Changes in the Transmission Dynamic of Chikungunya Virus in Southeastern Senegal
Abstract
1. Introduction
2. Materials and Methods
2.1. Serological Study
2.2. Environmental Data
2.3. Statistical Analysis
2.3.1. Descriptive Analysis
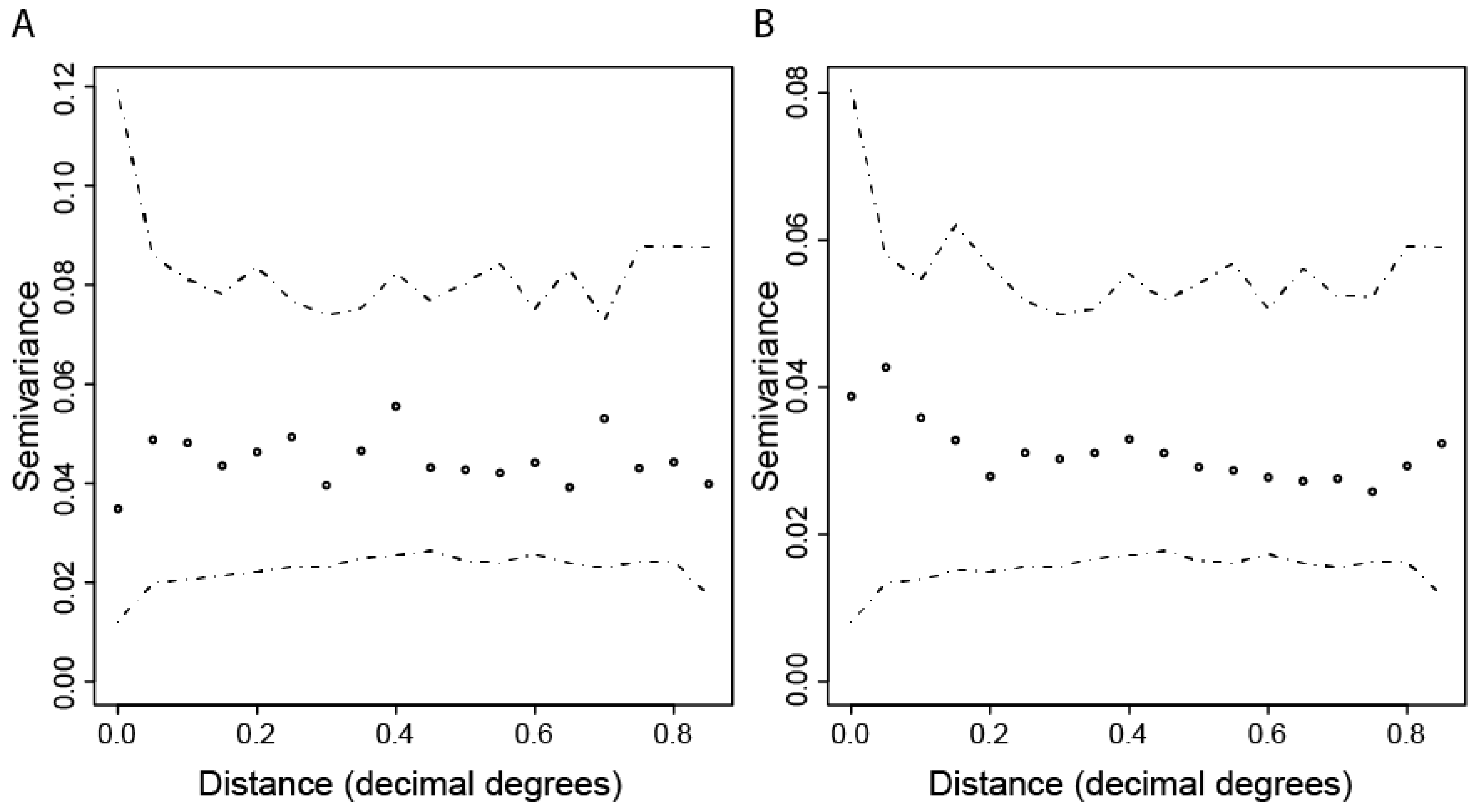
2.3.2. Spatial Patterns of Seroprevalence
2.3.3. Environmental Risk Factors
3. Results
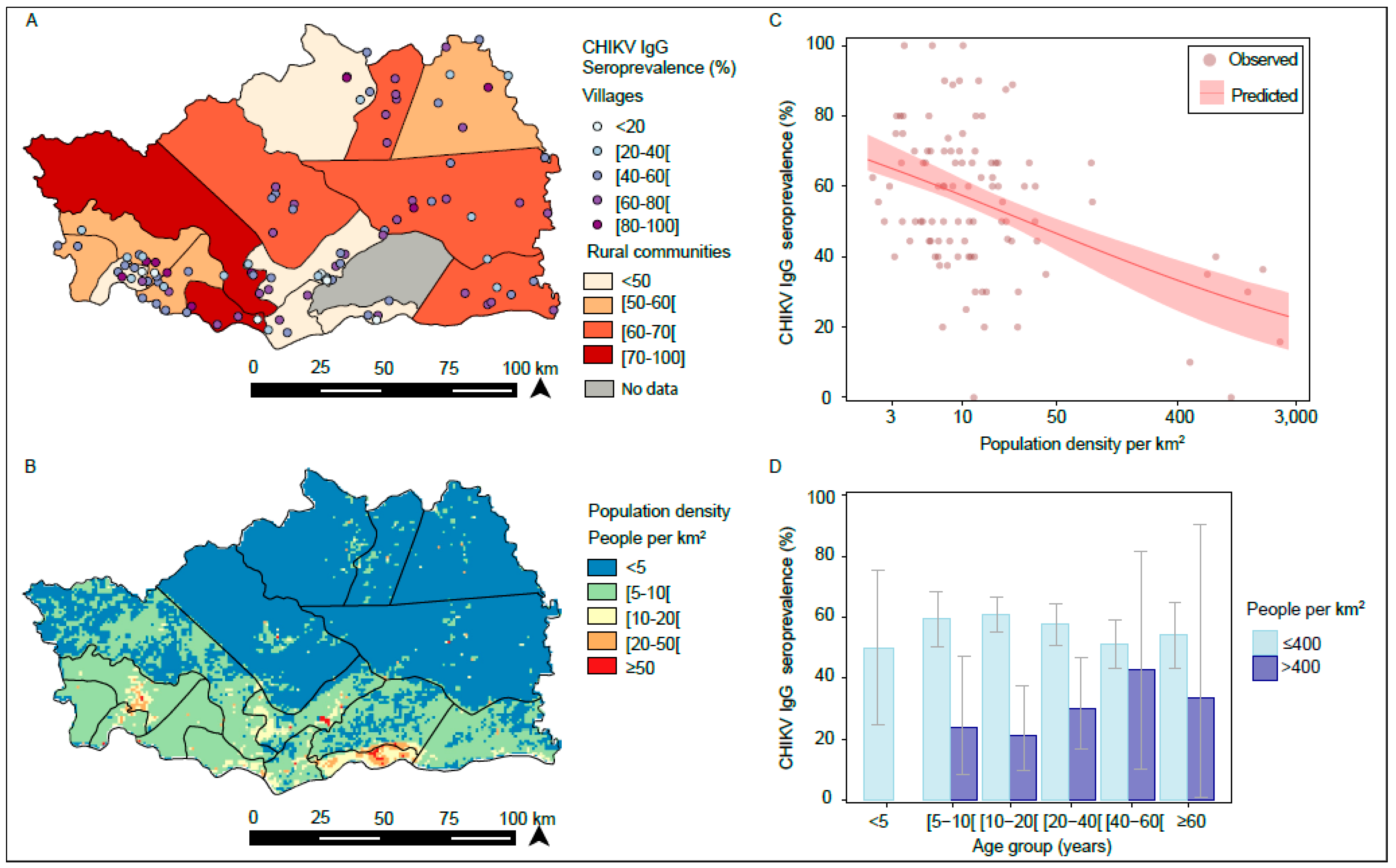
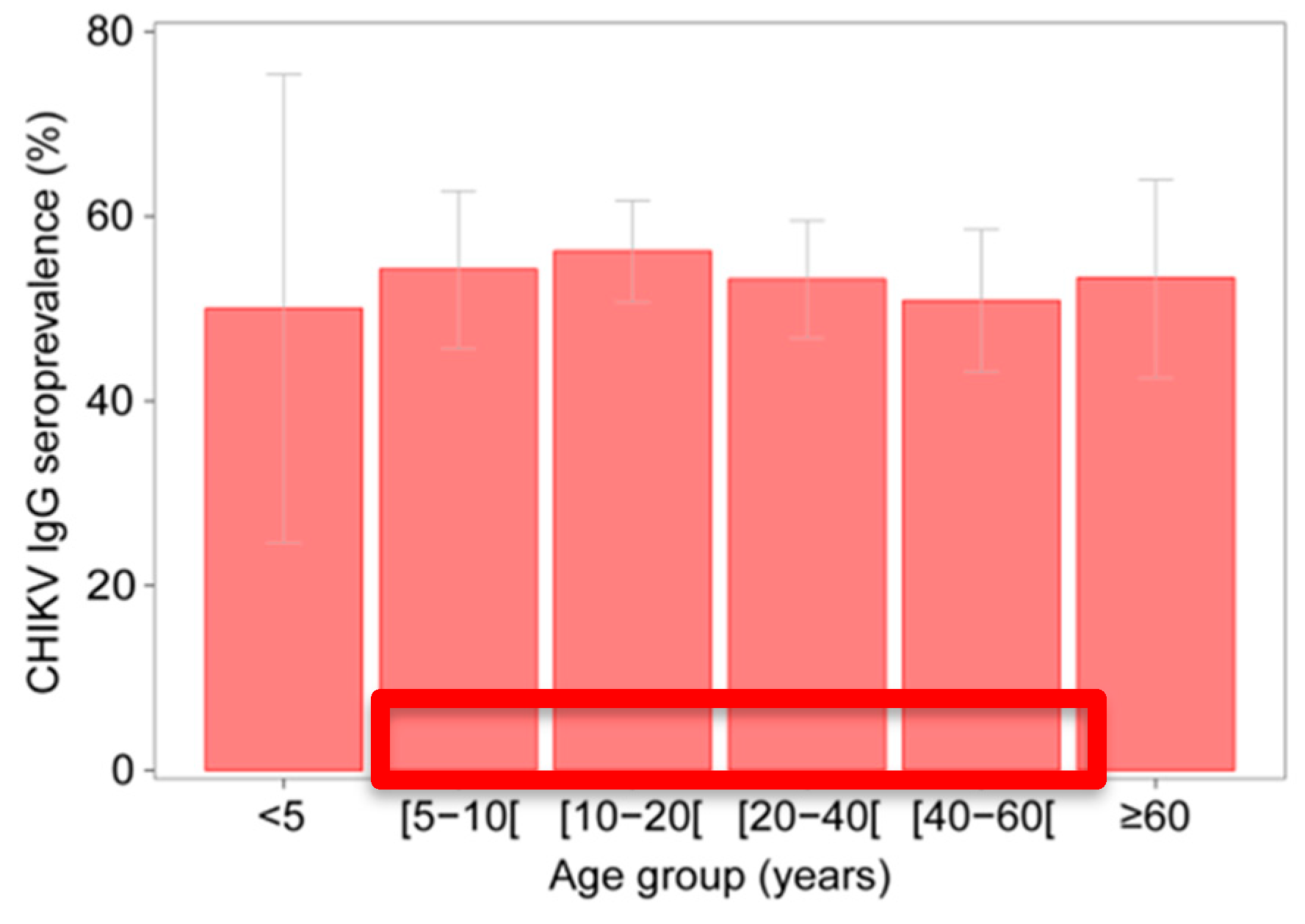
3.1. Serological Investigation for CHIKV IgG in 2012
3.2. Spatial Variation in CHIKV IgG Seroprevalence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| OR (95%CI) | LRT p-Value | AIC | |
|---|---|---|---|
| Environmental Variables | |||
| EVI_max (per 0.1 increase) | 1.79 (1.22; 2.56) | 0.002 | 1355 |
| EVI_mean (per 0.1 increase) | 2.84 (1.55; 5.22) | 0.001 | 1352 |
| EVI_sd (per 0.01 increase) | 1.17 (1.02; 1.31) | 0.018 | 1358 |
| NDVI_max (per 0.1 increase) | 2.58 (1.71; 3.97) | <0.001 | 1347 |
| NDVI_mean (per 0.1 increase) | 2.14 (1.54; 3.82) | <0.001 | 1350 |
| NDVI_sd (per 0.01 increase) | 1.16 (1.01; 1.30) | 0.028 | 1359 |
| MIR_max (per 0.1 increase) | 0.44 (0.18; 1.08) | 0.067 | 1360 |
| MIR_mean (per 0.1 increase) | 0.09 (0.03; 0.25) | <0.001 | 1347 |
| MIR_sd (per 0.01 increase) | 0.94 (0.72; 1.22) | 0.638 | 1363 |
| Distance to water bodies (km) | 1.01 (1.00; 1.03) | 0.044 | 1360 |
| Distance to rivers (km) | 1.02 (0.99; 1.05) | 0.196 | 1361 |
| Population density per km2 (log-transformed) | 0.73 (0.64; 0.81) | <0.001 | 1339 |
| Slope (degree) | 1.03 (0.90; 1.18) | 0.674 | 1363 |
