Antivirals Against Chikungunya Virus: Is the Solution in Nature?
Abstract
1. Introduction
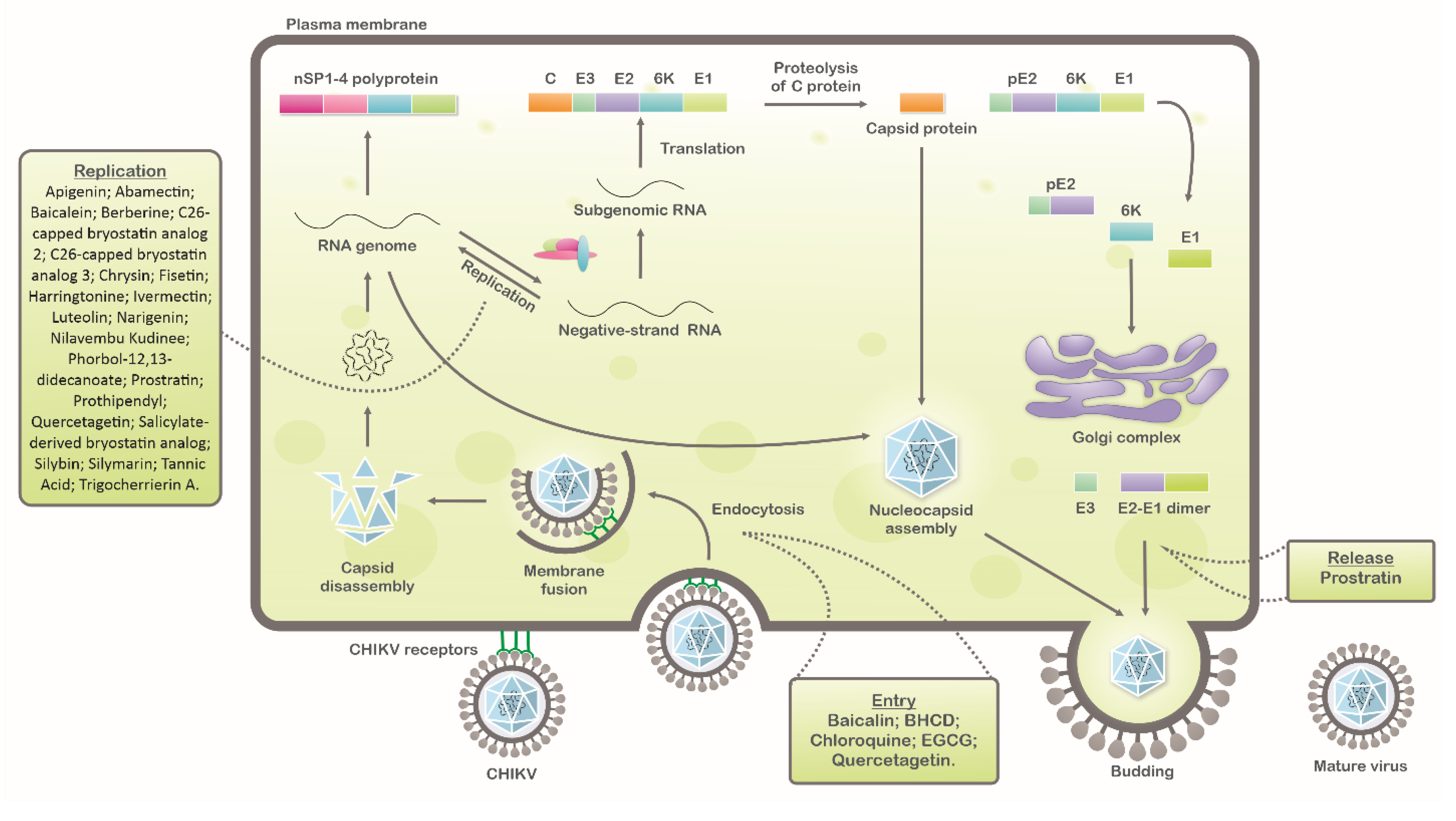
2. Inhibitors of CHIKV Replicative Cycle
2.1. Epigallocatechin Gallate (Green Tea)
2.2. Chloroquine
2.3. Apigenin, Chrysin, Luteonin, Narigerin, Silybin, and Prothipendyl
2.4. Flavaglines
2.5. Compounds from Tectona grandis
2.6. Trigocherrierin A
2.7. Harringtonine
2.8. Diterpene Ester (phorbol-12,13-didecanoate)
2.9. Daphanane Diterpenoid Ortho Esters
2.10. Aplysiatoxin-Related Compounds
2.11. Tannic Acid
2.12. Silymarin
2.13. Baicalein, Fisetin, and Quercetagetin
2.14. Bryostatin
2.15. Prostatin
2.16. Berberine
2.17. Avermectin Derivates
3. Prospects
4. Conclusions
Funding
Conflicts of Interest
References
- Ross, R.W. The virus: Isolation, Pathogenic properties and relationship to the epidemic. Newala Epidemic 1956, 177–191. [Google Scholar]
- Panning, M.; Grywna, K.; van Esbroeck, M.; Emmerich, P.; Drosten, C. Chikungunya Fever in Travelers Returning to Europe from the Indian Ocean Region, 2006. Emerg. Infect. Dis. 2008, 14, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Schuffenecker, I.; Frangeul, L.; Vaney, M.; Iteman, I.; Michault, A.; Lavenir, R.; Pardigon, N.; Reynes, J.; Biscornet, L.; Diancourt, L. Genome Microevolution of Chikungunya Viruses Causing the Indian Ocean Outbreak. PLoS Med. 2006, 3, 1058–1070. [Google Scholar] [CrossRef]
- Henry, M.; Francis, L.; Asin, V.; Polson-Edwards, K.; Olowokure, B. Chikungunya virus outbreak in Sint Maarten, 2013-2014. Rev. Panam. Salud Publica Pan Am. J. Public Health 2017, 41, e61. [Google Scholar] [CrossRef]
- Morens, D.M.; Fauci, A.S. Chikungunya at the Door — Déjà Vu All Over Again? N. Engl. J. Med. 2014, 371, 885–887. [Google Scholar] [CrossRef] [PubMed]
