1. Introduction
Restriction factors are a critical component of the innate immune response to cellular infection by viruses by inhibiting various stages of the viral replication cycle. The APOBEC3 (A3) family of cytidine deaminases are potent restriction factors against endogenous retroelements and retroviruses, with four members of this family (A3D, A3F, A3G, and A3H) demonstrating considerable antiviral activity against lentiviruses such as Simian Immunodeficiency Virus (SIV) and Human Immunodeficiency Virus (HIV) [
1]. The antiviral A3 proteins incorporate into nascent virions and act during the reverse transcription stage of retroviral infection to convert cytosines to uracils in viral single-stranded DNA [
2]. The result of successful A3 activity is lethal hypermutation of the retroviral genome, preventing the establishment of a productive infection. Thus, the A3 proteins act in concert to provide a barrier to both cross-species transmission and within-host cell-to-cell spread of infection.
All of the
A3 genes display strong signatures of positive selection throughout primate evolution, suggesting a longstanding genetic conflict between this family of restriction factors and the pathogens and endogenous retroelements they restrict [
3,
4,
5]. To overcome A3 restriction, lentiviruses have evolved the accessory protein, Vif, that potently inhibits the function of all antiviral A3 proteins simultaneously [
6,
7]. Vif hijacks a host cellular E3 ubiquitin ligase complex and acts as a substrate receptor for A3 proteins, promoting their ubiquitination and subsequent degradation via the proteasome [
8,
9,
10,
11]. The direct antagonistic relationship between host A3 and lentiviral Vif underlies an evolutionary arms race that selects for mutations in A3 that can escape Vif antagonism, and mutations in Vif that regain A3 binding [
12,
13]. Therefore, the A3 proteins confer selective pressure on lentiviruses to evolve Vif proteins that can overcome the host A3 defense.
Despite the selective pressure to retain host defense systems like the
A3 genes, restriction factors can become less effective due to circulating polymorphisms that reduce inherent antiviral function. It is possible that such polymorphisms may arise and become fixed in the population upon the loss of a selective pathogenic pressure, especially if the maintenance of a particular antiviral gene is deleterious to the host. A3H is a notable example of such a polymorphic restriction factor in humans, with four major A3H variants (haplotypes I, II, III, and IV) circulating in the human population, and at least eight other minor haplotypes identified to date [
14]. Among these, haplotype II is the only variant to encode a protein with potent activity against lentiviruses [
15,
16,
17,
18,
19]. In contrast, the protein encoded by haplotype I is far less antiviral and stable, and proteins encoded by haplotypes III and IV are not detectable at all [
15,
16,
17,
18,
20,
21]. Evolutionary evidence suggests that human A3H lost activity in two independent events since the last common ancestor with chimpanzees [
16]. Thus, the destabilization of haplotypes I, III, and IV are a consequence of two independent single nucleotide polymorphisms, R105G in haplotype I and a deletion of codon 15, N15del, in haplotypes III and IV. Each mutation is sufficient to render A3H ineffective against HIV [
15,
16,
18]. Importantly, the differences in protein expression are not explained by differences in transcript levels, as mRNA levels are comparable for both overexpressed stable and unstable alleles [
22] as well as endogenous transcripts of various alleles measured in primary T lymphocytes [
17]. Rather, the expression differences are due to reduced protein half-lives [
16].
Recent structural studies of pig-tailed macaque [
23], human [
24,
25], and chimpanzee [
26] A3H have established a unique RNA interaction mechanism central to the antiviral function of A3H. Two A3H monomers interact with opposite sides of a short RNA duplex, and the A3H monomers in this complex interact primarily with the RNA duplex and not with one another. Importantly, mutagenesis of residues in the interaction interface between A3H and duplex RNA results in a decrease in A3H expression similar to the unstable human haplotypes [
25,
26,
27]. Thus, the formation of the A3H-RNA complex may be a critical regulator of A3H stability. However, the exact mechanism in which R105G destabilizes A3H is not well understood, and even less is known of the effect of the N15del mutation in haplotypes III and IV.
In order to better understand the processes underlying the differential expression of different A3H haplotypes, we asked how ubiquitination, a posttranslational modification most widely known for its role in protein degradation, differs between A3H haplotypes. We found that the rates of ubiquitination were greater in unstable haplotypes I, III, and IV, while the stable haplotype II was largely protected from this modification. By genetically inhibiting ubiquitination through lysine mutagenesis, we were able to recover expression of the N15del-containing haplotypes III and IV to levels comparable to haplotype I. However, despite increasing protein expression, these changes did not restore antiviral activity of any of these haplotypes against HIV-1. Rather, stabilized versions of the proteins encoded by haplotypes III and IV were strictly localized to the nucleus and were unable to package into budding virions. Thus, the R105G and N15del mutations in A3H result in functional defects that cannot be restored by inhibiting ubiquitination alone. On the other hand, by fusing the stable and antiviral haplotype II to haplotype III, the deficiencies of the N15del mutation on expression, localization, and virion incorporation were reversed. However, we found that stabilized and packaged haplotype III was susceptible to processing by HIV-1 protease, and incapable of restricting HIV-1 when linked to an enzymatically inactive haplotype II. Taken together, these results suggest that both A3H stability and activity are tightly regulated by loss-of-function mutations, and selection for these mutations may serve to protect the host in the absence of a pathogenic pressure.