| Altitude (meters) | 1.00 (1.00; 1.00) | 0.825 | 1363 |
| Forest area (proportion, per 0.1 increase) | 1.07 (0.99; 1.16) | 0.071 | 1360 |
| Distance to forest (km) | 0.87 (0.75; 1.01) | 0.062 | 1360 |
| Accessibility (travel time to city per hour increase) | 1.00 (1.00; 1.00) | 0.966 | 1363 |
| Random Intercepts | |||
| Village only | NA | 0.003 | 1362 |
| Rural community only | NA | 0.094 | 1368 |
| Village and rural | NA | <0.001 | 1361 |
| Environmental Variables | OR (95%CI) | LRT p-Value | AIC |
|---|---|---|---|
| Population density per km2 (log-transformed) | 0.73 (0.64; 0.81) | <0.001 | 1337 |
| Sexe | Age Group | nb CHIKV_IgG (+) | nb Individual Surveyed | ChikV Seroprevalence Rate |
|---|---|---|---|---|
| male | (5–10) | 2 | 10 | 20% |
| (10–20) | 5 | 22 | 23% | |
| (20–40) | 10 | 22 | 45% | |
| (40–60) | 2 | 4 | 50% | |
| >60 | 0 | 1 | 0% | |
| Subtotal | 19 | 59 | 32% | |
| female | (5–10) | 3 | 11 | 27% |
| (10–20) | 3 | 16 | 19% | |
| (20–40) | 2 | 18 | 11% | |
| (40–60) | 1 | 3 | 33% | |
| >60 | 1 | 2 | 50% | |
| Subtotal | 10 | 50 | 20% |
References
- Schuffenecker, I.; Iteman, I.; Michault, A.; Murri, S.; Frangeul, L.; Vaney, M.C.; Lavenir, R.; Pardigon, N.; Reynes, J.M.; Pettinelli, F.; et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006, 3, e263. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.W. The Newala epidemic. III. The virus: Isolation, pathogenic properties and relationship to the epidemic. J. Hyg. 1956, 54, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.M.; Brault, A.C.; Shirako, Y.; Strauss, E.G.; Kang, W.; Strauss, J.H.; Weaver, S.C. Evolutionary relationships and systematics of the Alphaviruses. J. Virol. 2001, 75, 10118–10131. [Google Scholar] [CrossRef] [PubMed]
