Lactococcus Ceduovirus Phages Isolated from Industrial Dairy Plants—From Physiological to Genomic Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Bacteriophages, and Culture Conditions
2.2. Bacteriophage Propagation
2.3. Electron Microscopy
2.4. Host Range Studies
2.5. One-Step Growth Assay
2.6. PCR-Based Typing
2.7. Phage Genome Sequencing
2.8. Bioinformatic Analyses
2.9. Construction of Phage Ori+ Plasmids
2.10. Functional Analysis of Putative Phage Ori Regions
3. Results
3.1. Phage Classification and Morphological Analysis
3.2. Phage-Host Interactions
3.3. Infection Dynamics in One-Step Growth Tests
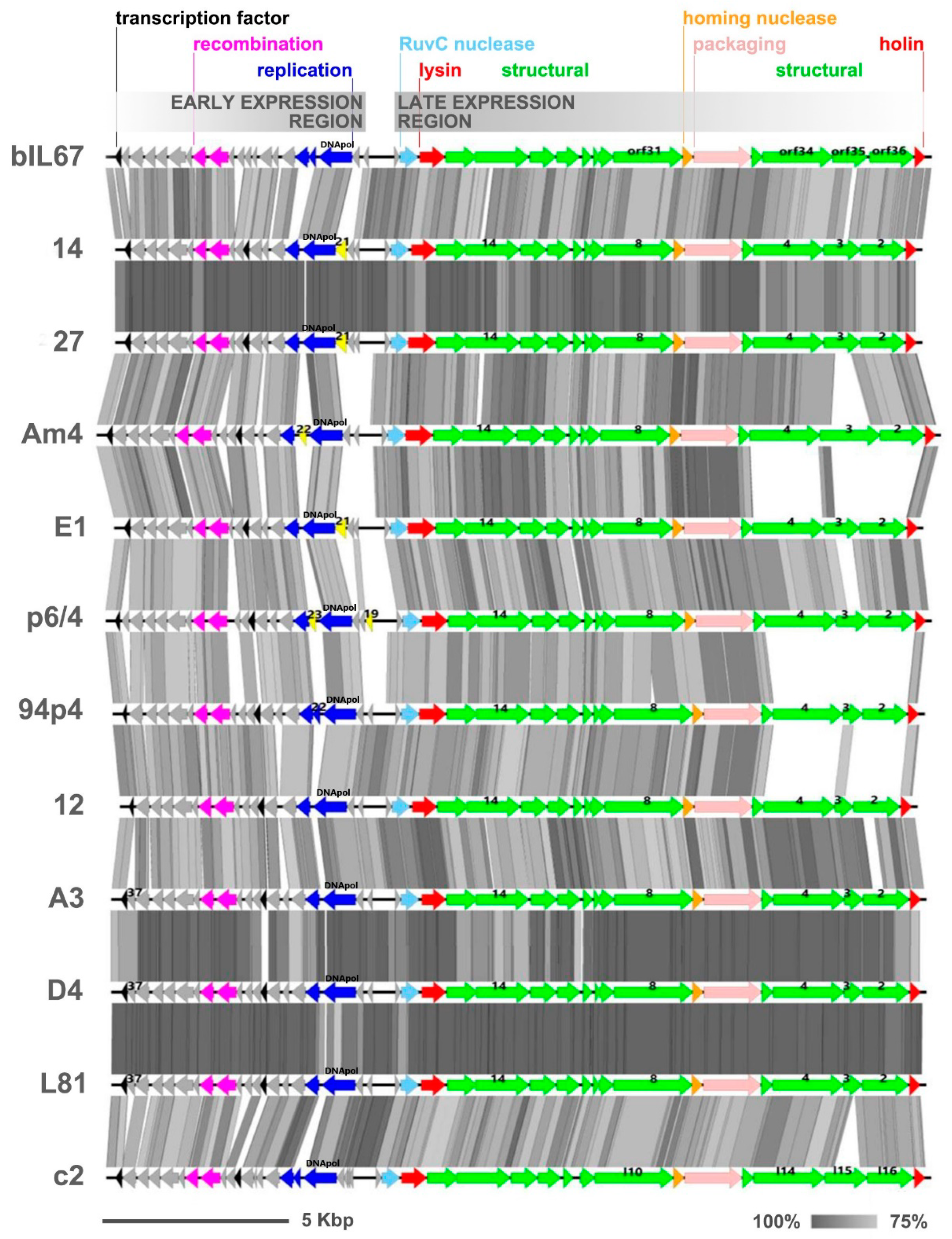
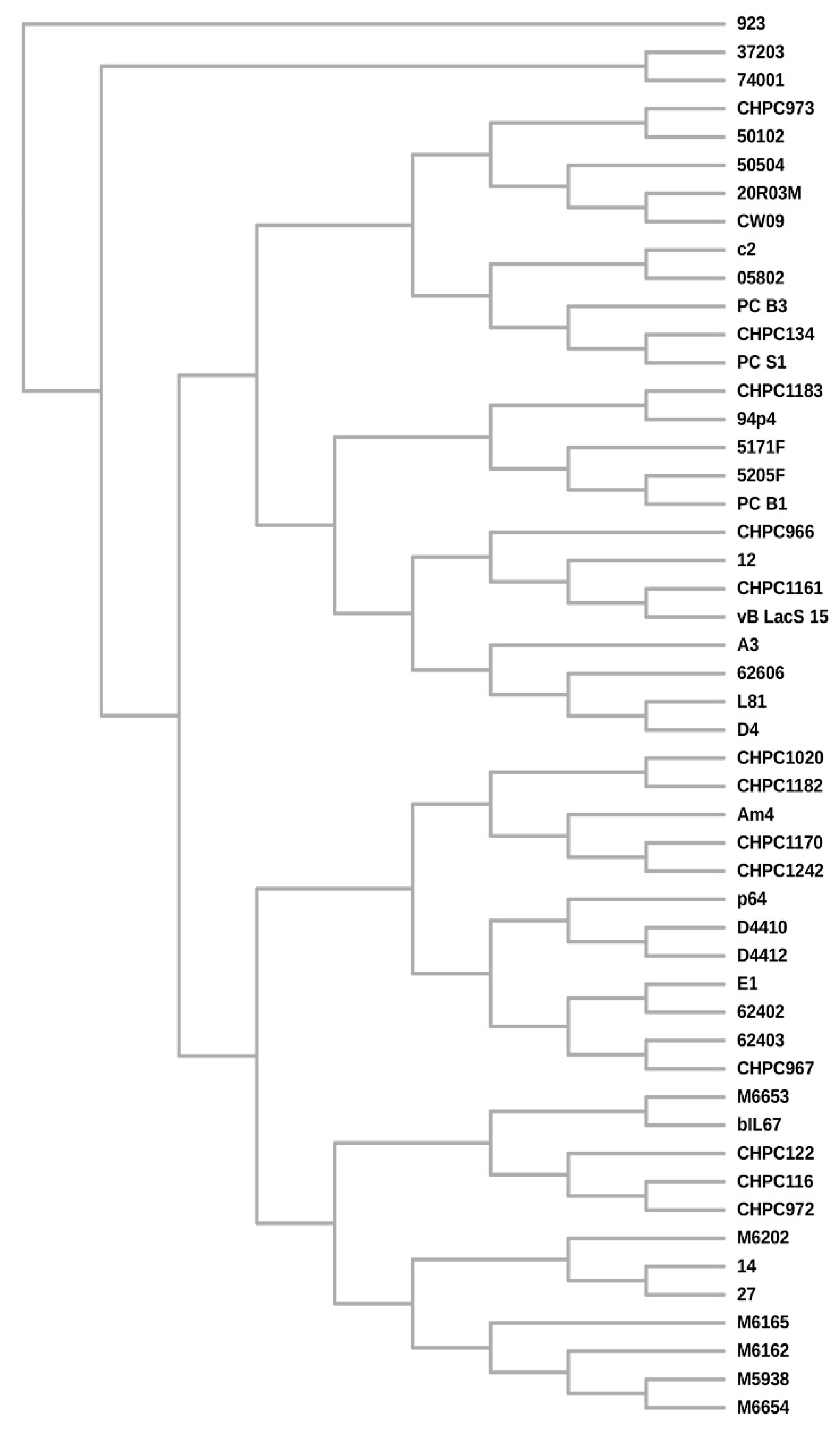
3.4. Phage Genome Sequencing and Comparative Analysis
3.5. Identification and Functional Analysis of the Origin of Phage Replication
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Labrie, S.; Moineau, S. Multiplex PCR for detection and identification of lactococcal bacteriophages. Appl. Environ. Microbiol. 2000, 66, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Deveau, H.; Labrie, S.J.; Chopin, M.C.; Moineau, S. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 2006, 72, 4338–4346. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, A.W.; Fitzgerald, G.F.; Mata, M.; Mercenier, A.; Neve, H.; Powell, I.B.; Ronda, C.; Saxelin, M.; Teuber, M. Species and type phages of lactococcal bacteriophages. Intervirology 1991, 32, 2–9. [Google Scholar] [CrossRef]
- McGrath, S.; Neve, H.; Seegers, J.F.; Eijlander, R.; Vegge, C.S. Anatomy of a lactococcal phage tail. J. Bacteriol. 2006, 188, 3972–3982. [Google Scholar] [CrossRef]