- PAHO PAHO WHO. Chikungunya. Data, Maps and Statistics. Available online: https://www.paho.org/hq/index.php%3Foption%3Dcom_topics%26view%3Drdmore%26cid%3D5927%26item%3Dchikungunya%26type%3Dstatistics%26Itemid%3D40931%26lang%3Den (accessed on 28 December 2019).
- Carbajo, A.E.; Vezzani, D. Waiting for chikungunya fever in Argentina: Spatio-temporal risk maps. Mem. Inst. Oswaldo Cruz 2015, 110, 259–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Badar, N.; Salman, M.; Ansari, J.; Ikram, A.; Qazi, J.; Alam, M.M. Epidemiological trend of chikungunya outbreak in Pakistan: 2016-2018. PLoS Negl. Trop. Dis. 2019, 13, 2018–2019. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.; Solet, J.; Sissoko, D.; Balleydier, E.; Larrieu, S.; Filleul, L.; Lassalle, C.; Thiria, J.; Rachou, E.; De Valk, H.; et al. A Major Epidemic of Chikungunya Virus Infection on Réunion Island. Am. Soc. Trop. Med. Hyg. 2007, 77, 727–731. [Google Scholar] [CrossRef]
- Thiberville, S.D.; Moyen, N.; Dupuis-Maguiraga, L.; Nougairede, A.; Gould, E.A.; Roques, P.; de Lamballerie, X. Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antivir. Res. 2013, 99, 345–370. [Google Scholar] [CrossRef]
- Song, H.; Zhao, Z.; Chai, Y.; Jin, X.; Li, C.; Yuan, F.; Liu, S.; Gao, Z.; Wang, H.; Song, J.; et al. Molecular Basis of Arthritogenic Alphavirus Receptor MXRA8 Binding to Chikungunya Virus Envelope Protein. Cell 2019, 177, 1714–1724.e12. [Google Scholar] [CrossRef]
- Zhang, R.; Earnest, J.T.; Kim, A.S.; Winkler, E.S.; Desai, P.; Adams, L.J.; Hu, G.; Bullock, C.; Gold, B.; Cherry, S.; et al. Expression of the Mxra8 Receptor Promotes Alphavirus Infection and Pathogenesis in Mice and Drosophila. Cell Rep. 2019, 28, 2647–2658.e5. [Google Scholar] [CrossRef] [PubMed]