- Deller, J.J., Jr.; Russell, P.K. An analysis of fevers of unknown origin in American soldiers in Vietnam. Ann. Intern. Med. 1967, 66, 1129–1143. [Google Scholar] [CrossRef]
- McGill, P.E. Viral infections: Alpha-viral arthropathy. Baillieres Clin. Rheumatol. 1995, 9, 145–150. [Google Scholar] [CrossRef]
- Adebajo, A.O. Rheumatic manifestations of tropical diseases. Curr. Opin. Rheumatol. 1996, 8, 85–89. [Google Scholar] [CrossRef]
- Althouse, B.M.; Guerbois, M.; Cummings, D.A.T.; Diop, O.M.; Faye, O.; Faye, A.; Diallo, D.; Sadio, B.D.; Sow, A.; Faye, O.; et al. Role of monkeys in the sylvatic cycle of chikungunya virus in Senegal. Nat. Commun. 2018, 9, 1046. [Google Scholar] [CrossRef]
- Ligon, B.L. Reemergence of an unusual disease: The chikungunya epidemic. Semin. Pediatr. Infect. Dis. 2006, 17, 99–104. [Google Scholar] [CrossRef]
- Diallo, M.; Thonnon, J.; Traore, L.M.; Fontenille, D. Vectors of Chikungunya virus in Senegal: Current data and transmission cycles. Am. J. Trop. Med. Hyg. 1999, 60, 281–286. [Google Scholar] [CrossRef]
- Volk, S.M.; Chen, R.; Tsetsarkin, K.A.; Adams, A.P.; Garcia, T.I.; Sall, A.A.; Nasar, F.; Schuh, A.J.; Holmes, E.C.; Higgs, S.; et al. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J. Virol. 2010, 84, 6497–6504. [Google Scholar] [CrossRef]