- Castro-Nallar, E.; Chen, H.; Gladman, S.; Moore, S.C.; Seemann, T.; Powell, I.B.; Hillier, A.; Crandall, K.A.; Chandry, P.S. Population genomics and phylogeography of an Australian dairy factory derived lytic bacteriophage. Genome Biol. Evol. 2012, 4, 382–393. [Google Scholar] [CrossRef]
- Murphy, J.; Bottacini, F.; Mahony, J.; Kelleher, P.; Neve, H.; Zomer, A.; Nauta, A.; van Sinderen, D. Comparative genomics and functional analysis of the 936 group of lactococcal Siphoviridae phages. Sci. Rep. 2016, 6, 21345. [Google Scholar] [CrossRef]
- Schouler, C.; Ehrlich, S.D.; Chopin, M.C. Sequence and organization of the lactococcal prolate-headed bIL67 phage genome. Microbiology 1994, 140, 3061–3069. [Google Scholar] [CrossRef]
- Lubbers, M.W.; Waterfield, N.R.; Beresford, T.P.; Le Page, R.W.; Jarvis, A.W. Sequencing and analysis of the prolate-headed lactococcal bacteriophage c2 genome and identification of the structural genes. Appl. Environ. Microbiol. 1995, 61, 4348–4356. [Google Scholar] [CrossRef]
- Millen, A.M.; Romero, D.A. Genetic determinants of lactococcal c2viruses for host infection and their role in phage evolution. J. Gen. Virol. 2016, 97, 1998–2007. [Google Scholar] [CrossRef]
- Oliveira, J.; Mahony, J.; Hanemaaijer, L.; Kouwen, T.R.H.M.; van Sinderen, D. Biodiversity of bacteriophages infecting Lactococcus lactis starter cultures. J. Dairy Sci. 2018, 101, 96–105. [Google Scholar] [CrossRef]
- Hayes Whey Sample Dairy Phages. Available online: https://www.ncbi.nlm.nih.gov/nuccore/?term=Hayes%20Whey%20sample%20dairy%20phages&utm_source=gquery&utm_medium=search (accessed on 2 March 2020).
