The N-glycosylation of Equine Tetherin Affects Antiviral Activity by Regulating Its Subcellular Localization
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. Cell Culture and Transfection
2.3. Western Blotting
2.4. Flow Cytometry
2.5. Immunofluorescence Assay
2.6. Antibody-Antigen Uptake Assay
2.7. Peptide-N-Glycosidase F (PNGase F) Digestion of eqTHN
2.8. RT Activity Assay
2.9. Statistical Analysis
3. Results
3.1. eqTHN is Modified by N-Glycosylation at Residues N51 and N78
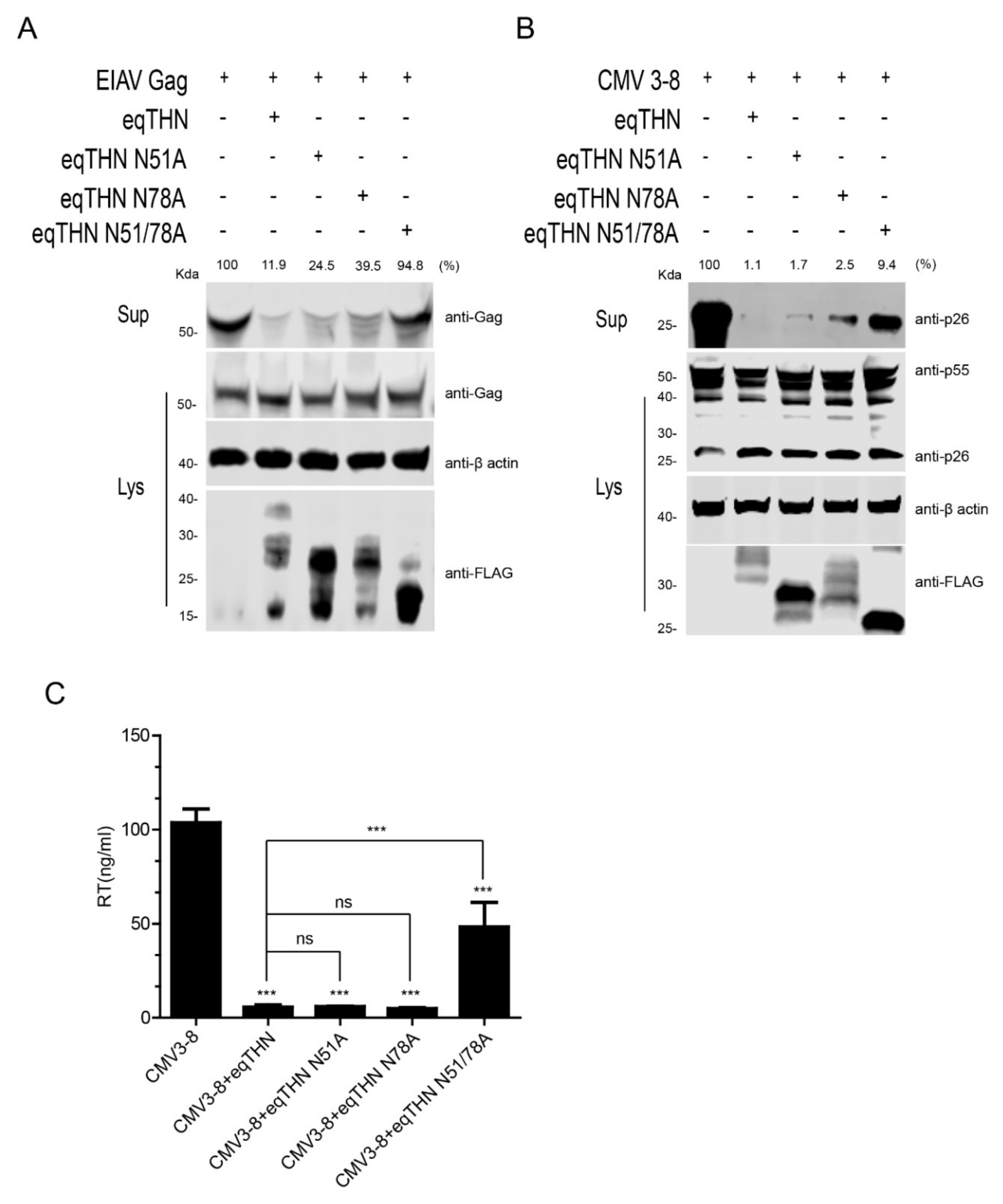
3.2. N-Glycosylation Affected the Antiviral Function of eqTHN
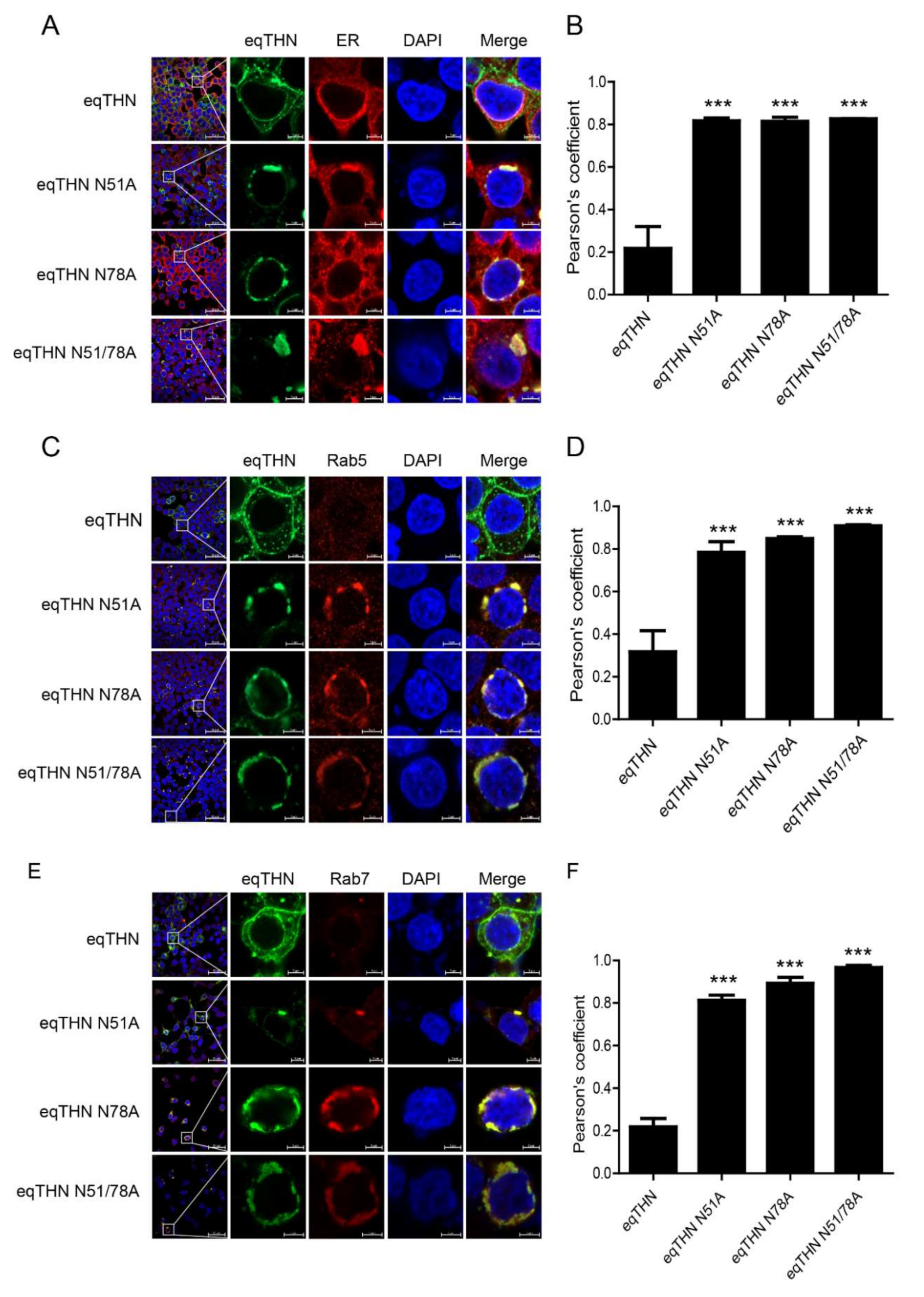
3.3. The N-Glycosylation of eqTHN is Required for Localization to the PM
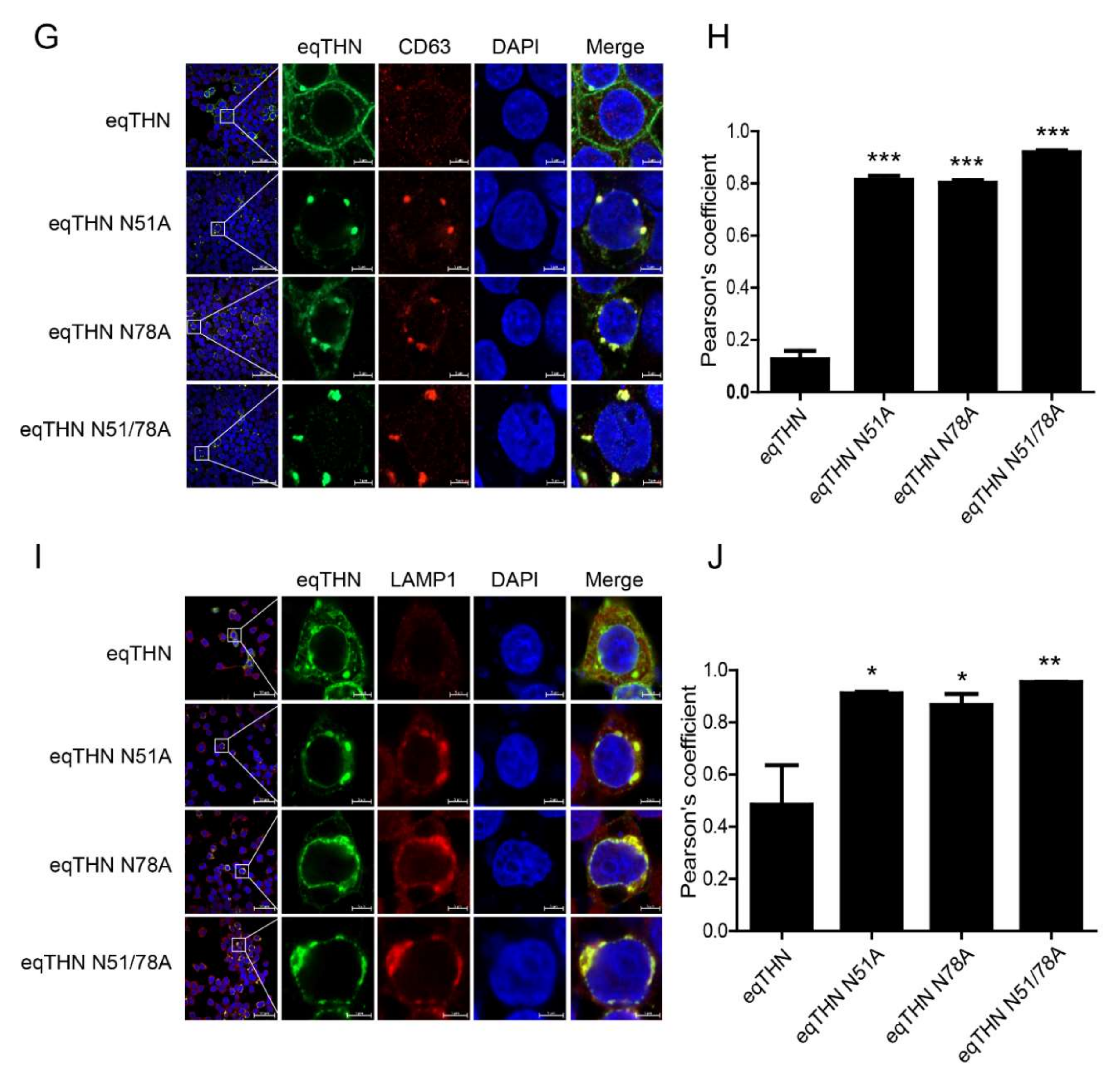
3.4. N-Glycosylation Impacts the Normal Trafficking of eqTHN
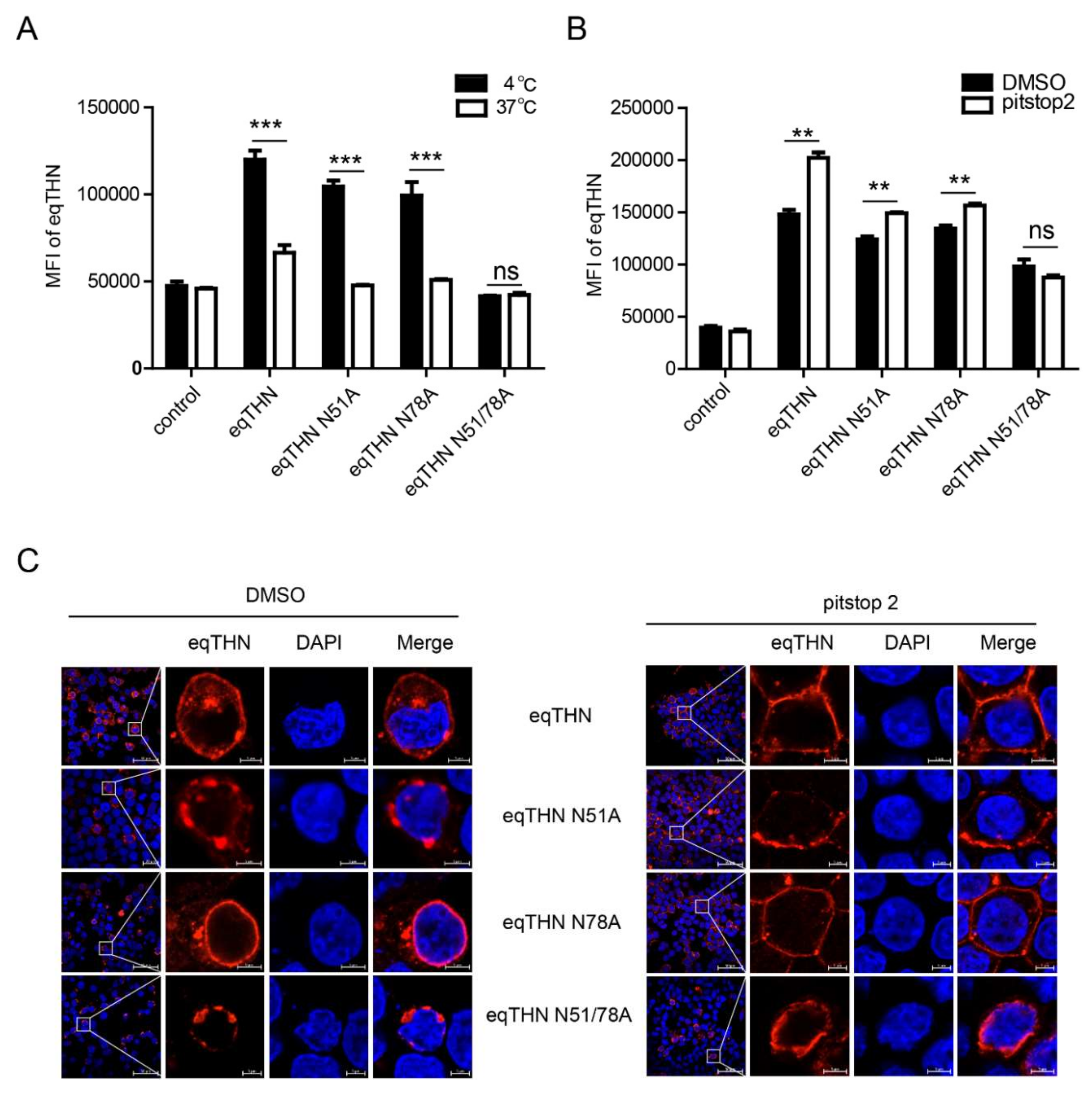
3.5. Anterograde Trafficking of eqTHN is Dependent on N-Glycosylation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Evans, D.T.; Serra-Moreno, R.; Singh, R.K.; Guatelli, J.C. BST-2/tetherin: A new component of the innate immune response to enveloped viruses. Trends Microbiol. 2010, 18, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.; Venkatesh, S.; Bieniasz, P.D. Mechanism of HIV-1 Virion Entrapment by Tetherin. PLoS Pathog. 2013, 9, e1003483. [Google Scholar]
- Neil, S.J.D.; Zang, T.; Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008, 451, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Jouvenet, N.; Neil, S.J.D.; Zhadina, M.; Zang, T.; Kratovac, Z.; Lee, Y.; McNatt, M.; Hatziioannou, T.; Bieniasz, P.D. Broad-Spectrum Inhibition of Retroviral and Filoviral Particle Release by Tetherin. J. Virol. 2008, 83, 1837–1844. [Google Scholar] [CrossRef]