- Barrett, A.D.T.; Weaver, S.C. Arboviruses: Alphaviruses, flaviviruses and bunyaviruses. In Medical Microbiology, 16th ed.; Greenwood, D., Slack, R.C.B., Peutherer, J.F., Eds.; Churchill Livingstone: London, UK, 2002; pp. 484–501. [Google Scholar]
- Brooks, G.F.; Butel, J.S.; Morse, S.A. Human arboviral infections. In Jawetz. Melnick and Adelberg’s Medical Microbiology, 23rd ed.; Mc Graw Hill: Singapore, 2004; pp. 514–524. [Google Scholar]
- Alessandra, L.P.; Alessia, L.; Eleonora, C.; Gianguglielmo, Z.; Massimo, C. Chikungunya virus, epidemiology, clinics and phylogenesis: A review. Asian Pac. J. Trop. Med. 2014, 7, 925–932. [Google Scholar]
- Thonnon, J.; Spiegel, A.; Diallo, M.; Diallo, A.; Fontenille, D. Chikungunya virus outbreak in Senegal in 1996 and 1997. Bull. Soc. Pathol. Exot. 1999, 92, 79–82. [Google Scholar] [PubMed]
- Pfeffer, M.; Dobler, G. Emergence of zoonotic arboviruses by animal trade and migration. ParasitVectors 2010, 3, 35. [Google Scholar] [CrossRef]
- Devaux, C.A. Emerging and re-emerging viruses: A global challenge illustrated by Chikungunya virus outbreaks. World J. Virol. 2012, 1, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Reisen, W.K. Present and Future Arboviral Threats. Antiviral Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Panella, A.J.; Velez, J.O.; Lambert, A.J.; Campbell, G.L. Chikungunya virus in US travelers returning from India, 2006. Emerg. Infect. Dis. 2007, 13, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Pistone, T.; Ezzedine, K.; Boisvert, M.; Receveur, M.C.; Schuffenecker, I.; Zeller, H.; Lafon, M.E.; Fleury, H.; Malvy, D. Cluster