2. Materials and Methods
2.1. Plasmids
Plasmids containing A3H haplotypes I through IV were previously described [
16], and used as templates to generate C-terminally HA-tagged constructs. All constructs used in this study were cloned into pcDNA3.1 (Thermo Fisher, Waltham, MA, USA) using EcoRI/XhoI restriction sites. Mutations were introduced using standard PCR or using the QuikChange Lightning Multi Site-Directed Mutagenesis Kit (Agilent, 210515, Santa Clara, CA, USA). Double A3H fusion constructs I–I, II–II, I–II, and II–I were previously described [
20]. The double A3H fusion constructs unique to this study, III–III, III–II, and II–III were made through amplification of haplotypes II and III with a 5’ or 3’ primer containing the linker sequence GGT GGT GGT GGT GGC GCC (Gly-Gly-Gly-Gly-Gly-Ala). The 5’ and 3’ haplotype domains were digested using the KasI restriction site in the linker, and EcoRI (for 5’ domains) or XhoI (for 3’ domains). The 5’ domains, 3’ domains, and the EcoRI/XhoI double digested pcDNA3.1 vector were joined using T4 DNA Ligase (New England BioLabs, M0202, Ipswich, MA, USA). Infectivity experiments were performed using the HIV-1 molecular clone pLAIΔenvLuc2Δvif, which was previously described [
28].
2.2. Cell Lines and Transfections
293T, HeLa, and SupT1 cells were obtained from ATCC (CRL-3216, CCL-2, and CRL-1942, respectively). The 293T and HeLa cells were cultured in DMEM, high glucose (Thermo Fisher, 11965092) supplemented with 10% HyClone Bovine Growth Serum (GE Healthcare Life Sciences, SH30541.03, Chicago, IL, USA) and 1× Penicillin-Streptomycin (Thermo Fisher, 15140122). SupT1 cells were cultured in RPMI-1640 (Thermo Fisher, 11875093) supplemented with 10% HyClone Fetal Bovine Serum (GE Healthcare Life Sciences, SH3091003) and 1× Penicillin-Streptomycin. Cell lines were mycoplasma free, as determined by the Fred Hutchinson Cancer Research Center Specimen Processing/Research Cell Bank core facility. Cells were incubated at 37 °C and 5% CO2 in a humidified incubator and maintained for under thirty passages before returning to a lower passage stock.
Transfections were performed using TransIT-LT1 Transfection Reagent (Mirus, MIR 2305, Madison, WI, USA) according to the manufacturer’s protocol. For Western blotting, 293T cells were plated at 1.5 × 105/mL 24 h prior to transfection. Transfections in 12-well plates were performed using 0.5 µg/well of A3H plasmid and 3 µL/well of transfection reagent. For ubiquitination experiments, 6-well plates were transfected with 1.0 µg/well of A3H plasmid, 1.0 µg/well of myc-Ubiquitin, and 6 µL/well of transfection reagent. For infectivity experiments, 293T cells in 96-well plates (3.75 × 104/well) were reverse-transfected with 60 ng/well pLAIΔenvLuc2Δvif, 30 ng/well A3H plasmid, and 10 ng/well L-VSV-G. For virion incorporation assays, transfections were performed in 6-well plates using 0.5 µg/well of A3H plasmid, 1.0 µg/well pLAIΔenvLuc2Δvif and 6 µL/well of transfection reagent. For immunofluorescence microscopy, HeLa cells were plated at 5.0 × 104/mL on glass cover slips in a 12-well plate 24 h prior to transfection with 0.5 µg/mL of A3H plasmid and 3 µL/well of transfection reagent. In all cases, transfected cells were incubated for 48 h prior to downstream applications.
2.3. Viral Infectivity Assays
Viruses were propagated in 293T cells reverse-transfected with pLAIΔenvLuc2Δvif, A3H plasmid, and L-VSV-G for pseudotyping. Virus-containing supernatants were harvested 48 h after transfection, transferred to a V-bottom plate, and clarified of cells and debris by centrifugation at 1000×
g for 3 min at 25 °C. An amount of 10 µL of supernatant was added to flat-bottom 96-well plates containing SupT1 cells (3.75 × 10
4 and 90 µL/well) pretreated with 20 µg/mL DEAE/Dextran and mixed by repipetting. An amount of 5 µL of supernatant was saved for quantification of reverse transcriptase (RT) activity, as described previously [
29]. Infected SupT1 cells were incubated for 48 h and lysed in 100 µL of Bright-Glo Luciferase Reagent (Promega, E2610, Madison, WI, USA). Infection was assessed by luciferase activity using a LUMIstar Omega microplate luminometer (BMG Labtech, Ortenberg, Germany), and raw luciferase values were normalized to 2000 mU RT activity.