- Wintachai, P.; Wikan, N.; Kuadkitkan, A.; Jaimipuk, T.; Ubol, S.; Pulmanausakakil, R.; Auewarakul, P.; Kasinrerk, W.; Weng, W.-Y.; Panyasrivanit, M.; et al. Identification of Prohibitin as a Chikungunya Virus Receptor Protein. J. Med. Virol. 2012, 84, 1757–1770. [Google Scholar] [CrossRef] [PubMed]
- Moller-Tank, S.; Kondratowicz, A.S.; Davey, R.A.; Rennert, P.D.; Maury, W. Role of the Phosphatidylserine Receptor TIM-1 in Enveloped-Virus Entry. J. Virol. 2013, 87, 8327–8341. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Khomandiak, S.; Ashbrook, A.W.; Weller, R.; Heise, M.T.; Morrison, T.E.; Dermody, T.S.; Lyles, D.S. A Single-Amino-Acid Polymorphism in Chikungunya Virus E2 Glycoprotein Influences Glycosaminoglycan Utilization. J. Virol. 2014, 88, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Fongsaran, C.; Jirakanwisal, K.; Kuadkitkan, A.; Wikan, N.; Wintachai, P.; Thepparit, C.; Ubol, S.; Phaonakrop, N.; Roytrakul, S.; Smith, D.R. Involvement of ATP synthase β subunit in chikungunya virus entry into insect cells. Arch. Virol. 2014, 159, 3353–3364. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Neyts, J.; Delang, L. Towards antivirals against chikungunya virus. Antivir. Res. 2015, 121, 59–68. [Google Scholar] [CrossRef]
- Gould, E.A.; Coutard, B.; Malet, H.; Morin, B.; Jamal, S.; Weaver, S.; Gorbalenya, A.; Moureau, G.; Baronti, C.; Delogu, I.; et al. Understanding the alphaviruses: Recent research on important emerging pathogens and progress towards their control. Antivir. Res. 2010, 87, 111–124. [Google Scholar] [CrossRef]
- De Lima, M.E.S.; Bachur, T.P.R.; Aragão, G.F. Guillain-Barre syndrome and its correlation with dengue, Zika and chikungunya viruses infection based on a literature review of reported cases in Brazil. Acta Trop. 2019, 197, 105064. [Google Scholar] [CrossRef]
- W.H.O. Chikungunya. Available online: https://www.who.int/news-room/fact-sheets/detail/chikungunya (accessed on 28 December 2019).