of chikungunya virus infection in travelers returning from Senegal, 2006. J. Travel Med. 2009, 16, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Moro, M.L.; Gagliotti, C.; Silvi, G.; Angelimni, R.; Sambri, V.; Rezza, G.; Massimiliani, E.; Mattivi, A.; Grilli, E.; Finarelli, A.C.; et al. Chikungunya virus in north-eastern Italy: A seroprevalence survey. Am. J. Trop. Med. Hyg. 2010, 82, 508–511. [Google Scholar] [CrossRef]
- Grandadam, M.; Caro, V.; Plumet, S.; Thiberge, J.M.; Souarès, Y.; Failloux, A.B.; Tolou, H.J.; Budelot, M.; Cosserat, D.; Goffart, I.L.; et al. Chikungunya Virus, Southeastern France. Emerg. Infect. Dis. 2011, 17, 910–913. [Google Scholar] [CrossRef]
- Powell, J. New contender for most lethal animal. Nature 2016, 540, 525. [Google Scholar] [CrossRef]
- Reiter, P. Aedes albopictus and the world trade in used tires, 1988-1995: The shape of things to come? J. Am. Mosq. Control. Assoc. 1998, 14, 83–94. [Google Scholar] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Schaffner, F.; Versteirt, V.; Hendrickx, G.; Zeller, H.; Bortel, W.V. A review of the invasive mosquitoes in Europe: Ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012, 12, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Bres, P.; Chambon, L.; Pape, Y.; Michel, R. Isolement de plusieurs souches d’arbovirus à partir des glandes salivaires de chauves-souris. Bul. Soc. Med. Afr. Noire. Langue. Fr. 1963, 8, 710–712. [Google Scholar]