2.4. Immunoprecipitation and Western Blotting
For experiments involving MG132, cells were treated with 10 µM MG132 (Selleckchem, S2619, Houston, TX, USA), or an equal volume of DMSO, in fresh media for 18 h after the initial 48 h transfection. For all other experiments, cells were harvested 48 h post-transfection. Cells were washed twice with PBS, and lysed on ice with RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1.0% Triton X-100, 0.5% sodium deoxycholate, 1 mM EDTA, 1 mM MgCl2) for 20 min. For ubiquitination assays, transfected cells were washed twice with PBS and lysed in 150 µL of 1% SDS buffer (50 mM Tris-HCl pH 7.4, 1.0% SDS) at 95 °C for 10 min. Lysate was passed through a 30-gauge needle to sheer DNA. An amount of 50 µg of lysate was saved for input. An equal concentration of lysate for immunoprecipitation was diluted in 100 µL of 1% SDS buffer, which was further diluted in 900 µL ice cold RIPA buffer lacking SDS containing 15 µL EZview Red Anti-HA Affinity Gel (Sigma-Adrich, E6779, St. Louis, MO, USA). Lysate was immunoprecipitated for 1 h at 4 °C with nutation, washed three times with ice cold RIPA buffer lacking SDS, and eluted in 40 µL 2× Laemmli Sample Buffer (BIO-RAD, 1610737, Hercules, CA, USA). Lysis buffers were supplemented with cOmplete Protease Inhibitor Cocktail (Roche, 11697498001, Basel, Switzerland), 10 µM MG132, and 50 µM PR-619 deubiquitinase inhibitor (Selleckchem, S7130). For virion incorporation assays, viral supernatants were passed through a 0.22 micron filter, and virus was pelleted at 21,000× g for 1 h at 4 °C then resuspended in 30 µL 4% SDS in PBS and 10 uL of NuPAGE 4× LDS Sample Buffer (Thermo Fisher, NP0007).
10 µg per lysate, 10 µL of immunoprecipitated sample, or 10 µL of viral lysate was resolved on a NuPAGE 4–12% Bis-Tris Protein Gel (Thermo Fisher, NP0336). Western blotting was performed using the primary antibodies anti-HA (Proteintech, 51064-2-AP, Rosemont, IL, USA), anti-myc (Proteintech, 16286-1-AP), anti-A3H (P1H6-1, [
28]), anti-Lamin B1 (Proteintech, 66095-1-Ig), and anti-HIV-1 p24 (NIH AIDS Reagent Program, #3537, [
30,
31]) at a dilution of 1:2000. Secondary antibodies StarBright Blue 520 Goat Anti-Rabbit IgG (BIO-RAD, 12005869) and StarBright Blue 700 Goat Anti-Mouse IgG (BIO-RAD, 12005866) were used at a dilution of 1:10,000. Densitometric analysis was performed using ImageJ software [
32]. All immunoblots are representative of at least three independent experiments.
2.5. Fluorescence Microscopy
Transfected cells on glass coverslips were washed twice with PBS and fixed in 4% paraformaldehyde, permeabilized in 0.1% Triton X-100 in PBS, and blocked in 2% Bovine Serum Albumin in PBS for 10 min each at room temperature. Primary antibodies anti-HA.11 (BioLegend, 901514, San Diego, CA, USA) or anti-A3H were used at a dilution at 1:500 and 1:50, respectively, in 0.1% Triton X-100 in PBS and incubated with cells for 20 min at room temperature. Cells were washed five times with 0.1% Triton X-100 in PBS prior to incubation with Alexa Fluor 488-labeled anti-mouse secondary antibody (Thermo Fisher, A-11001) at a 1:1000 dilution in 0.1% Triton X-100 in PBS. Cells were again washed five times prior to mounting glass coverslips in ProLong Gold antifade reagent containing DAPI (Thermo Fisher, P36935). Images were obtained using a Nikon E800 microscope at 40× magnification.
4. Discussion
In the present study, we provide evidence that steady-state ubiquitination differs between the major A3H haplotypes circulating in the human population. These differences among A3H haplotypes in humans result in loss of protein expression that, only in the case of the N15del mutation, can be restored by mutation of lysines within the protein. However, the resultant protein is localized to the nucleus, fails to incorporate into virions, and does not have anti-HIV-1 activity. Our results indicate that the A3H antiviral activity was lost during human evolution, and that the inactive A3H proteins encoded by much of the human population cannot be easily restored by simply increasing the expression or by finding means to stabilize these proteins.