- Yactayo, S.; Staples, J.E.; Millot, V.; Cibrelus, L.; Ramon-Pardo, P. Epidemiology of Chikungunya in the Americas. J. Infect. Dis. 2016, 214, S441–S445. [Google Scholar] [CrossRef]
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; AbuBakar, S. Novel antiviral activity of baicalein against dengue virus. BMC Complement. Altern. Med. 2012, 12, 1185. [Google Scholar] [CrossRef]
- Jain, J.; Kumar, A.; Narayanan, V.; Ramaswamy, R.S.; Sathiyarajeswaran, P.; Shree Devi, M.S.; Kannan, M.; Sunil, S. Antiviral activity of ethanolic extract of Nilavembu Kudineer against dengue and chikungunya virus through in vitro evaluation. J. Ayurveda Integr. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Calderón, C.; Mesa-Castro, C.; Robledo, S.; Gómez, S.; Bolivar-Avila, S.; Diaz-Castillo, F.; Martínez-Gutierrez, M. Antiviral effect of compounds derived from the seeds of Mammea americana and Tabernaemontana cymosa on Dengue and Chikungunya virus infections. BMC Complement. Altern. Med. 2017, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, E.; Pezzullo, M.; De burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K.; De lamballerie, X.; Neyts, J.; Hanson, A.M.; Frick, D.N.; et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G. Experimental therapies for yellow fever. Antivir. Res. 2013, 97, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Danielle, V.; Muller, M.; Rinaldi, R.; Cristina, A.; Cintra, O.; Aurélio, M.; Alves-paiva, R.D.M.; Tadeu, L.; Figueiredo, M.; Vilela, S.; et al. Toxicon Crotoxin and phospholipases A 2 from Crotalus durissus terri fi cus showed antiviral activity against dengue and yellow fever viruses. Toxicon 2012, 59, 507–515. [Google Scholar]
- Calland, N.; Dubuisson, J.; Rouillé, Y.; Séron, K. Hepatitis C virus and natural compounds: A new antiviral approach? Viruses 2012, 4, 2197–2217. [Google Scholar] [CrossRef]
- Campos, G.R.F.; Bittar, C.; Jardim, A.C.G.; Shimizu, J.F.; Batista, M.N.; Paganini, E.R.; de Assis, L.R.; Bartlett, C.; Harris, M.; da Bolzani, V.S.; et al. Hepatitis C virus in vitro replication is efficiently inhibited by acridone Fac4. J. Gen. Virol. 2017, 98, 1693–1701. [Google Scholar] [CrossRef]
- Stankiewicz-drogon, A.; Palchykovska, L.G.; Kostina, V.G.; Alexeeva, I.V.; Shved, A.D.; Boguszewska-chachulska, A.M. New acridone-4-carboxylic acid derivatives as potential inhibitors of Hepatitis C virus infection. Bioorg. Med. Chem. 2008, 16, 8846–8852. [Google Scholar] [CrossRef]
- Jardim, A.C.G.; Igloi, Z.; Shimizu, J.F.; Santos, V.A.F.F.M.; Felippe, L.G.; Mazzeu, B.F.; Amako, Y.; Furlan, M.; Harris, M.; Rahal, P. Natural compounds isolated from Brazilian plants are potent inhibitors of hepatitis C virus replication in vitro. Antivir. Res. 2015, 115, 39–47. [Google Scholar] [CrossRef]
- Shimizu, J.F.; Lima, C.S.; Pereira, C.M.; Bittar, C.; Batista, M.N.; Nazaré, A.C.; Polaquini, C.R.; Zothner, C.; Harris, M.; Rahal, P.; et al. Flavonoids from Pterogyne nitens Inhibit Hepatitis C Virus Entry. Sci. Rep. 2017, 7, 16127. [Google Scholar] [CrossRef]
- Varghese, F.S.; Rausalu, K.; Hakanen, M.; Saul, S.; Kümmerer, B.M.; Susi, P.; Merits, A.; Ahola, T. Obatoclax Inhibits Alphavirus Membrane Fusion by Neutralizing the Acidic Environment of Endocytic Compartments. Antimicrob. Agents Chemother. 2017, 61, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Deng, Y.; Wang, S.; Ma, F.; Aliyari, R.; Huang, X.-Y.; Zhang, N.-N.; Watanabe, M.; Dong, H.-L.; Liu, P.; et al. 25-Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity 2017, 46, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The green tea molecule EGCG inhibits Zika virus entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Sliva, K.; Von Rhein, C.; Kümmerer, B.M.; Schnierle, B.S. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antivir. Res. 2015, 113, 1–3. [Google Scholar] [CrossRef]