- Robin, Y.; Bres, P. Arboviruses in Senegal. Current status. Bull. Soc. Med. Afr. Noire. Lang. 1969, 14, 722–728. [Google Scholar]
- Roche, S.; Robin, Y. Human infections by CHIK virus in Rufisque (Senegal). Bul. Soc. Med. Afr. Noire. Langue. Fr. 1966, 12, 490–496. [Google Scholar]
- Saluzzo, J.F.; Cornet, M.; Digoutte, J.P. Outbreak of a CHIK virus epidemic in western Senegal in 1982. Dakar Med. 1983, 28, 497–500. [Google Scholar]
- Monlun, E.; Zeller, H.; Le Guenno, B.; Traoré, L.M.; Hervy, J.P. Surveillance de la circulation des arbovirus d’intérêt médical dans la région du Sénégal Oriental (1988–1991). Bull. Soc. Pathol. Exot. 1993, 86, 21–28. [Google Scholar]
- Cornet, M.; Robin, Y.; Chateau, R.; Héme, G.; Adam, C.; Valade, M.; Gonidec, G.L.; Jan, C.; Renaudet, J.; Dieng, P.L.; et al. Isolement d’arbovirus au Sénégal oriental à partir de moustiques (1972-1977) et notes sur l’épidémiologie des virus transmis par les Aedes, en particulier du virus Amaril. Cah. O.R.S.T.O.M. Sér. Ent. Méd. Parasitol. 1979, 17, 149–163. [Google Scholar]
- Diallo, M.; Sall, A.A.; Moncayo, A.C.; Ba, Y.; Fernandez, Z.; Ortiz, D.; Coffey, L.L.; Mathiot, C.; Tesh, B.B.; Weaver, S.C. Potential role of sylvatic and domestic African mosquito species in dengue emergence. Am. J. Trop. Med. Hyg. 2005, 73, 445–449. [Google Scholar] [CrossRef]
- CRORA (Centre Collaborateur OMS de Référence et de Recherche pour les Arbovirus et Virus de Fièvres Hémorragiques). Available online: http://www.pasteur.fr/recherche/banques/CRORA/virus (accessed on 1 October 2017).