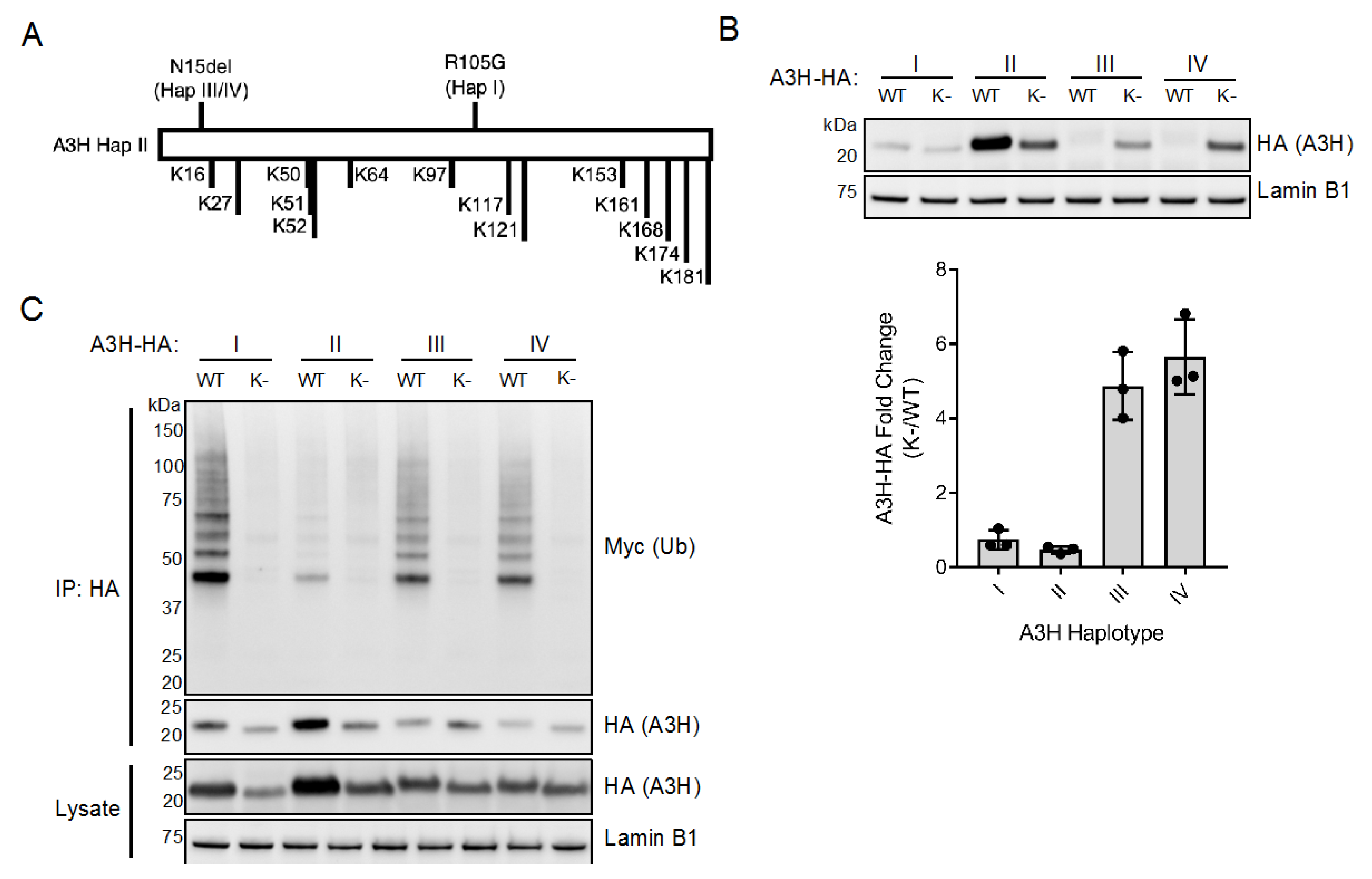
Ubiquitination most commonly results in proteasomal or lysosomal degradation of target proteins, although several other functions such as protein trafficking and inflammatory signaling have been described [
37]. The function of ubiquitin modification on target proteins depends on the type of linkage (e.g., lysine-48 and lysine-64) and the particular lysine residues modified on the target protein. However, due to the partial recovery of unstable A3H haplotypes with the proteasomal inhibitor MG132 (
Figure 1A), and the apparent lack of ubiquitination of the most stable A3H haplotype II (
Figure 1B), it is likely that ubiquitination of haplotypes I, III, and IV primarily direct A3H for proteasomal degradation. It is important to note that each haplotype has three major splice variants that determine the length of the C-terminus, the most common two across all haplotypes being the 182 and 183 amino acid isoforms (SV182 and SV183, respectively) [
14,
15]. Interestingly, A3H haplotype II also expresses a 200 amino acid isoform at a frequency comparable to SV182 and SV183 [
14]. While our study compares the ubiquitination of the SV183 isoform of all four haplotypes, how the ubiquitination of SV182 and SV200 may differ from SV183 is an interesting avenue of future research.