- Khan, M.; Santhosh, S.R.; Tiwari, M.; Rao, P.V.L.; Parida, M. Assessment of In Vitro Prophylactic and Therapeutic Efficacy of Chloroquine Against Chikungunya Virus in Vero Cells. J. Med. Virol. 2010, 824, 817–824. [Google Scholar] [CrossRef]
- Pohjala, L.; Utt, A.; Varjak, M.; Lulla, A.; Merits, A.; Ahola, T.; Tammela, P. Inhibitors of Alphavirus Entry and Replication Identified with a Stable Chikungunya Replicon Cell Line and Virus-Based Assays. PLoS ONE 2011, 6, e28923. [Google Scholar] [CrossRef]
- Murali, K.S.; Sivasubramanian, S.; Vincent, S.; Murugan, S.B.; Giridaran, B.; Dinesh, S.; Gunasekaran, P.; Krishnasamy, K.; Sathishkumar, R. Anti—chikungunya activity of luteolin and apigenin rich fraction from Cynodon dactylon. Asian Pac. J. Trop. Med. 2015, 8, 352–358. [Google Scholar] [CrossRef]
- Wintachai, P.; Thuaud, F.; Basmadjian, C.; Roytrakul, S.; Ubol, S.; Désaubry, L.; Smith, D.R. Assessment of flavaglines as potential chikungunya virus entry inhibitors. Microbiol. Immunol. 2015, 59, 129–141. [Google Scholar] [CrossRef]
- Sangeetha, K.; Purushothaman, I.; Rajarajan, S. Spectral characterisation, antiviral activities, in silico ADMET and molecular docking of the compounds isolated from Tectona grandis to chikungunya virus. Biomed. Pharmacother. 2017, 87, 302–310. [Google Scholar]
- Bourjot, M.; Leyssen, P.; Neyts, J.; Dumontet, V.; Litaudon, M. Trigocherrierin A, a potent inhibitor of chikungunya virus replication. Molecules 2014, 19, 3617–3627. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Thiruchelvan, M.; Lee, R.C.H.; Chen, H.; Chen, K.C.; Ng, M.L.; Chu, J.J.H. Inhibition of Chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob. Agents Chemother. 2013, 57, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Allard, P.M.; Leyssen, P.; Martin, M.T.; Bourjot, M.; Dumontet, V.; Eydoux, C.; Guillemot, J.C.; Canard, B.; Poullain, C.; Guéritte, F.; et al. Antiviral chlorinated daphnane diterpenoid orthoesters from the bark and wood of Trigonostemon cherrieri. Phytochemistry 2012, 84, 160–168. [Google Scholar] [CrossRef]
- Nothias-Scaglia, L.-F.; Pannecouque, C.; Renucci, F.; Delang, L.; Neyts, J.; Roussi, F.; Costa, J.; Leyssen, P.; Litaudon, M.; Paolini, J. Antiviral Activity of Diterpene Esters on Chikungunya Virus and HIV Replication. J. Nat. Prod. 2015, 78, 1277–1283. [Google Scholar] [CrossRef]
- Gupta, D.K.; Kaur, P.; Leong, S.T.; Tan, L.T.; Prinsep, M.R.; Chu, J.J.H. Anti-Chikungunya viral activities of aplysiatoxin-related compounds from the marine cyanobacterium Trichodesmium erythraeum. Mar. Drugs 2014, 12, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, R.; Hagedorn, C.H. Tannic Acid Inhibits Hepatitis C Virus Entry into Huh7.5 Cells. PLoS ONE 2015, 10, e0131358. [Google Scholar] [CrossRef]
- Orłowski, P.; Kowalczyk, A.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Węgrzyn, A.; Grzesiak, J.; Celichowski, G.; Grobelny, J.; Eriksson, K.; Krzyzowska, M. Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis. Viruses 2018, 10, 524. [Google Scholar] [CrossRef]
- Konishi, E.; Hotta, S. Effects of Tannic Acid and Its Related Compounds upon Chikungunya Virus. Microbiol. Immunol. 1979, 23, 659–667. [Google Scholar] [CrossRef]
- Wagoner, J.; Negash, A.; Kane, O.J.; Martinez, L.E.; Nahmias, Y.; Bourne, N.; Owen, D.M.; Grove, J.; Brimacombe, C.; McKeating, J.A.; et al. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatol. Baltim. Md 2010, 51, 1912–1921. [Google Scholar] [CrossRef]