- Radoshitzky, S.R.; Dong, L.; Chi, X.; Clester, J.C.; Retterer, C.; Spurgers, K.; Kuhn, J.H.; Sandwick, S.; Ruthel, G.; Kota, K.; et al. Infectious Lassa virus, but not filoviruses, is restricted by BST-2/tetherin. J. Virol. 2010, 84, 10569–10580. [Google Scholar] [CrossRef]
- Kaletsky, R.L.; Francica, J.R.; Agrawal-Gamse, C.; Bates, P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. USA 2009, 106, 2886–2891. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Wang, X. Strain-Specific Antagonism of the Human H1N1 Influenza A Virus against Equine Tetherin. Viruses 2018, 10, 264. [Google Scholar] [CrossRef]
- Perez-Caballero, D.; Zang, T.; Ebrahimi, A.; McNatt, M.W.; Gregory, D.A.; Johnson, M.C.; Bieniasz, P.D. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 2009, 139, 499–511. [Google Scholar] [CrossRef]
- Hauser, H.; Lopez, L.A.; Yang, S.J.; Oldenburg, J.E.; Exline, C.M.; Guatelli, J.C.; Cannon, P.M. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology 2010, 7, 51. [Google Scholar] [CrossRef]
- Hoffmann, M.; Nehlmeier, I.; Brinkmann, C.; Krahling, V.; Behner, L.; Moldenhauer, A.S.; Kruger, N.; Nehls, J.; Schindler, M.; Hoenen, T.; et al. Tetherin Inhibits Nipah Virus but Not Ebola Virus Replication in Fruit Bat Cells. J. Virol. 2019, 93, e01821-18. [Google Scholar] [CrossRef]
