Late-Relapsing Hepatitis after Yellow Fever
Abstract
1. Introduction
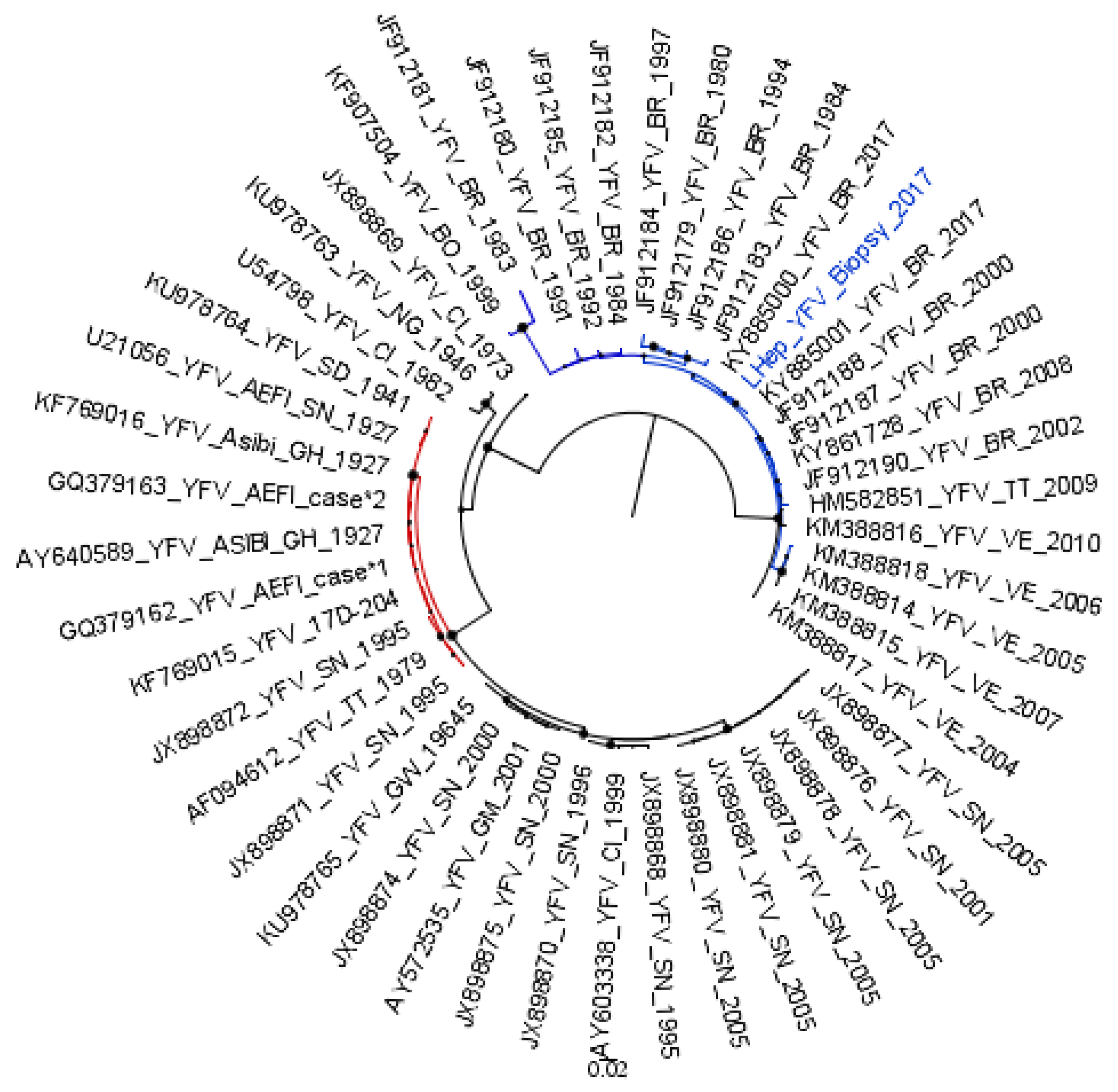
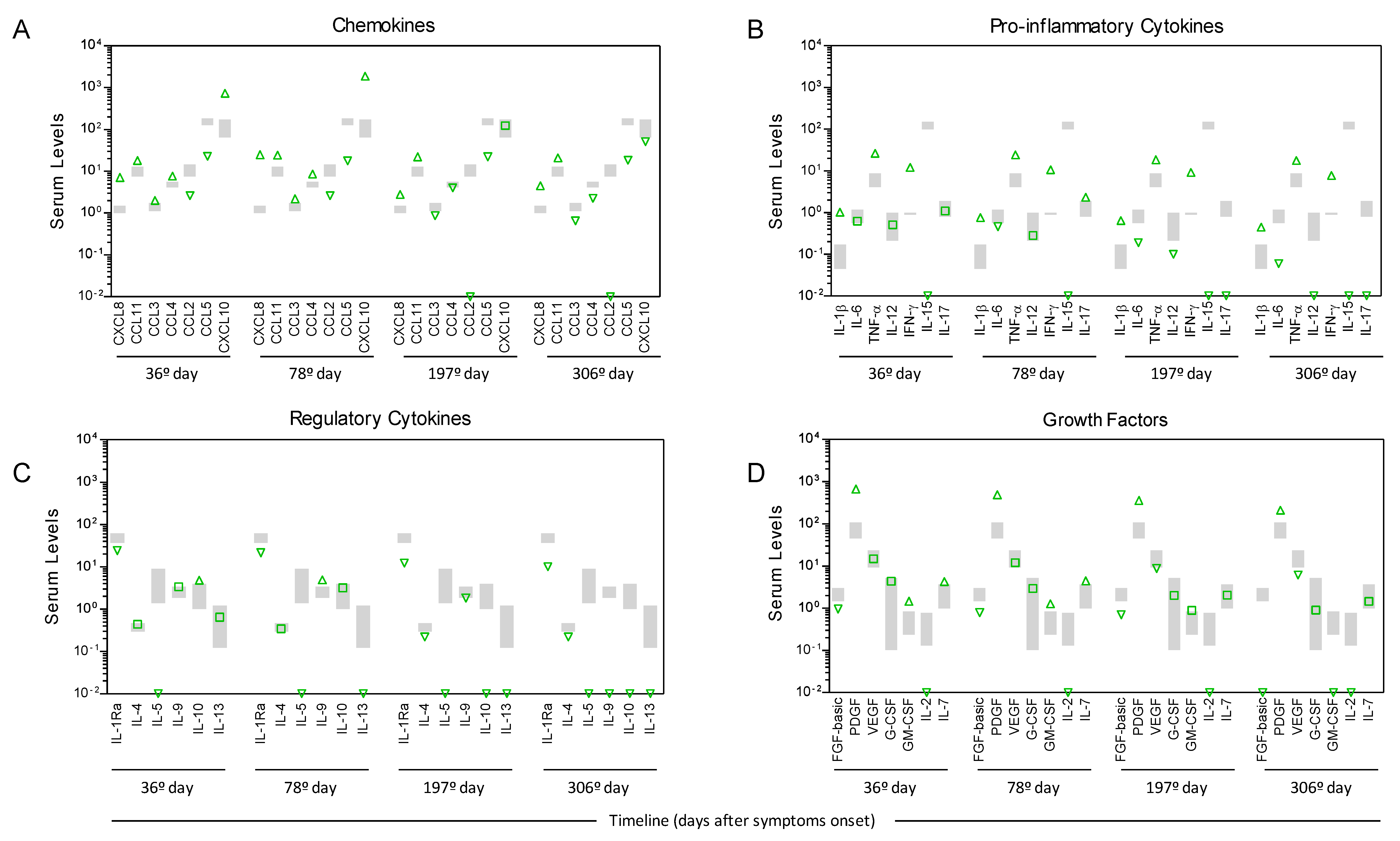
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monath, T.P. Yellow fever: An update. Lancet Infect. Dis. 2001, 1, 11–20. [Google Scholar] [CrossRef]
- Oudart, J.L.; Rey, M. Proteinuria, proteinaemia, and serum transaminase activity in 23 confirmed cases of yellow fever. Bull. World Health Organ. 1970, 42, 95–102. [Google Scholar] [PubMed]
- Kallas, E.G.; D’Elia Zanella, L.G.F.A.B.; Moreira, C.H.V.; Buccheri, R.; Diniz, G.B.F.; Castiñeiras, A.C.P.; Costa, P.R.; Dias, J.Z.C.; Marmorato, M.P.; Song, A.T.W.; et al. Predictors of mortality in patients with yellow fever: An observational cohort study. Lancet Infect. Dis. 2019, 19, 750–758. [Google Scholar] [CrossRef]
- Denis, B.; Chirio, D.; Ponscarme, D.; Brichler, S.; Colin de Verdière, N.; Simon, F.; Molina, J.-M. Hepatitis Rebound after Infection with Yellow Fever Virus. Emerg. Infect. Dis. 2019, 25, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Patel, P.; Yillah, J.; Weidmann, M.; Mendez, J.A.; Nakoune, E.R.; Niedrig, M. Advanced Yellow Fever Virus Genome Detection in Point-of-Care Facilities and Reference Laboratories. J. Clin. Microbiol. 2012, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Alm, E.; Lindegren, G.; Falk, K.I.; Lagerqvist, N. One-step real-time RT-PCR assays for serotyping dengue virus in clinical samples. BMC Infect. Dis. 2015, 15, 493. [Google Scholar] [CrossRef] [PubMed]
- Santiago, G.A.; Vergne, E.; Quiles, Y.; Cosme, J.; Vazquez, J.; Medina, J.F.; Medina, F.; Colón, C.; Margolis, H.; Muñoz-Jordán, J.L. Analytical and Clinical Performance of the CDC Real Time RT-PCR Assay for Detection and Typing of Dengue Virus. PLoS Negl. Trop. Dis. 2013, 7, e2311. [Google Scholar] [CrossRef]