- Sow, A.; Faye, O.; Diallo, M.; Diallo, D.; Cheng, R.; Faye, O.; Diagne, C.T.; Guerbois, M.; Weidmann, M.; Ndiaye, Y.; et al. Chikungunya Outbreak in Kedougou, Southeastern Senegal in 2009–2010. Open Forum Infect Dis. 2018, 5. [Google Scholar] [CrossRef]
- Agence nationale de la Démographie et de la Statistique (2015). Service Régional de la Statistique et de la Démographie de Kédougou. Rapport Annuel de la Situation Economique et Régionale. Kedougou, Sénégal. Aboubacar Sédikh BEYE. 138p. Available online: http://www.ansd.sn/ressources/ses/SES-Kedougou-2013.pdf (accessed on 17 July 2017).
- Traore, L.M.; Fontenille, D.; Zeller, H.G.; Mondo, M.; Diallo, M.; Adam, F.; Eyraud, M.; Maiga, A.; Digoutte, J.P. Surveillance for yellow fever virus in eastern Senegal during 1993. J. Med. Entomol. 1996, 33, 760–765. [Google Scholar] [CrossRef] [PubMed]
- NASA EOSDIS. Land Processes DAAC, USGS Earth Resources Observation and Science (EROS) Center, Sioux Falls. Available online: https://lpdaac.usgs.gov (accessed on 15 September 2017).
- Hay, S.I.; Tatem, A.J.; Graham, A.J.; Goetz, S.J.; Rogers, D.J. Global environmental data for mapping infectious disease distribution. Adv Parasitol. 2006, 62, 37–77. [Google Scholar] [PubMed]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Farr, T.G.; Kobrick, M. Shuttle Radar Topography Mission produces a wealth of data. Am. Geophys. Union Eos 2000, 81, 583–585. [Google Scholar] [CrossRef]
- Lunn, D.J.; Thomas, A.; Best, N.; Spiegelhalter, D. WinBUGS—A Bayesian modelling framework: Concepts, structure, and extensibility. Stat. Comput. 2000, 10, 325–337. [Google Scholar] [CrossRef]
- Diallo, D.; Sall, A.A.; Buenemann, M.; Chen, R.; Faye, O.; Diagne, C.T.T.; Faye, O.; Ba, Y.; Dia, I.; Watts, D.; et al. Landscape Ecology of Sylvatic Chikungunya Virus and mosquito Vectors in Southeastern Senegal. PLoS Negl. Trop. Dis. 2012, 6, e1649. [Google Scholar] [CrossRef]
- The WorldPop Demography Project. Available online: http://www.worldpop.org.uk/ (accessed on 15 September 2016).
- Sow, A.; Faye, O.; Diallo, D.; Chen, R.; Faye, O.; Diagne, C.T.T.; Ndiaye, Y.; Senghor, C.S.; Abdourahmane Faye, A.; Diop, O.M.; et al. Re-emergence of Yellow fever in Kedougou, Southeastern Senegal in 2010–2011. Int. J. Sc. Res. 2017. [Google Scholar] [CrossRef][Green Version]
- Travel Time to Major Cities: A Global Map of Accessibility. Available online: http://bioval.jrc.ec.europa.eu/products/gam/index.htm (accessed on 15 September 2018).