- Habermann, A.; Krijnse-Locker, J.; Oberwinkler, H.; Eckhardt, M.; Homann, S.; Andrew, A.; Strebel, K.; Krausslich, H.G. CD317/Tetherin Is Enriched in the HIV-1 Envelope and Downregulated from the Plasma Membrane upon Virus Infection. J. Virol. 2010, 84, 4646–4658. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 2011, 3, a005199. [Google Scholar] [CrossRef] [PubMed]
- Kupzig, S.; Korolchuk, V.; Rollason, R.; Sugden, A.; Wilde, A.; Banting, G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 2003, 4, 694–709. [Google Scholar] [CrossRef]
- Sauter, D. Counteraction of the multifunctional restriction factor tetherin. Front. Microbiol. 2014, 5, 163. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Qu, M.; Li, X.; Zhang, J.; Zhang, H.; Wu, J.; Yu, B.; Wu, H.; Kong, W.; et al. Viral Restriction Activity of Feline BST2 Is Independent of Its N-Glycosylation and Induction of NF-kappaB Activation. PLoS ONE 2015, 10, e0138190. [Google Scholar]
- Tokarev, A.; Suarez, M.; Kwan, W.; Fitzpatrick, K.; Singh, R.; Guatelli, J. Stimulation of NF- B Activity by the HIV Restriction Factor BST2. J. Virol. 2012, 87, 2046–2057. [Google Scholar] [CrossRef]
- Andrew, A.J.; Miyagi, E.; Kao, S.; Strebel, K. The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu. Retrovirology 2009, 6, 80. [Google Scholar] [CrossRef]
- Sakuma, T.; Noda, T.; Urata, S.; Kawaoka, Y.; Yasuda, J. Inhibition of Lassa and Marburg Virus Production by Tetherin. J. Virol. 2008, 83, 2382–2385. [Google Scholar] [CrossRef]