- Lani, R.; Hassandarvish, P.; Chiam, C.W.; Moghaddam, E.; Chu, J.J.H.; Rausalu, K.; Merits, A.; Higgs, S.; Vanlandingham, D.; Abu Bakar, S.; et al. Antiviral activity of silymarin against chikungunya virus. Sci. Rep. 2015, 5, 11421. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wu, T.; Jin, Y.; Cheng, J.; Wan, C.; Qian, W.; Xing, F.; Shi, W. The Antiviral Effect of Baicalin on Enterovirus 71 In Vitro. Viruses 2015, 7, 4756–4771. [Google Scholar] [CrossRef] [PubMed]
- Lani, R.; Hassandarvish, P.; Shu, M.-H.; Phoon, W.H.; Chu, J.J.H.; Higgs, S.; Vanlandingham, D.; Abu Bakar, S.; Zandi, K. Antiviral activity of selected flavonoids against Chikungunya virus. Antivir. Res. 2016, 133, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Halford, B. THE BRYOSTATINS’ TALE. Chem. Eng. News Arch. 2011, 89, 10–17. [Google Scholar] [CrossRef]
- Plimack, E.R.; Tan, T.; Wong, Y.-N.; von Mehren, M.M.; Malizzia, L.; Roethke, S.K.; Litwin, S.; Li, T.; Hudes, G.R.; Haas, N.B. A Phase I Study of Temsirolimus and Bryostatin-1 in Patients With Metastatic Renal Cell Carcinoma and Soft Tissue Sarcoma. Oncologist 2014, 19, 354–355. [Google Scholar] [CrossRef]
- Schrott, L.M.; Jackson, K.; Yi, P.; Dietz, F.; Johnson, G.S.; Basting, T.F.; Purdum, G.; Tyler, T.; Rios, J.D.; Castor, T.P.; et al. Acute oral Bryostatin-1 administration improves learning deficits in the APP/PS1 transgenic mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 22–31. [Google Scholar] [CrossRef]
- Mehla, R.; Bivalkar-Mehla, S.; Zhang, R.; Handy, I.; Albrecht, H.; Giri, S.; Nagarkatti, P.; Nagarkatti, M.; Chauhan, A. Bryostatin Modulates Latent HIV-1 Infection via PKC and AMPK Signaling but Inhibits Acute Infection in a Receptor Independent Manner. PLoS ONE 2010, 5, e11160. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Staveness, D.; Near, K.E.; Wender, P.A.; Delang, L.; Neyts, J.; Leyssen, P. Comparative analysis of the anti-chikungunya virus activity of novel bryostatin analogs confirms the existence of a PKC-independent mechanism. Biochem. Pharmacol. 2016, 120, 15–21. [Google Scholar] [CrossRef]
- Bourjot, M.; Delang, L.; Nguyen, V.H.; Neyts, J.; Guéritte, F.; Leyssen, P.; Litaudon, M. Prostratin and 12- O -Tetradecanoylphorbol 13-Acetate Are Potent and Selective Inhibitors of Chikungunya Virus Replication. J. Nat. Prod. 2012, 75, 2183–2187. [Google Scholar] [CrossRef]
- Varghese, F.S.; Thaa, B.; Amrun, S.N.; Simarmata, D.; Rausalu, K.; Nyman, T.A.; Merits, A.; McInerney, G.M.; Ng, L.F.P.; Ahola, T. The Antiviral Alkaloid Berberine Reduces Chikungunya Virus-Induced Mitogen-Activated Protein Kinase Signaling. J. Virol. 2016, 90, 9743–9757. [Google Scholar] [CrossRef]
- Campbell, W.C.; Fisher, M.H.; Stapley, E.O.; Albers-Schönberg, G.; Jacob, T.A. Ivermectin: A potent new antiparasitic agent. Science 1983, 221, 823–828. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Muhammed Ameen, S.; Drancourt, M. Ivermectin lacks antituberculous activity. J. Antimicrob. Chemother. 2013, 68, 1936–1937. [Google Scholar] [CrossRef]
- Laing, R.; Gillan, V.; Devaney, E. Ivermectin – Old Drug, New Tricks? Trends Parasitol. 2017, 33, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.S.; Kaukinen, P.; Gläsker, S.; Bespalov, M.; Hanski, L.; Wennerberg, K.; Kümmerer, B.M.; Ahola, T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir. Res. 2016, 117–124. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee on Applications of Toxicogenomic Technologies to Predictive Toxicology. Application to the Study of Mechanisms of Action; National Academies Press: Washington, DC, USA, 2007.