- Marcelli, B.; de Jong, A.; Kuipers, O.P. Genome Sequences of 31 Lactococcus lactis Bacteriophages Isolated from Foods. Available online: https://www.ncbi.nlm.nih.gov/nuccore/?term=Genome+sequences+of+31+Lactococcus+lactis+bacteriophages+isolated+from+foods (accessed on 2 March 2020).
- Damnjanovic, D.M. Lactococcus Phage vB_LacS_15, Complete Genome. Available online: https://www.ncbi.nlm.nih.gov/nuccore/1740136454? report=genbank (accessed on 3 January 2019).
- Rakonjac, J.; Ward, L.J.H.; Schiemann, A.H.; Gardner, P.P.; Lubbers, M.W.; O’Toole, P.W. Sequence diversity and functional conservation of the origin of replication in lactococcal prolate phages. Appl. Environ. Microbiol. 2003, 69, 5104–5114. [Google Scholar] [CrossRef] [PubMed]
- Stuer-Lauridsen, B.; Janzen, T.; Schnabl, J.; Johansen, E. Identification of the host determinant of two prolate-headed phages infecting lactococcus lactis. Virology 2003, 309, 10–17. [Google Scholar] [CrossRef]
- Mahony, J.; Oliveira, J.; Collins, B.; Hanemaaijer, L.; Lugli, G.A.; Neve, H.; Ventura, M.; Kouwen, T.R.; Cambillau, C.; van Sinderen, D. Genetic and functional characterisation of the lactococcal P335 phage-host interactions. Bmc Genom. 2017, 18, 146. [Google Scholar] [CrossRef]
- Hayes, S.; Duhoo, Y.; Neve, H.; Murphy, J.; Noben, J.P.; Franz, C.M.A.P.; Cambillau, C.; Mahony, J.; Nauta, A.; van Sinderen, D. Identification of dual receptor binding protein systems in lactococcal 936 group phages. Viruses 2018, 10, 668. [Google Scholar] [CrossRef]
- Rakonjac, J.; O’Toole, P.W.; Lubbers, M. Isolation of lactococcal prolate phage-phage recombinants by an enrichment strategy reveals two novel host range determinants. J. Bacteriol. 2005, 187, 3110–3121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ainsworth, S.; Sadovskaya, I.; Vinogradov, E.; Courtin, P.; Guerardel, Y.; Mahony, J.; Grard, T.; Cambillau, C.; Chapot-Chartier, M.F.; van Sinderen, D. Differences in lactococcal cell wall polysaccharide structure are major determining factors in bacteriophage sensitivity. MBio 2014, 5, e00880-e14. [Google Scholar] [CrossRef] [PubMed]
- Chapot-Chartier, M.P.; Kulakauskas, S. Cell wall structure and function in lactic acid bacteria. Microb. Cell Fact. 2014, 13 (Suppl. 1), S9. [Google Scholar] [CrossRef] [PubMed]
