999, 73, 510–518. [Google Scholar] [CrossRef]
- Hooper, D.C. The role of immune responses in the pathogenesis of rabies. J. Neurovirology 2005, 11, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Ubogu, E.E.; Callahan, M.K.; Tucky, B.H.; Ransohoff, R.M. Determinants of ccl5-driven mononuclear cell migration across the blood–brain barrier. Implications for therapeutically modulating neuroinflammation. J. Neuroimmunol. 2006, 179, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Bartosik-Psujek, H.; Stelmasiak, Z. The levels of chemokines cxcl8, ccl2 and ccl5 in multiple sclerosis patients are linked to the activity of the disease. Eur. J. Neurol. 2005, 12, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Dénes, Á.; Humphreys, N.; Lane, T.E.; Grencis, R.; Rothwell, N. Chronic systemic infection exacerbates ischemic brain damage via a ccl5 (regulated on activation, normal t-cell expressed and secreted)-mediated proinflammatory response in mice. J. Neurosci. 2010, 30, 10086–10095. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.E.; Guabiraba, R.; Russo, R.C.; Teixeira, M.M. Targeting ccl5 in inflammation. Expert Opin. Ther. Targets 2013, 17, 1439–1460. [Google Scholar] [CrossRef] [PubMed]
- Glass, W.G.; Hickey, M.J.; Hardison, J.L.; Liu, M.T.; Manning, J.E.; Lane, T.E. Antibody targeting of the cc chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J. Immunol. 2004, 172, 4018–4025. [Google Scholar] [CrossRef]
- Di Prisco, S.; Merega, E.; Milanese, M.; Summa, M.; Casazza, S.; Raffaghello, L.; Pistoia, V.; Uccelli, A.; Pittaluga, A. Ccl5-glutamate interaction in central nervous system: Early and acute presynaptic defects in eae mice. Neuropharmacology 2013, 75, 337–346. [Google Scholar] [CrossRef]
- Pittaluga, A. Ccl5–glutamate cross-talk in astrocyte-neuron communication in multiple sclerosis. Front. Immunol. 2017, 8, 1079. [Google Scholar] [CrossRef]
- Grassi-Oliveira, R.; Brieztke, E.; Teixeira, A.; Pezzi, J.C.; Zanini, M.; Lopes, R.P.; Bauer, M.E. Peripheral chemokine levels in women with recurrent major depression with suicidal ideation. Rev. Bras. De Psiquiatr. 2012, 34, 71–75. [Google Scholar] [CrossRef]
- Musante, V.; Longordo, F.; Neri, E.; Pedrazzi, M.; Kalfas, F.; Severi, P.; Raiteri, M.; Pittaluga, A. Rantes modulates the release of glutamate in human neocortex. J. Neurosci. 2008, 28, 12231–12240. [Google Scholar] [CrossRef]
- Campbell, L.A.; Avdoshina, V.; Day, C.; Lim, S.T.; Mocchetti, I. Pharmacological induction of ccl5 in vivo prevents gp120-mediated neuronal injury. Neuropharmacology 2015, 92, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, A.; Robert, J.; Gregory-Evans, C.; Schaller, H. Rantes stimulates ca2+ mobilization and inositol trisphosphate (ip3) formation in cells transfected with g protein-coupled receptor 75. Br. J. Pharmacol. 2006, 149, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Chitu, V.; Gokhan, Ş.; Nandi, S.; Mehler, M.F.; Stanley, E.R. Emerging roles for csf-1 receptor and its ligands in the nervous system. Trends Neurosci. 2016, 39, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Funk, K.E.; Klein, R.S. Csf1r antagonism limits local restimulation of antiviral cd8+ t cells during viral encephalitis. J. Neuroinflammation 2019, 16, 22. [Google Scholar] [CrossRef]
- Akimoto, N.; Ifuku, M.; Mori, Y.; Noda, M. Effects of chemokine (c–c motif) ligand 1 on microglial function. Biochem. Biophys. Res. Commun. 2013, 436, 455–461. [Google Scholar] [CrossRef]
- Gomez-Nicola, D.; Valle-Argos, B.; Nieto-Sampedro, M. Blockade of il-15 activity inhibits microglial activation through the nfκb, p38, and erk1/2 pathways, reducing cytokine and chemokine release. Glia 2010, 58, 264–276. [Google Scholar] [CrossRef]