- Kaur, G.; Dufour, J.M. Cell lines. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, A.B.; Pour, P.M. Cell Lines. In Encyclopedia of Genetics; Brenner, S., Miller, J.H., Eds.; Academic Press: New York, NY, USA, 2001; pp. 310–311. [Google Scholar]
- Slater, A.F. Chloroquine: Mechanism of drug action and resistance in Plasmodium falciparum. Pharmacol. Ther. 1993, 57, 203–235. [Google Scholar] [CrossRef]
- Roques, P.; Thiberville, S.-D.; Dupuis-Maguiraga, L.; Lum, F.-M.; Labadie, K.; Martinon, F.; Gras, G.; Lebon, P.; Ng, L.F.P.; de Lamballerie, X.; et al. Paradoxical Effect of Chloroquine Treatment in Enhancing Chikungunya Virus Infection. Viruses 2018, 10, 268. [Google Scholar] [CrossRef]
| Compound | Structure | Inhibition | SI or EC50 | Cell Line |
|---|---|---|---|---|
| Abamectin [66] | 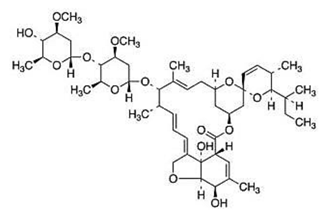 | Replication | 1.5 µM | BHK-21 |
| Apigenin [39,40] | 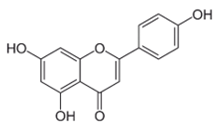 | Infection/Replication | 70.8 µM | BHK 21 |
| Baicalein [54] | 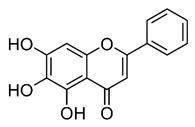 | Infection and replication | 1.891 µg/mL | BHK-21 |
| Baicalein [54] | 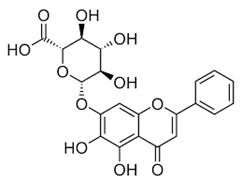 | Entry, binding | 6.997 µM | Vero |
| Berberine [61] | 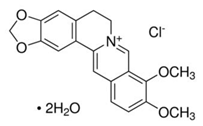 | Replication (interfering in host components) | ≤35.3 µM | CRL-2522, HEK-293T, and HOS |
| BHCD [42] | 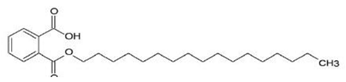 | Entry | 116 (Asian strain) and 4.66 (ECSA) | Vero and in silico |
| C26-capped bryostatin analog 2 [59] | 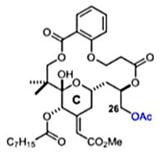 | Replication | 8 µM | Vero |
| C26-capped bryostatin analog 3 [59] | 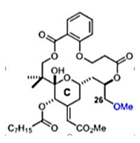 | Replication | 7.5 µM | Vero |
| Chloroquine [38] | 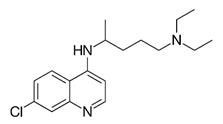 | Entry | 37.14 | Vero |
| Chrysin [39] | 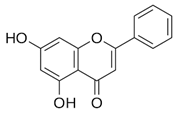 | Infection | 126.6 µM | BHK 21 |
| EGCG [37] | 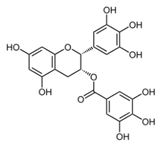 | Entry steps; cell attachment | 6.54 µg/mL | HEK 293T |
| Fisetin [54] | 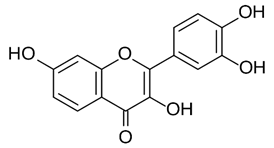 | Replication | 8.44 µg/mL | BHK-21 |
| Harringtonine [44] | 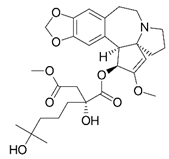 | Early stages of replication | 0.24 µM | BHK 21 |
| Ivermectin [66] | 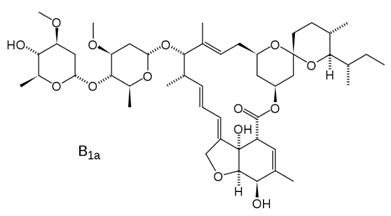 | Replication | 0.6 µM | BHK-21 |