While we attempted to map the dominant lysine residues ubiquitinated in A3H, we were only able to see a loss of ubiquitination when all lysines were mutated. From this, we suspect that multiple lysines can be modified, and there is flexibility in lysine utilization in our more targeted lysine mutants. We found an intriguing phenotype by which mutating all lysines to arginine only increases expression of haplotypes III and IV and decreases expression of I and II (
Figure 2B). The decrease in expression observed with lysine-less haplotypes I and II was unexpected, as treatment with MG132 resulted in an increase of these two haplotypes (
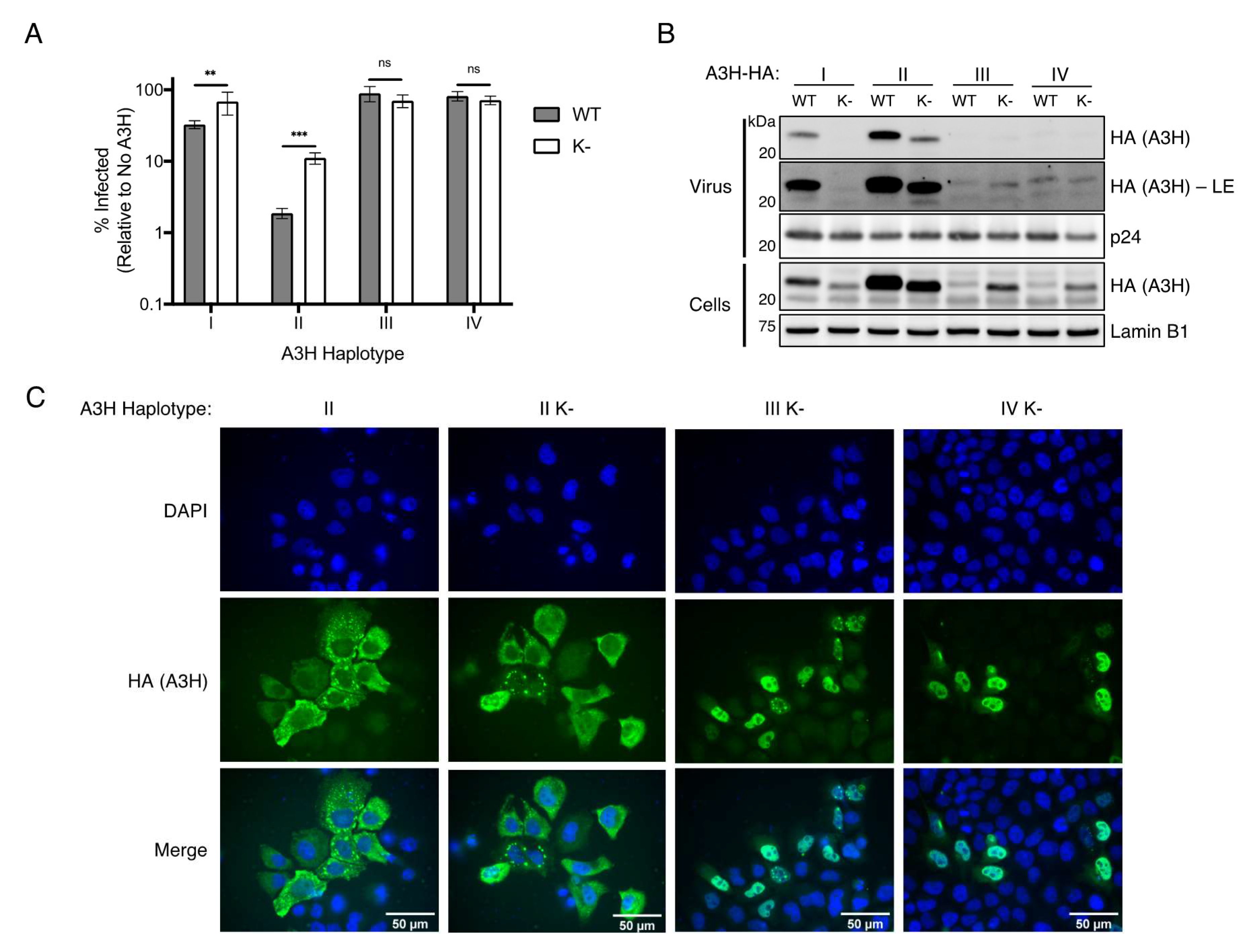
Figure 1A). Therefore, it is possible that haplotypes I and II are regulated by ubiquitin in a manner independent from proteasomal degradation or that the presence of lysine residues are otherwise important for the stability of these haplotypes. Nonetheless, lysine-less A3H haplotype II is still potently antiviral in relation to its expression (
Figure 3A) and maintains its cytoplasmic localization (
Figure 3C). However, lysine-less haplotypes III and IV express detectable proteins that allowed us to assess known functionally relevant characteristics of A3H (
Figure 2B). Lysine-less haplotypes III and IV are almost exclusively nuclear, are unable to package into virions and have no antiviral activity (
Figure 3). In contrast, haplotype I has some cytoplasmic localization [
20,
38], and low but detectable viral packaging (
Figure 3B) and antiviral activity (
Figure 3A,
Figure 5A). Taken together, these results reveal key differences between the R105G and N15del mutations in unstable A3H haplotypes, and suggest that the R105G mutation of haplotype I, although destabilizing, has more functional characteristics in common with the stable haplotype II than it does to the N15del-containing haplotypes III and IV.