- McCabe, O.; Spinelli, S.; Farenc, C.; Labbé, M.; Tremblay, D.; Blangy, S.; Oscarson, S.; Moineau, S.; Cambillau, C. The targeted recognition of Lactococcus lactis phages to their polysaccharide receptors. Mol. Microbiol. 2015, 96, 875–886. [Google Scholar] [CrossRef]
- Waterfield, N.R.; Lubbers, M.W.; Polzin, K.M.; Le Page, R.W.; Jarvis, A.W. An origin of DNA replication from Lactococcus lactis bacteriophage c2. Appl. Environ. Microbiol. 1996, 62, 1452–1453. [Google Scholar] [CrossRef]
- Lubbers, M.W.; Schofield, K.; Waterfield, N.R.; Polzin, K.M. Transcription analysis of the prolate-headed lactococcal bacteriophage c2. J. Bacteriol. 1998, 180, 4487–4496. [Google Scholar] [CrossRef]
- Del Solar, G.; Giraldo, R.; Ruiz-Echevarria, M.J.; Espinosa, M.; Diaz-Orejas, R. Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev. 1998, 62, 434–464. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, A.; Rakonjac, J.; Callanan, M.; Gordon, J.; Polzin, K.; Lubbers, M.W.; O’Toole, P.W. Essentiality of the early transcript in the replication origin of the lactococcal prolate phage c2. J. Bacteriol. 2004, 186, 8010–8017. [Google Scholar] [CrossRef] [PubMed]
- Chopin, A.; Chopin, M.-C.; Moillo-Batt, A.; Langella, P. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid 1984, 11, 260–263. [Google Scholar] [CrossRef]
- Gasson, M.J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 1983, 154, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maguin, E.; Duwat, P.; Hege, T.; Ehrlich, D.; Gruss, A. New thermosensitive plasmid for gram-positive bacteria. J. Bacteriol. 1992, 174, 5633–5638. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.; Uhlén, M.; Josephson, S.; Gatenbeck, S.; Philipson, L. An improved positive selection plasmid vector constructed by oligonucleotide mediated mutagenesis. NAR 1983, 11, 8019–8030. [Google Scholar] [CrossRef][Green Version]
- Dupont, K.; Vogensen, F.K.; Josephsen, J. Detection of lactococcal 936-species bacteriophages in whey by magnetic capture hybridization PCR targeting a variable region of receptor-binding protein genes. J. Appl. Microbiol. 2005, 98, 1001–1009. [Google Scholar] [CrossRef]
- Chmielewska-Jeznach, M.; Bardowski, J.K.; Szczepankowska, A.K. Molecular, physiological and phylogenetic traits of Lactococcus 936-type phages from distinct dairy environments. Sci. Rep. 2018, 22, 12540. [Google Scholar] [CrossRef]