| Luteolin [40] | 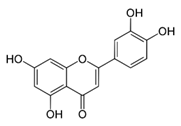 | Replication | NS | Vero |
| Narigenin [39] | 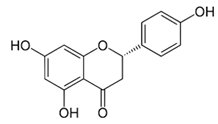 | Infection | 118.4 µM | BHK 21 |
| Prostratin [60] | 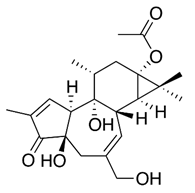 | Replication and release | 2,6 µM and ± 8 µM | Vero, BGM, and HEL |
| Prothipendyl [39] | 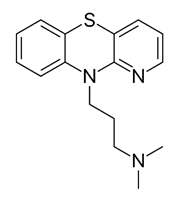 | Replication | 97.3 µM | BHK 21 |
| Quercetagetin [54] | 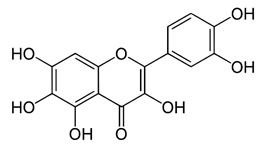 | Entry and binding | 43.52 µM | Vero |
| Quercetagetin [54] |  | Entry and replication | 13.85 µg/mL | BHK-21 |
| Salicylate-derived bryostatin analog [59] | 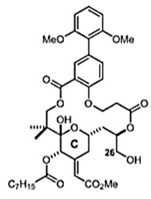 | Entry and replication | 4 µM | Vero |
| Silybin [39] | 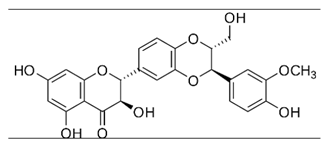 | Infection | 92.3 µM | BHK 21 |
| Silymarin [52] | 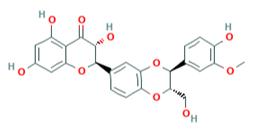 | Replication | 16.9 µg/mL | BHK-21 and Vero |
| Tannic Acid [50] | 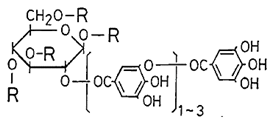 | Replication | NS | BHK-21 |
| Phorbol-12,13-dideca-noate [46] | 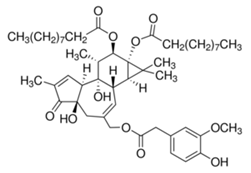 | Replication | 6 ± 0.9 nM | Vero |
| Trigocherrierin [43] | 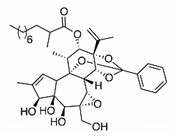 | Replication | 0.6 ± 0.1 µM | Vero |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira Silva Martins, D.; de Andrade Santos, I.; Moraes de Oliveira, D.; Riquena Grosche, V.; Carolina Gomes Jardim, A. Antivirals Against Chikungunya Virus: Is the Solution in Nature? Viruses 2020, 12, 272. https://doi.org/10.3390/v12030272
Oliveira Silva Martins D, de Andrade Santos I, Moraes de Oliveira D, Riquena Grosche V, Carolina Gomes Jardim A. Antivirals Against Chikungunya Virus: Is the Solution in Nature? Viruses. 2020; 12(3):272. https://doi.org/10.3390/v12030272
Chicago/Turabian StyleOliveira Silva Martins, Daniel, Igor de Andrade Santos, Débora Moraes de Oliveira, Victória Riquena Grosche, and Ana Carolina Gomes Jardim. 2020. "Antivirals Against Chikungunya Virus: Is the Solution in Nature?" Viruses 12, no. 3: 272. https://doi.org/10.3390/v12030272
APA StyleOliveira Silva Martins, D., de Andrade Santos, I., Moraes de Oliveira, D., Riquena Grosche, V., & Carolina Gomes Jardim, A. (2020). Antivirals Against Chikungunya Virus: Is the Solution in Nature? Viruses, 12(3), 272. https://doi.org/10.3390/v12030272