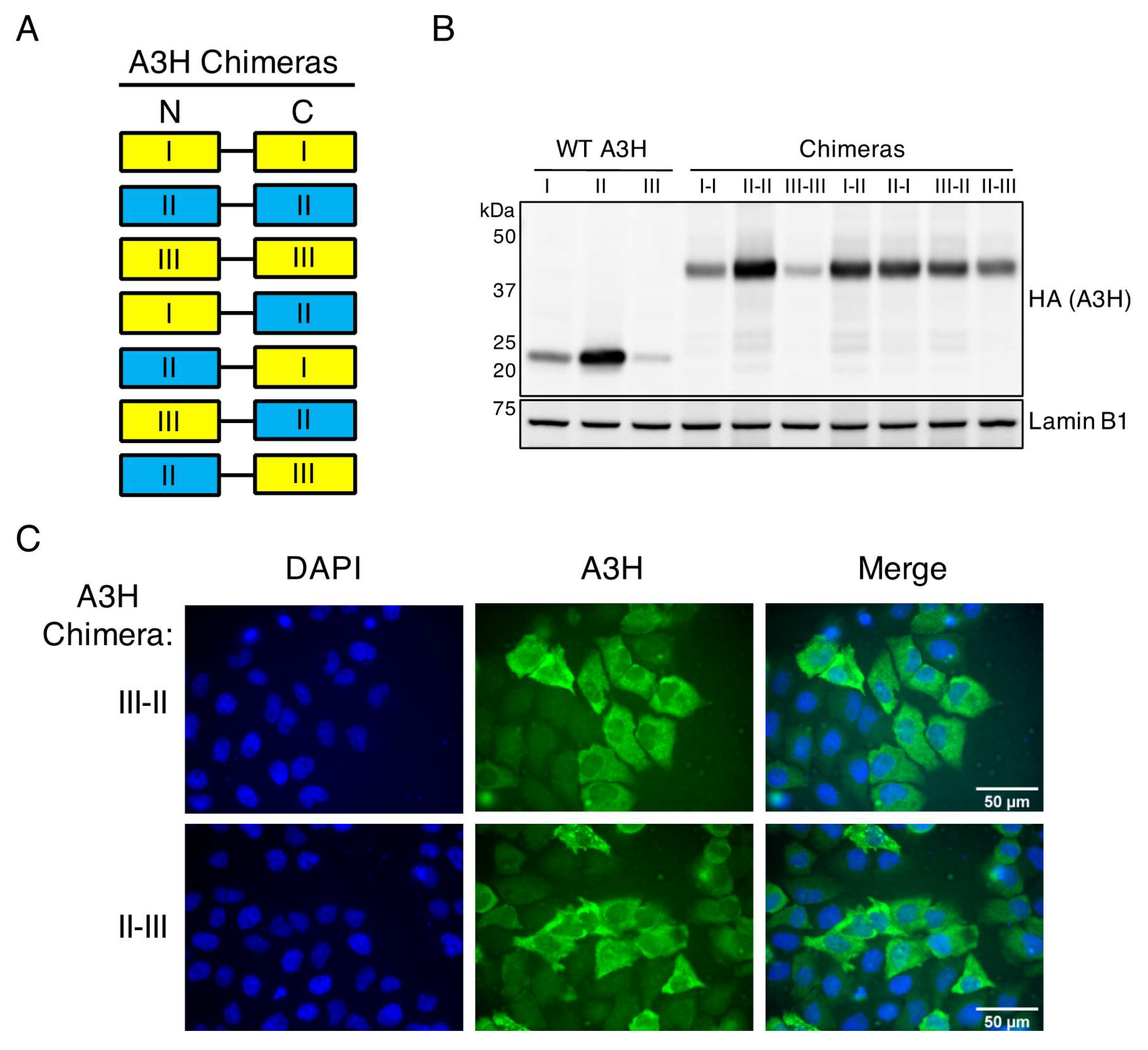
Previous findings from our group demonstrated that the stability, cytoplasmic retention, and antiviral activity of haplotype II was dominant over the instability and nuclear bias of haplotype I by linking these haplotypes in either orientation [
20]. The N15del mutation of haplotypes III and IV results in a protein that is less stable, more nuclear, and non-antiviral in comparison to haplotype I, suggesting that these haplotypes are more prone to negative regulation. Despite greater instability and functional deficiencies of N15del-containing haplotypes, we found that linking haplotype III to haplotype II in either orientation results in a stable, antiviral protein that localizes to the cytoplasm (
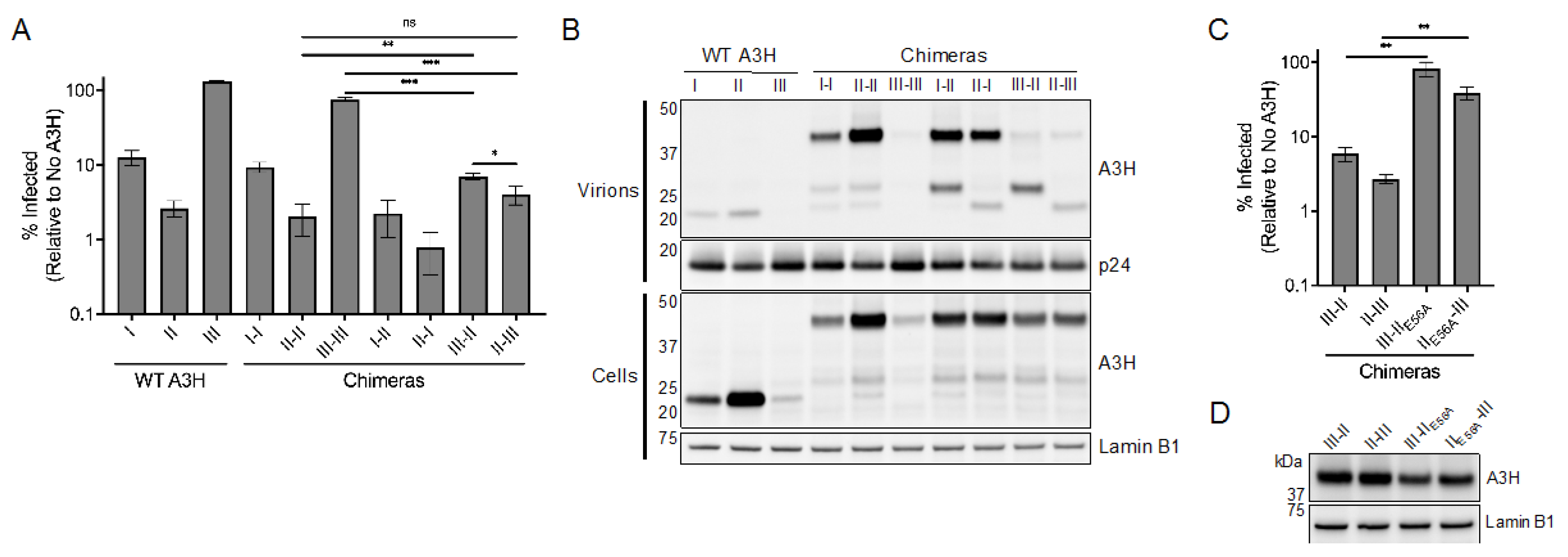
Figure 4 and
Figure 5). These findings suggest that A3H variants expressing the R105G and N15del mutations may share a common negative regulatory mechanism that affects the stability and localization of these mutants, and the N15del mutation leads to greater utilization of this mechanism.
We also observed an intriguing phenotype of our chimeric A3H proteins in that the packaging of full-length chimeras differs depending on the haplotypes linked. Unexpectedly, the stable and antiviral III–II and II–III chimeras were not detected at the expected molecular weight in Western blots of viral lysates, and only truncated A3H products were present (
Figure 5B). There is precedent for HIV protease processing of one particular spliced isoform of A3H [
14], and we suspect that these chimeras are more prone to cleavage by the HIV-1 protease than wild-type A3H. We also found truncated products of chimeras I–II and II–I in virions, but a large number of full-length chimeras were still present, suggesting that susceptibility to HIV-1 protease is also an important feature distinguishing the stable and unstable haplotypes (
Figure 5B). Despite being stable, antivirally active, and presumably incorporated into virions, we found that the catalytic activity of haplotype II was required for the restriction capacity of haplotype III-containing chimeras (
Figure 5C,D). This result suggests that the bulk of antiviral activity exhibited by chimeras III–II and II–III is conferred by haplotype II, and not from a stabilized and packaged haplotype III. These results indicate that the N15del mutation renders A3H nonfunctional even when stabilized and packaged into virions.