| Environmental Variables | OR (95%CI) | LRT p-Value | AIC |
|---|---|---|---|
| EVI_max (per 0.1 increase) | 1.54 (1.16; 2.02) | 0.002 | 1354 |
| EVI_mean (per 0.1 increase) | 2.23 (1.44; 3.47) | <0.001 | 1351 |
| EVI_sd (per 0.01 increase) | 1.14 (1.03; 1.27) | 0.010 | 1357 |
| NDVI_max (per 0.1 increase) | 1.90 (1.42; 2.57) | <0.001 | 1348 |
| NDVI_mean (per 0.1 increase) | 1.85 (1.35; 2.52) | <0.001 | 1350 |
| NDVI_sd (per 0.01 increase) | 1.16 (1.03; 1.30) | 0.012 | 1357 |
| MIR_max (per 0.1 increase) | 0.68 (0.35; 1.35) | 0.258 | 1362 |
| MIR_mean (per 0.1 increase) | 0.22 (0.10; 0.51) | <0.001 | 1352 |
| MIR_sd (per 0.01 increase) | 1.02 (0.82; 1.28) | 0.829 | 1363 |
| Distance to water bodies (km) | 1.01 (1.00; 1.03) | 0.048 | 1360 |
| Distance to rivers (km) | 1.02 (0.99; 1.05) | 0.202 | 1361 |
| Population density per km2 (log-transformed) | 0.76 (0.69; 0.84) | <0.001 | 1340 |
| Slope (degree) | 1.12 (0.98; 1.29) | 0.089 | 1360 |
| Altitude (meters) | 1.00 (1.00; 1.00) | 0.937 | 1363 |
| Forest area (proportion, per 0.1 increase) | 1.06 (0.99; 1.13) | 0.081 | 1360 |
| Distance to forest (km) | 0.86 (0.76; 0.98) | 0.023 | 1358 |
| Accessibility (travel time to city per hour increase) | 1.00 (1.00; 1.00) | 0.985 | 1363 |
| Random Intercepts: | |||
| Village only | NA | 0.003 | 1362 |
| Rural community only | NA | 0.094 | 1368 |
| Village and rural | NA | <0.001 | 1361 |
| Environmental Variables | OR (95%CI) | LRT p-Value | AIC |
|---|---|---|---|
| Population density per km2 (log-transformed) | 0.76 (0.69; 0.84) | 0.008 | 1338 |
| NDVI_max (per 0.1 increase) | 1.17 (0.76; 1.81) | 0.485 | 1340 |
| Distance to forest (km) | 0.97 (0.86; 1.10) | 0.614 | 1340 |
| Distance to water bodies (km) | 1.00 (0.99; 1.01) | 0.735 | 1340 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sow, A.; Nikolay, B.; Faye, O.; Cauchemez, S.; Cano, J.; Diallo, M.; Faye, O.; Sadio, B.; Ndiaye, O.; Weaver, S.C.; et al. Changes in the Transmission Dynamic of Chikungunya Virus in Southeastern Senegal. Viruses 2020, 12, 196. https://doi.org/10.3390/v12020196
Sow A, Nikolay B, Faye O, Cauchemez S, Cano J, Diallo M, Faye O, Sadio B, Ndiaye O, Weaver SC, et al. Changes in the Transmission Dynamic of Chikungunya Virus in Southeastern Senegal. Viruses. 2020; 12(2):196. https://doi.org/10.3390/v12020196
Chicago/Turabian StyleSow, Abdourahmane, Birgit Nikolay, Oumar Faye, Simon Cauchemez, Jorge Cano, Mawlouth Diallo, Ousmane Faye, Bakary Sadio, Oumar Ndiaye, Scott C. Weaver, and et al. 2020. "Changes in the Transmission Dynamic of Chikungunya Virus in Southeastern Senegal" Viruses 12, no. 2: 196. https://doi.org/10.3390/v12020196
APA StyleSow, A., Nikolay, B., Faye, O., Cauchemez, S., Cano, J., Diallo, M., Faye, O., Sadio, B., Ndiaye, O., Weaver, S. C., Dia, A. T., Sall, A. A., & Malvy, D. (2020). Changes in the Transmission Dynamic of Chikungunya Virus in Southeastern Senegal. Viruses, 12(2), 196. https://doi.org/10.3390/v12020196