- Kar, P.K.; Nagpal, B.N.; Dua, V.K.; Ghosh, S.K.; Raghavendra, K.; Bhatt, R.M.; Anvikar, A.; Das, A. Molecular characterization of chikungunya virus from Andhra Pradesh, India. Indian J. Med. Res. 2009, 129, 335–338. [Google Scholar] [PubMed]
- Patel, P.; Landt, O.; Kaiser, M.; Faye, O.; Koppe, T.; Lass, U.; Sall, A.A.; Niedrig, M. Development of one-step quantitative reverse transcription PCR for the rapid detection of flaviviruses. Virol. J. 2013, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Wieten, R.W.; Jonker, E.F.F.; Pieren, D.K.J.; Hodiamont, C.J.; van Thiel, P.P.A.M.; van Gorp, E.C.M.; de Visser, A.W.; Grobusch, M.P.; Visser, L.G.; Goorhuis, A. Comparison of the PRNT and an immune fluorescence assay in yellow fever vaccinees receiving immunosuppressive medication. Vaccine 2016, 34, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- American Board of Internal Medicine (ABIM) Laboratory Test Reference Ranges. Available online: https://www.abim.org/~/media/ABIMPublic/Files/pdf/exam/laboratory-reference-ranges.pdf (accessed on 13 January 2020).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, J.J.; Rojas, A.; Pinsky, B.A. Yellow Fever Virus: Diagnostics for a Persistent Arboviral Threat. J. Clin. Microbiol. 2018, 56, e00827-18. [Google Scholar] [CrossRef] [PubMed]
- Francis, T.I.; Moore, D.L.; Edington, G.M.; Smith, J.A. A clinicopathological study of human yellow fever. Bull. World Health Organ. 1972, 46, 659–667. [Google Scholar] [PubMed]