- Mahony, J.; Kot, W.; Murphy, J.; Ainsworth, S.; Neve, H.; Hansen, L.H.; Heller, K.J.; Sørensen, S.J.; Hammer, K.; Cambillau, C.; et al. Investigation of the relationship between lactococcal host cell wall polysaccharide genotype and 936 phage receptor binding protein phylogeny. Appl. Environ. Microbiol. 2013, 79, 4385–4392. [Google Scholar] [CrossRef]
- Szczepanska, A.K.; Hejnowicz, M.S.; Kołakowski, P.; Bardowski, J. Biodiversity of Lactococcus lactis bacteriophages in Polish dairy environment. Acta Biochim. Pol. 2007, 54, 151–158. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M. The RAST Server: Rapid annotations using subsystems technology. Bmc Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]
- Zimmermann, L.; Stephens, A.; Nam, S.Z.; Rau, D.; Kübler, J.; Lozajic, M.; Gabler, F.; Söding, J.; Lupas, A.N.; Alva, V. A completely reimplemented MPI Bioinformatics Toolkit with a new HHpred server at its core. J. Mol. Biol. 2018, 430, 2237–2243. [Google Scholar] [CrossRef]
- Kelley, L.A.; Sternberg, M.J.E. Protein structure prediction on the web: A case study using the Phyre server. Nat. Protoc. 2009, 4, 363–371. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.G.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualiser. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar] [CrossRef] [PubMed]
- Górecki, P.; Tiuryn, J. URec: A system for unrooted reconciliation. Bioinformatics 2007, 23, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Krysa, W. Identification and Characterization of Virulent Lactococcal Bacteriophages. Master’s Thesis, University of Warsaw and the Institute of Biochemistry and Biophysics, Warsaw, Poland, 26 September 2002. (In Polish). [Google Scholar]
- Monteville, M.R.; Ardestani, B.; Geller, B.L. Lactococcal bacteriophages require host cell wall carbohydrate and a plasma membrane protein for adsorption and ejection of DNA. Appl. Environ. Microbiol. 1994, 60, 3204–3211. [Google Scholar] [CrossRef]
- Dupont, K.; Janzen, T.; Vogensen, F.K.; Josephsen, J.; Stuer-Lauridsen, B. Identification of Lactococcus lactis genes required for bacteriophage adsorption. Appl. Environ. Microbiol. 2004, 70, 5825–5832. [Google Scholar] [CrossRef]
- Perrin, R.; Billard, P.; Branlant, C. Comparative analysis of the genomic DNA terminal regions of the lactococcal bacteriophages from species c2. Res. Microbiol. 1997, 148, 573–583. [Google Scholar] [CrossRef]