Several groups have recently described the mechanism by which stable A3H dimerizes through its interaction with duplex RNA, and this complex is maintained primarily through protein-RNA contacts [
23,
25,
26,
27]. Interaction with duplex RNA is mediated by aromatic and positively-charged residues in Loop 1, Loop 7, and the C-terminal α6 helix [
23,
25,
26,
27]. It is possible that the R105G and N15del mutations disrupt RNA binding and A3H-RNA complex formation, interfering with active stabilization and cytoplasmic retention observed with haplotype II. The R105G mutation is situated in β4 immediately upstream of the putative RNA-binding region (110-RLYYHW-115) in Loop 7 [
39]. Although residue 105 is not directly implicated in RNA interaction, it is possible that mutations at R105 may alter the positioning of RNA-interacting residues in Loop 7. Importantly, the N15 residue is positioned within Loop 1, and deletion of this residue in haplotypes III and IV may directly prevent binding of A3H to its duplex RNA substrate and lead to greater instability and loss of function than what is conferred by the R105G mutation. Disrupting RNA binding prevents dimerization, and monomeric A3H may be susceptible to increased processing by ubiquitination. Indeed, mutagenesis of RNA binding residues results in decreased expression, nuclear localization, and a loss of packaging and antiviral activity [
25,
26,
27,
40,
41], suggesting a shared mechanism of instability and loss of function to the R105G and N15del mutations. Recent biochemical work has more directly implicated nucleic acid binding as critical for A3H antiviral activity and is required for stabilization, virion incorporation, deaminase-independent inhibition of reverse transcriptase, and ssDNA substrate recognition [
42]. Moreover, residues within Loop 1 play a dual role in both RNA binding and recognition of ssDNA, underscoring the importance of this region for the regulation of expression and antiviral function of A3H [
42]. Thus, disruption of nucleic acid binding might explain all of the phenotypes observed for the unstable and inactive haplotypes I, III, and IV, as well as the functional differences observed between the R105G and N15del mutations.
Furthermore, the active mechanism by which haplotype II is stabilized might also be explained by RNA binding. Interestingly, five lysine residues are present in the C-terminal α6 helix in a span of 20 amino acids (
Figure 2A), and ubiquitination of these residues may prevent duplex RNA binding. An intriguing model is that stabilization of haplotype II may result from a higher affinity of this haplotype for duplex RNA, which requires hydrogen bonding of arginine and lysine residues to RNA phosphates [
23] and may preclude ubiquitination of these residues. While RNA-mediated dimerization may protect A3H from steady-state ubiquitination and degradation, recent work suggests that dimerized A3H is more susceptible to Vif-mediated degradation. HIV-1 Vif recognizes A3H in part due to its cytoplasmic localization resulting from RNA-mediated dimerization and binds to A3H in a manner that does not impact its interaction with RNA [
41]. Thus, stable and active A3H may be protected from steady-state ubiquitination by nature of its unique RNA-mediated dimerization mechanism, while HIV-1 Vif has evolved to recognize and degrade the stabilized, cytoplasmic, and antiviral form of A3H.
Interestingly, the loss of A3H activity is not unique to humans, as an evolutionary analysis of A3H in Old World monkeys found that A3H activity has been lost in multiple primate species [
43]. Moreover, several residues in the putative RNA binding region of Loop 1 were found to influence the antiviral potency of African green monkey and patas monkey A3H, providing further evidence that mutations introduced in this loop are a recurrent evolutionary mechanism that results in a loss of function [
43]. It is unknown why these loss-of-function mutations of A3H arise and become fixed in multiple primate species despite the established importance of this restriction factor in the control of retroviruses. It is possible that the selective pressure to maintain A3H function has been lost due to the loss of an unknown pathogen or due to redundancy of more potent antiretroviral restriction factors such as A3G. On the other hand, functional A3H may come at a fitness cost in the absence of a pathogenic selective pressure. Although A3H haplotype I has lost much of its stability and antiviral activity, this variant has been associated with breast and lung cancer similar to the well-established cancer driver A3B [
44,
45]. Interestingly, a similar association has not been made for A3H haplotypes II, III, and IV, which may suggest a unique dysregulation of haplotype I promotes A3H-mediated genomic mutations driving these cancers. In contrast, the complete instability and loss of function coinciding with the N15del mutation of haplotypes III and IV may dually work to constrain a redundant restriction factor while protecting the host from genomic mutations. Thus, although unstable A3H haplotypes are all ineffective against HIV, a careful examination of these variants reveals differences in function that may have a significant influence on host fitness.