| Timeline Month/DPS | Laboratory Tests (Followed by Normal Range Values/unit) # | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AST | ALT | TBil | DBil | APhos | GGT | INR | Urea | Cr | CK | ||
| 10–40 U/L | 10–40 U/L | 0.3–1.0 mg/dL | 0.1–0.3 mg/dL | 30–120 U/L | 9–50 U/L * | 8–20 mg/dL | 0.7–1.30 mg/dL * | 55–170 U/L * | |||
| 4 | 4560 | 3710 | na | na | na | na | na | 118 | 2.17 | na | |
| 7 A | 781 | 999 | 9.8 | 8.9 | 139 | 495 | 1 | 85.1 | 2 | 42 | |
| 8 B | 537 | 674 | 9.5 | 8.4 | 144 | 855 | 0.88 | 47 | 1.3 | na | |
| Jan | 9 | 434 | 571 | 9.1 | 8 | 154 | 9399 | na | 53.6 | 1.4 | na |
| 10 | 375 | 508 | 8.3 | 7.2 | 145 | 1057 | 1 | 39.1 | 1.2 | 38 | |
| 11 | 297 | 456 | 6.7 | 5.8 | 159 | 1103 | na | na | na | na | |
| 12 | 235 | 401 | 5.2 | 4.4 | 173 | 1074 | na | 40.2 | 1.4 | na | |
| 14 C | 253 | 371 | 5.1 | 4.2 | 149 | 869 | 1 | 39.7 | 1.2 | na | |
| Feb | 36 D | 233 | 353 | 1.5 | 0.9 | 147 | 530 | 1.02 | 33.5 | 1.5 | na |
| 64 | 677 | 797 | 10.4 | 6.5 | 142 | 355 | 1 | 44 | 1.3 | na | |
| 70 | 655 | 819 | 13.9 | 7 | 485 | 401 | 1.06 | 51 | 1.2 | na | |
| Mar | 78 E | 744 | 862 | 7.2 | 6.2 | 210 | 431 | 1.03 | 43.5 | 1.4 | na |
| 80 F | 660 | 1007 | 6.5 | 5.5 | 164 | 624 | 1 | na | na | na | |
| 82 G | 793 | 973 | 6.4 | 5.5 | 163 | 447 | 1.24 | na | na | na | |
| 83 H | 903 | 1235 | 6.1 | 5.2 | 171 | 572 | 1 | na | na | na | |
| 85 I | 987 | 1285 | 5.7 | 4.9 | 182 | 448 | 1 | na | na | na | |
| Apr | 87 | 1002 | 1666 | 6.5 | 5.4 | 193 | 444 | 1 | na | na | na |
| 89 J | 883 | 1252 | 5.9 | 4.9 | 192 | 525 | 1 | 48 | 1.4 | na | |
| 92 | 769 | 904 | 3.8 | 3.3 | 207 | 554 | 1 | 51.4 | 1.4 | na | |
| 93 | na | na | na | na | na | na | na | na | na | na | |
| 95 | 743 | 863 | 3.6 | 3.1 | 229 | 547 | na | 45 | 1.3 | na | |