- Gill, J.J.; Berry, J.D.; Russell, W.K.; Lessor, L.; Escobar-Garcia, D.A.; Hernandez, D.; Kane, A.; Keene, J.; Maddox, M.; Martin, R. The Caulobacter crescentus phage phiCbK: Genomics of a canonical phage. Bmc Genom. 2012, 13, 542. [Google Scholar] [CrossRef]
- Lubbers, M.W.; Wardm, L.J.; Beresford, T.P.; Jarvis, B.D.; Jarvis, A.W. Sequencing and analysis of the cos region of the lactococcal bacteriophage c2. Mol. Gen. Genet. 1994, 245, 160–166. [Google Scholar] [CrossRef]
- Boucher, I.; Moineau, S. Phages of Lactococcus lactis: An ecological and economical equilibrium. Recent Res. Dev. Virol. 2001, 3, 243–256. [Google Scholar]
- Raiski, A.; Belyasova, N. Biodiversity of Lactococcus lactis bacteriophages in the Republic of Belarus. Int. J. Food Microbiol. 2009, 130, 1–5. [Google Scholar] [CrossRef]
- Heap, H.A.; Jarvis, A.W. A comparison of prolate- and isometric- headed lactic streptococcal bacteriophages. N. Z. J. Dairy Sci. Technol. 1980, 15, 75–81. [Google Scholar]
- Moineau, S.; Fortier, J.; Ackermann, H.W.; Pandian, S. Characterization of lactococcal bacteriophages from Quebec cheese plants. Can. J. Microbiol. 1992, 38, 875–882. [Google Scholar] [CrossRef]
- Depuis, M.-E.; Moineau, S. Genome organization and characterization of the virulent lactococcal phage 1358 and its similarities to Listeria phage. Appl. Environ. Microbiol. 2010, 76, 1623–1632. [Google Scholar] [CrossRef]
- Pires, D.P.; Oliveira, H.; Melo, L.D.R.; Sillankorva, S.; Azeredo, J. Bacteriophage-encoded depolymerases: Their diversityand biotechnological applications. Appl. Microbiol. Biotechnol. 2016, 100, 2141–2151. [Google Scholar] [CrossRef]
- Aucouturier, A.; Chain, F.; Langella, P.; Bidnenko, E. Characterization of a prophage-free derivative strain of Lactococcus lactis ssp. lactis IL1403 reveals the importance of prophages for phenotypic plasticity of the host. Front. Microbiol. 2018, 9, 2032. [Google Scholar] [CrossRef] [PubMed]
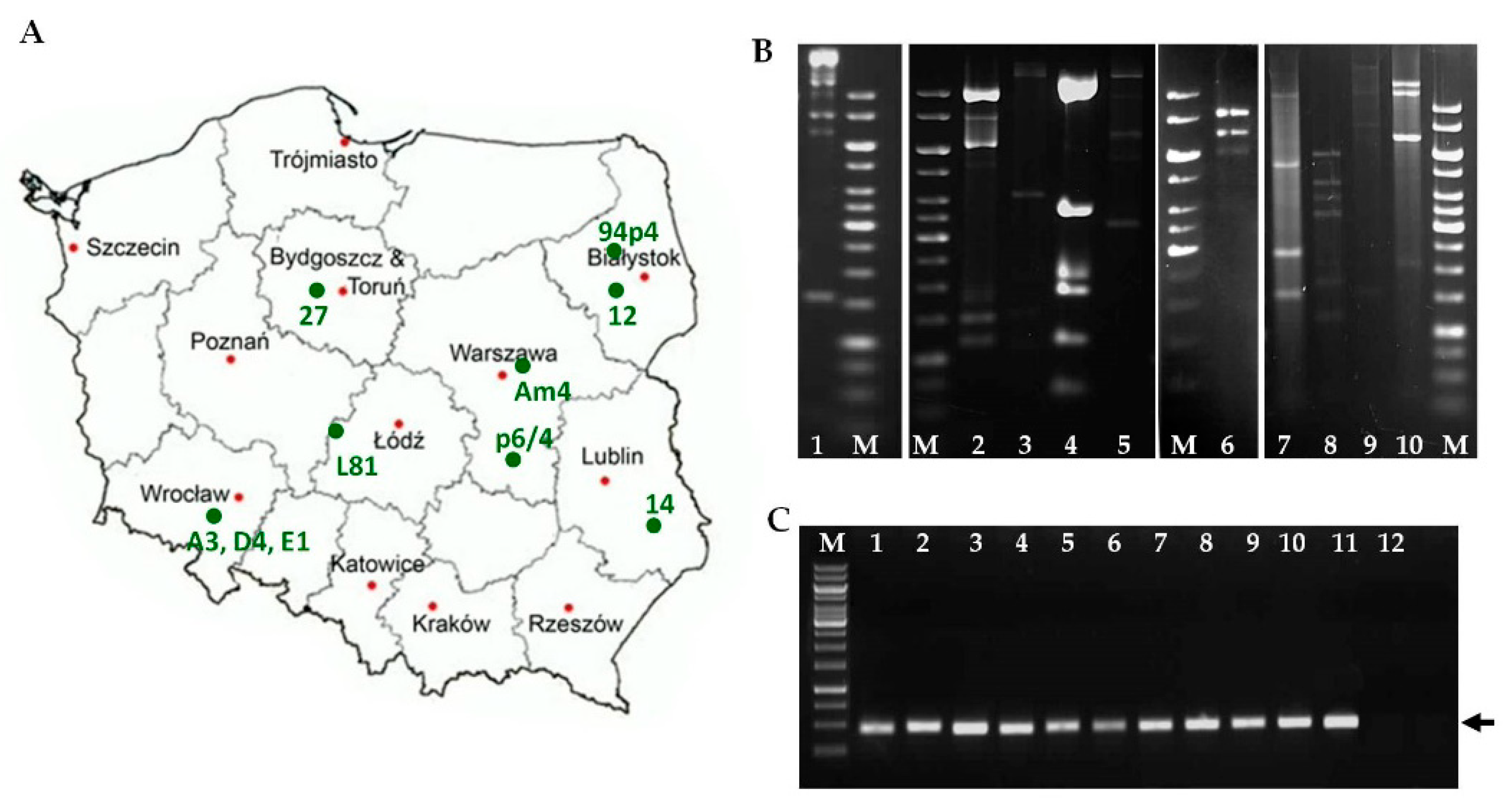
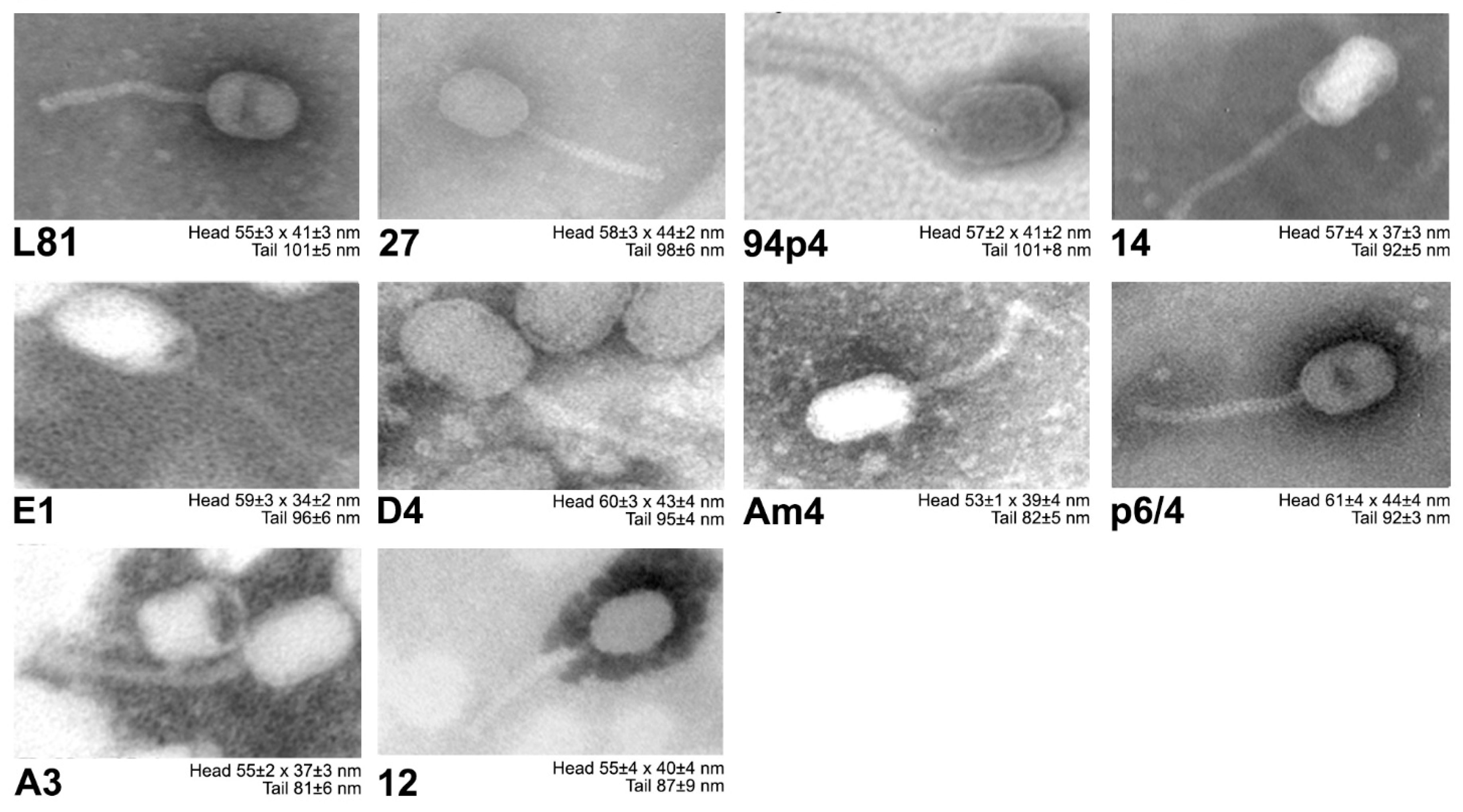
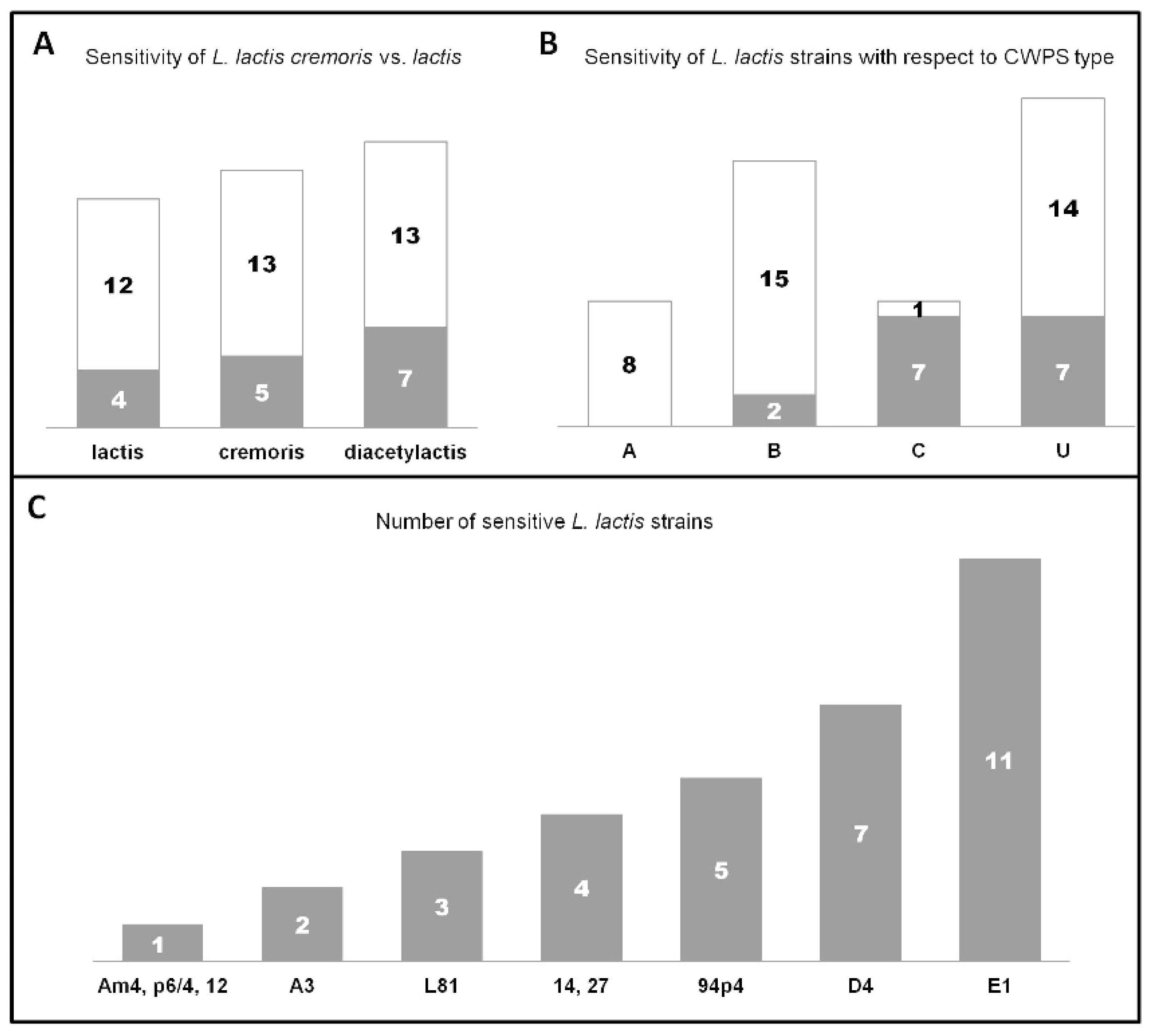
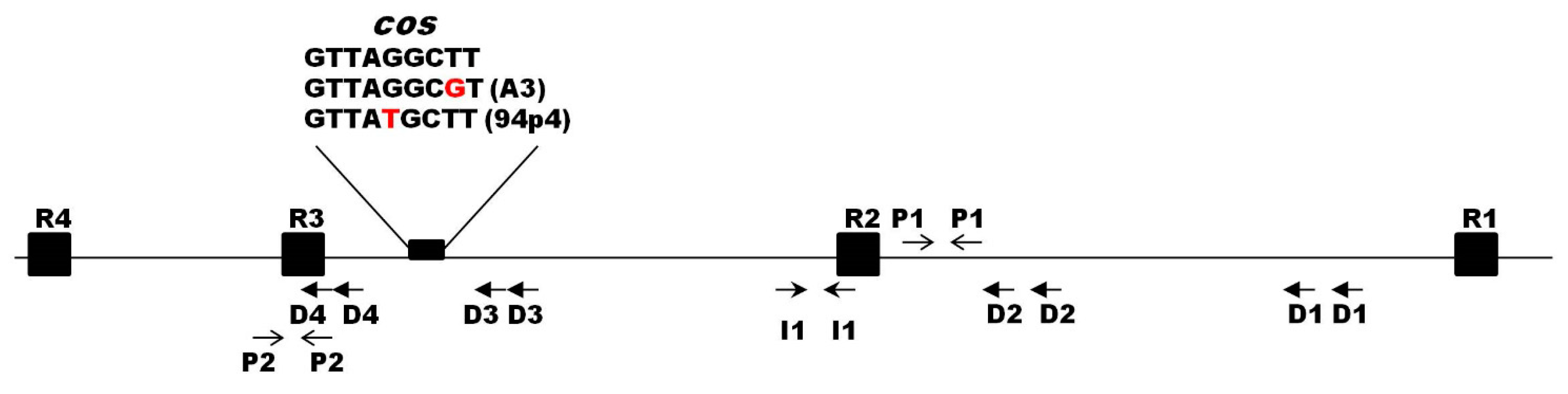
| Bacterial Strains | Relevant Features | Source/Reference |
| IL1403 | L. lactis subsp. lactis wild-type strain | [26] |
| MG1363 | L. lactis subsp. cremoris wild-type strain | [27] |
| NZ9000 | L. lactis subsp. cremoris MG1363 derivative pepN::nisRnisK strain | MoBiTec® |