- Liva, S.M.; de Vellis, J. Il-5 induces proliferation and activation of microglia via an unknown receptor. Neurochem. Res. 2001, 26, 629–637. [Google Scholar] [CrossRef]
- Fülle, L.; Offermann, N.; Hansen, J.N.; Breithausen, B.; Erazo, A.B.; Schanz, O.; Radau, L.; Gondorf, F.; Knöpper, K.; Alferink, J. Ccl17 exerts a neuroimmune modulatory function and is expressed in hippocampal neurons. Glia 2018, 66, 2246–2261. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Zhou, M.; Zhang, Y.; Yang, J.; Cao, Y.; Wang, K.; Cui, M.; Chen, H.; Fu, Z.F. A novel rabies vaccine expressing cxcl13 enhances humoral immunity by recruiting both t follicular helper and germinal center b cells. J. Virol. 2017, 91, e01956. [Google Scholar] [CrossRef]
- Sawant, K.V.; Poluri, K.M.; Dutta, A.K.; Sepuru, K.M.; Troshkina, A.; Garofalo, R.P.; Rajarathnam, K. Chemokine cxcl1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci. Rep. 2016, 6, 33123. [Google Scholar] [CrossRef]
- Lokensgard, J.R.; Mutnal, M.B.; Prasad, S.; Sheng, W.; Hu, S. Glial cell activation, recruitment, and survival of b-lineage cells following mcmv brain infection. J. Neuroinflammation 2016, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, D.S.; Horvath, C.M. A road map for those who don’t know jak-stat. Science 2002, 296, 1653–1655. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of type-i-and type-ii-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375. [Google Scholar] [CrossRef] [PubMed]
- Barkhouse, D.A.; Garcia, S.A.; Bongiorno, E.K.; Lebrun, A.; Faber, M.; Hooper, D.C. Expression of interferon gamma by a recombinant rabies virus strongly attenuates the pathogenicity of the virus via induction of type i interferon. J. Virol. 2015, 89, 312–322. [Google Scholar] [CrossRef] [PubMed]
- MacGibeny, M.A.; Koyuncu, O.O.; Wirblich, C.; Schnell, M.J.; Enquist, L.W. Retrograde axonal transport of rabies virus is unaffected by interferon treatment but blocked by emetine locally in axons. Plos Pathog. 2018, 14, e1007188. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Pulmanausahakul, R.; Nagao, K.; Prosniak, M.; Rice, A.B.; Koprowski, H.; Schnell, M.J.; Dietzschold, B. Identification of viral genomic elements responsible for rabies virus neuroinvasiveness. Proc. Natl. Acad. Sci. 2004, 101, 16328–16332. [Google Scholar] [CrossRef]
- Reardon, T.R.; Murray, A.J.; Turi, G.F.; Wirblich, C.; Croce, K.R.; Schnell, M.J.; Jessell, T.M.; Losonczy, A. Rabies virus cvs-n2cδg strain enhances retrograde synaptic transfer and neuronal viability. Neuron 2016, 89, 711–724. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundaramoorthy, V.; Godde, N.; J. Farr, R.; Green, D.; M. Haynes, J.; Bingham, J.; O’Brien, C.M.; Dearnley, M. Modelling Lyssavirus Infections in Human Stem Cell-Derived Neural Cultures. Viruses 2020, 12, 359. https://doi.org/10.3390/v12040359
Sundaramoorthy V, Godde N, J. Farr R, Green D, M. Haynes J, Bingham J, O’Brien CM, Dearnley M. Modelling Lyssavirus Infections in Human Stem Cell-Derived Neural Cultures. Viruses. 2020; 12(4):359. https://doi.org/10.3390/v12040359
Chicago/Turabian StyleSundaramoorthy, Vinod, Nathan Godde, Ryan J. Farr, Diane Green, John M. Haynes, John Bingham, Carmel M. O’Brien, and Megan Dearnley. 2020. "Modelling Lyssavirus Infections in Human Stem Cell-Derived Neural Cultures" Viruses 12, no. 4: 359. https://doi.org/10.3390/v12040359
APA StyleSundaramoorthy, V., Godde, N., J. Farr, R., Green, D., M. Haynes, J., Bingham, J., O’Brien, C. M., & Dearnley, M. (2020). Modelling Lyssavirus Infections in Human Stem Cell-Derived Neural Cultures. Viruses, 12(4), 359. https://doi.org/10.3390/v12040359