- Han, Z.; Lv, M.; Shi, Y.; Yu, J.; Niu, J.; Yu, X.-F.; Zhang, W. Mutation of Glycosylation Sites in BST-2 Leads to Its Accumulation at Intracellular CD63-Positive Vesicles without Affecting Its Antiviral Activity against Multivesicular Body-Targeted HIV-1 and Hepatitis B Virus. Viruses 2016, 8, 62. [Google Scholar] [CrossRef]
- Yin, X.; Hu, Z.; Gu, Q.; Wu, X.; Zheng, Y.H.; Wei, P.; Wang, X. Equine tetherin blocks retrovirus release and its activity is antagonized by equine infectious anemia virus envelope protein. J Virol. 2014, 88, 1259–1270. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, J.; Zhang, X.; Su, C.; Yao, Q.-C.; Wang, X.; Ross, S.R. Equine Infectious Anemia Virus Gag Assembly and Export Are Directed by Matrix Protein throughtrans-Golgi Networks and Cellular Vesicles. J. Virol. 2016, 90, 1824–1838. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelieres, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shi, N.; Jiang, C.-G.; Lin, Y.-Z.; Wang, X.-F.; Wang, S.; Lv, X.-L.; Zhao, L.-P.; Shao, Y.-M.; Kong, X.-G.; et al. A proviral derivative from a reference attenuated EIAV vaccine strain failed to elicit protective immunity. Virology 2011, 410, 96–106. [Google Scholar] [CrossRef]
- Wang, X.F.; Bai, B.; Lin, Y.; Qi, T.; Du, C.; Song, M.; Wang, X. High-Efficiency Rescue of Equine Infectious Anemia Virus from a CMV-Driven Infectious Clone. Virol. Sin. 2019, 34, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Na, L.; Ren, H.; Wang, Y.; Wang, X. Equine Myxovirus Resistance Protein 2 Restricts Lentiviral Replication by Blocking Nuclear Uptake of Capsid Protein. J. Virol. 2018, 92, e00499-18. [Google Scholar] [CrossRef]