| ER2655 | E. coli strain F- λ- fhuA2 [lon] ompT lacZ::T7p07 gal sulA11 Δ(mcrC-mrr)114::IS10 R(mcr-73::miniTn10-TetS)2 R(zgb-210::Tn10)(TetS) endA1 [dcm] | NEB® |
| IBB338, 339, 342, 695-700, 704, 732-739, 741-758, 760-764, 1219, 1267, 1280, 1283, 1298, 1784, 1787-1792, 1796 | L. lactis strains | IBB PAS collection |
| Plasmids | Relevant Features | Source/Reference |
| pG+host3 | E. coli vector, Ts derivative of pGK12, catR | [28] |
| pUN121 | E. coli vector, tetR, ampR non-replicative in L. lactis cells | [29] |
| pUN121:catR:ori27 | recombinant plasmid with putative ori from L. lactis phage 27, catR | this study |
| pUN121:catR:ori94p4 | recombinant plasmid with putative ori from L. lactis phage 94p4, catR | this study |
| pUN121:catR:orip6/4 | recombinant plasmid with putative ori from L. lactis phage p6/4, catR | this study |
| pUN121:catR:oric2 | recombinant plasmid with putative ori from L. lactis phage c2, catR | this study |
| Bacteriophages | GenBank Accession Number* | Source/Reference |
| bIL67 | NC_001629.1 | [7] |
| c2 | NC_001706.1 | [8] |
| bIBB_L81 (L81) | MH779526 * | this study |
| bIBB_12 (12) | MH779518 * | “ |
| bIBB_14 (14) | MH779519 * | “ |
| bIBB_94p4 (94p4) | MH779521 * | “ |
| bIBB_27 (27) | MH779520 * | “ |
| bIBB_p6/4 (p6/4) | MH779527 * | “ |
| bIBB_A3 (A3) | MH779522 * | “ |
| bIBB_D4 (D4) | MH779524 * | “ |
| bIBB_Am4 (Am4) | MH779523 * | “ |
| bIBB_E1 (E1) | MH779525 * | “ |
| Name | 5′-3′ Sequence * |
|---|---|
| c-2for | CAGGTGTAAAAGTTCGAGAACT |
| c-2rev | CAGATAATGCACCTGAATCA |
| ori27_F | CCGGAATTCCAAGCTATCAAATATTTC |
| ori27_R | CCCAAGCTTCATACCAACAAAAG |
| orip6/4_F | CCCGATATCCAATGTGTTTTTGTG |
| orip6/4_R | CCCAAGCTTCCTACAAAAAATTTAG |
| ori94p4_F | CCGGAATTCTARTTACYTTGCTAAAGGG |
| ori94p4_R | CCCAAGCTTTCCCTCWTTGATTATG |
| oric2_F | CCGGAATTCTARTTACYTTGCTAAAGGG |
| oric2_R | CCCAAGCTTTCCCTCWTTGATTATG |
| catR_F | CGCGGATCCATGAAGAAAGCAGACAAGTAAG |
| catR_R | ACGCGTCGACGTAAAAAGTACAGTC |
| pUN121_F | GTCTGGCTATGCAGAAATCC |
| pUN121_R | GCTTATAACGCCGCATTGCT |
| pUN121_catR_F | TATCGACTACGCGATCATGG |
| pUN121_catR_R | GCGTGCAAGATTCCGAATAC |
| CWPS_Afor | GTGCCTATGCTCCGTTAGTC |
| CWPS_Arev | CGAGGGCCAATCTCTTTACC |
| CWPS_Bfor | GATTCAGTTGCACGGCCG |
| CWPS_Brev | AGTAAGGGGGCGGATTGTG |
| CWPS_Cfor | AAAGCTCATCTTTCCCCTGTTGT |
| CWPS_Crev | GCACCATAGTCTGGAATAAGACC |
| CTL_for (U-type) | GTACACTATGTTTATAACAATCATCCAG |
| CTL_rev (U-type) | GCAAACCAGATTCAAAGTCAGTATG |