| May | 124 | 78 | 108 | 1.3 | 1 | 139 | 199 | 1 | 34.9 | 1.2 | na |
| Jul | 186 | 21 | 28 | 0.84 | 0.2 | na | 71 | na | 44 | 1.05 | na |
| 197 K | 27 | 39 | 1 | 0.4 | 65 | 72 | 1 | 43.1 | 1.2 | na | |
| Nov | 306 L | 22 | 26 | 0.8 | 0.2 | 72 | na | 1.12 | 37.1 | 1.1 | na |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezende, I.M.; Pereira, L.S.; Fradico, J.R.B.; Pascoal Xavier, M.A.; Alves, P.A.; Campi-Azevedo, A.C.; Speziali, E.; dos Santos, L.Z.M.; Albuquerque, N.S.; Penido, I.; et al. Late-Relapsing Hepatitis after Yellow Fever. Viruses 2020, 12, 222. https://doi.org/10.3390/v12020222
Rezende IM, Pereira LS, Fradico JRB, Pascoal Xavier MA, Alves PA, Campi-Azevedo AC, Speziali E, dos Santos LZM, Albuquerque NS, Penido I, et al. Late-Relapsing Hepatitis after Yellow Fever. Viruses. 2020; 12(2):222. https://doi.org/10.3390/v12020222
Chicago/Turabian StyleRezende, Izabela Maurício, Leonardo Soares Pereira, Jordana Rodrigues Barbosa Fradico, Marcelo Antônio Pascoal Xavier, Pedro Augusto Alves, Ana Carolina Campi-Azevedo, Elaine Speziali, Lívia Zignago Moreira dos Santos, Natalia Soares Albuquerque, Indiara Penido, and et al. 2020. "Late-Relapsing Hepatitis after Yellow Fever" Viruses 12, no. 2: 222. https://doi.org/10.3390/v12020222
APA StyleRezende, I. M., Pereira, L. S., Fradico, J. R. B., Pascoal Xavier, M. A., Alves, P. A., Campi-Azevedo, A. C., Speziali, E., dos Santos, L. Z. M., Albuquerque, N. S., Penido, I., Santos, T. A., Bom, A. P. D. A., da Silva, A. M. V., Fernandes, C. B., Calzavara, C. E., Kroon, E. G., Martins-Filho, O. A., Teixeira-Carvalho, A., & Drumond, B. P. (2020). Late-Relapsing Hepatitis after Yellow Fever. Viruses, 12(2), 222. https://doi.org/10.3390/v12020222