- Le Tortorec, A.; Willey, S.; Neil, S.J. Antiviral inhibition of enveloped virus release by tetherin/BST-2: Action and counteraction. Viruses 2011, 3, 520–540. [Google Scholar] [CrossRef]
- Masuyama, N.; Kuronita, T.; Tanaka, R.; Muto, T.; Hirota, Y.; Takigawa, A.; Fujita, H.; Aso, Y.; Amano, J.; Tanaka, Y. HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with alpha-adaptin. J. Biol. Chem. 2009, 284, 15927–15941. [Google Scholar] [CrossRef]
- Dutta, D.; Williamson, C.D.; Cole, N.B.; Donaldson, J.G. Pitstop 2 is a potent inhibitor of clathrin-independent endocytosis. PLoS ONE 2012, 7, e45799. [Google Scholar] [CrossRef]
- Chu, H.; Wang, J.J.; Qi, M.; Yoon, J.J.; Chen, X.; Wen, X.; Hammonds, J.; Ding, L.; Spearman, P. Tetherin/BST-2 is essential for the formation of the intracellular virus-containing compartment in HIV-infected macrophages. Cell Host Microbe 2012, 12, 360–372. [Google Scholar] [CrossRef]
- Wujek, P.; Kida, E.; Walus, M.; Wisniewski, K.E.; Golabek, A.A. N-glycosylation is crucial for folding, trafficking, and stability of human tripeptidyl-peptidase I. J. Biol. Chem. 2004, 279, 12827–12839. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kwak, M.S.; Park, J.B.; Lee, S.A.; Choi, J.E.; Cho, H.S.; Shin, J.S. N-linked glycosylation plays a crucial role in the secretion of HMGB1. J. Cell Sci. 2016, 129, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, T.; Peng, J.; Xu, P.; Dong, N.; Chen, S.; Wu, Q. Distinct Roles ofN-Glycosylation at Different Sites of Corin in Cell Membrane Targeting and Ectodomain Shedding. J. Biol. Chem. 2015, 290, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ropert, N.; Koulakoff, A.; Giaume, C.; Oheim, M. Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 7648–7658. [Google Scholar] [CrossRef]
- Lee, J.; Ye, Y. The Roles of Endo-Lysosomes in Unconventional Protein Secretion. Cells 2018, 7, 198. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, B.; Wang, X.-F.; Zhang, M.; Na, L.; Zhang, X.; Zhang, H.; Yang, Z.; Wang, X. The N-glycosylation of Equine Tetherin Affects Antiviral Activity by Regulating Its Subcellular Localization. Viruses 2020, 12, 220. https://doi.org/10.3390/v12020220
Bai B, Wang X-F, Zhang M, Na L, Zhang X, Zhang H, Yang Z, Wang X. The N-glycosylation of Equine Tetherin Affects Antiviral Activity by Regulating Its Subcellular Localization. Viruses. 2020; 12(2):220. https://doi.org/10.3390/v12020220
Chicago/Turabian StyleBai, Bowen, Xue-Feng Wang, Mengmeng Zhang, Lei Na, Xiangmin Zhang, Haili Zhang, Zhibiao Yang, and Xiaojun Wang. 2020. "The N-glycosylation of Equine Tetherin Affects Antiviral Activity by Regulating Its Subcellular Localization" Viruses 12, no. 2: 220. https://doi.org/10.3390/v12020220
APA StyleBai, B., Wang, X.-F., Zhang, M., Na, L., Zhang, X., Zhang, H., Yang, Z., & Wang, X. (2020). The N-glycosylation of Equine Tetherin Affects Antiviral Activity by Regulating Its Subcellular Localization. Viruses, 12(2), 220. https://doi.org/10.3390/v12020220