| Phage | Burst Size * [Plaques Per Infected Cell] | Latent Period * [min] | Burst Time * [min] | Plaque Diameter [mm] | Halo [mm] |
|---|---|---|---|---|---|
| 14 | 66 ± 3 | 20 ± 0 | 32 ± 1 | 2.5 | 0.5 |
| 27 | 67 ± 2 | 20 ± 0 | 29 ± 3 | 2 | 0.5 |
| 94p4 | 64 ± 3 | 28 ± 0 | 31 ± 0 | 2 | nd |
| L81 | 32 ± 3 | 20 ± 0 | 27 ± 2 | 2 | 1 |
| p6/4 | 34 ± 3 | 22 ± 3 | 29 ± 1 | 1.5 | nd |
| 12 | 197 ± 17 | 22 ± 3 | 29 ± 2 | 3 | nd |
| E1 | 109 ± 8 | 26 ± 2 | 33 ± 0 | 5.3 | nd |
| D4 | 94 ± 4 | 19 ± 2 | 28 ± 3 | 5 | 1 |
| Am4 | 33 ± 3 | 20 ± 0 | 29 ± 2 | 3.5 | 0.5 |
| A3 | 91 ± 3 | 23 ± 3 | 33 ± 0 | 4 | 0.5 |
| Phage (GenBank Accession No.) | Propagation Strain (Phage Titer; PFU mL−1) | Genome Length (bp) | GC Content (%) | Orf Count |
|---|---|---|---|---|
| 14 (MH779519) | 738 (2.4 × 1010) | 21,710 | 36 | 37 |
| 27 (MH779520) | 738 (8 × 108) | 21,713 | 36.1 | 37 |
| 94p4 (MH779521) | 753 (1.1 × 1010) | 21,834 | 36.1 | 38 |
| L81 (MH779526) | MG1363 (8.4 × 109) | 21,915 | 36.0 | 38 |
| p6/4 (MH779527) | 739 (1.3 × 109) | 22,220 | 36.0 | 39 |
| 12 (MH779418) | 1787 (6.1 × 109) | 21,458 | 36.2 | 36 |
| E1 (MH779525) | 1283 (1.3 × 1010) | 21,749 | 36.1 | 37 |
| D4 (MH779524) | 1280 (1.4 × 1010) | 21,907 | 36.0 | 38 |
| Am4 (MH779523) | 1219 (2.4 × 109) | 22,703 | 35.9 | 38 |
| A3 (MH779522) | 1267 (7.2 × 109) | 21,906 | 36.2 | 39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielewska-Jeznach, M.; Bardowski, J.K.; Szczepankowska, A.K. Lactococcus Ceduovirus Phages Isolated from Industrial Dairy Plants—From Physiological to Genomic Analyses. Viruses 2020, 12, 280. https://doi.org/10.3390/v12030280
Chmielewska-Jeznach M, Bardowski JK, Szczepankowska AK. Lactococcus Ceduovirus Phages Isolated from Industrial Dairy Plants—From Physiological to Genomic Analyses. Viruses. 2020; 12(3):280. https://doi.org/10.3390/v12030280
Chicago/Turabian StyleChmielewska-Jeznach, Magdalena, Jacek K. Bardowski, and Agnieszka K. Szczepankowska. 2020. "Lactococcus Ceduovirus Phages Isolated from Industrial Dairy Plants—From Physiological to Genomic Analyses" Viruses 12, no. 3: 280. https://doi.org/10.3390/v12030280
APA StyleChmielewska-Jeznach, M., Bardowski, J. K., & Szczepankowska, A. K. (2020). Lactococcus Ceduovirus Phages Isolated from Industrial Dairy Plants—From Physiological to Genomic Analyses. Viruses, 12(3), 280. https://doi.org/10.3390/v12030280





