Genomic Characterization, Formulation and Efficacy in Planta of a Siphoviridae and Podoviridae Protection Cocktail against the Bacterial Plant Pathogens Pectobacterium spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates and Media
2.2. Isolation, Purification and Enrichment of Bacteriophages
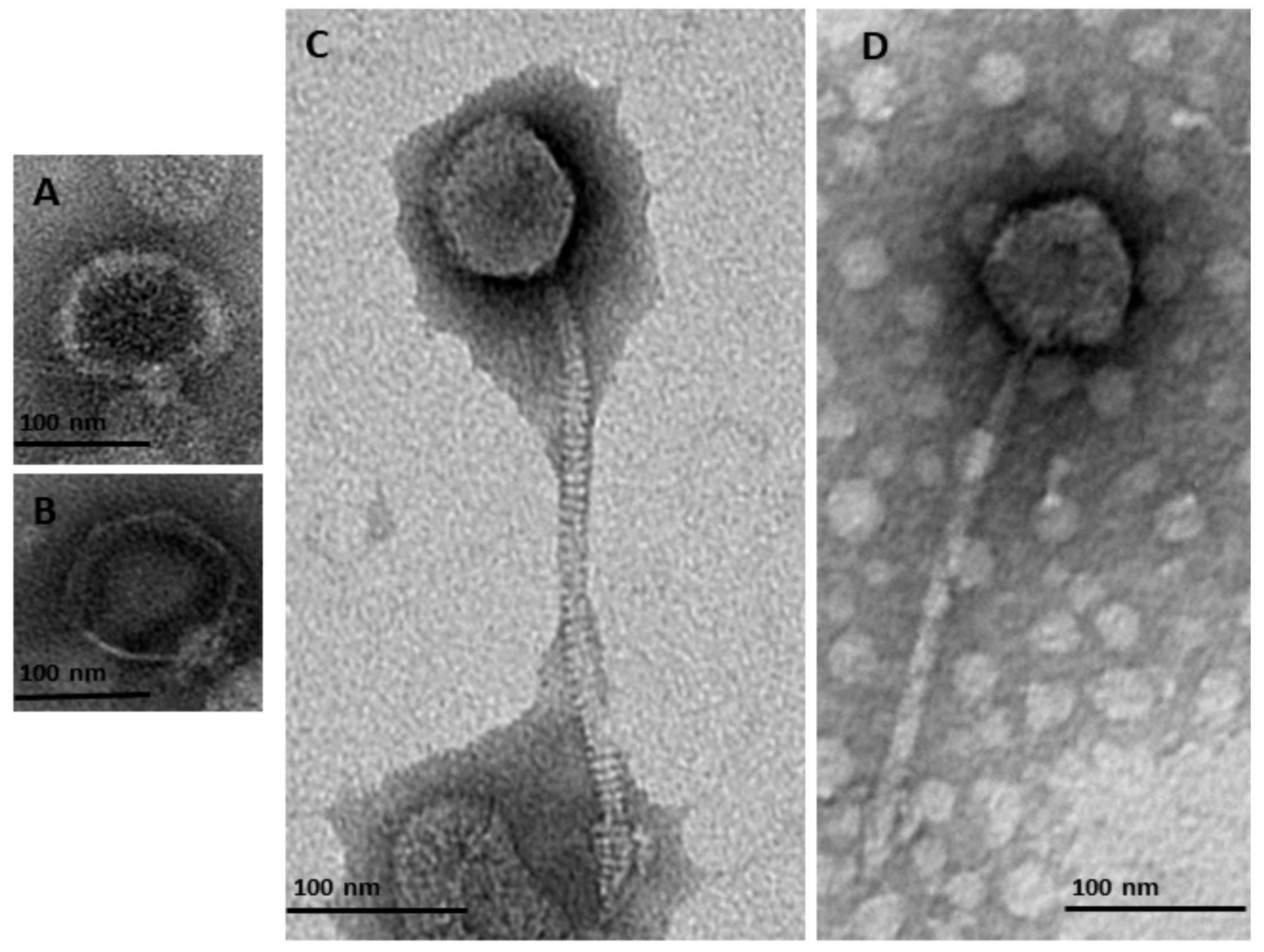
2.3. TEM
2.4. Screening Bacteriophages against Soft Rot Bacteria Species for Cocktail Formulation
2.5. DNA Extraction, Purification and DNA Sequencing
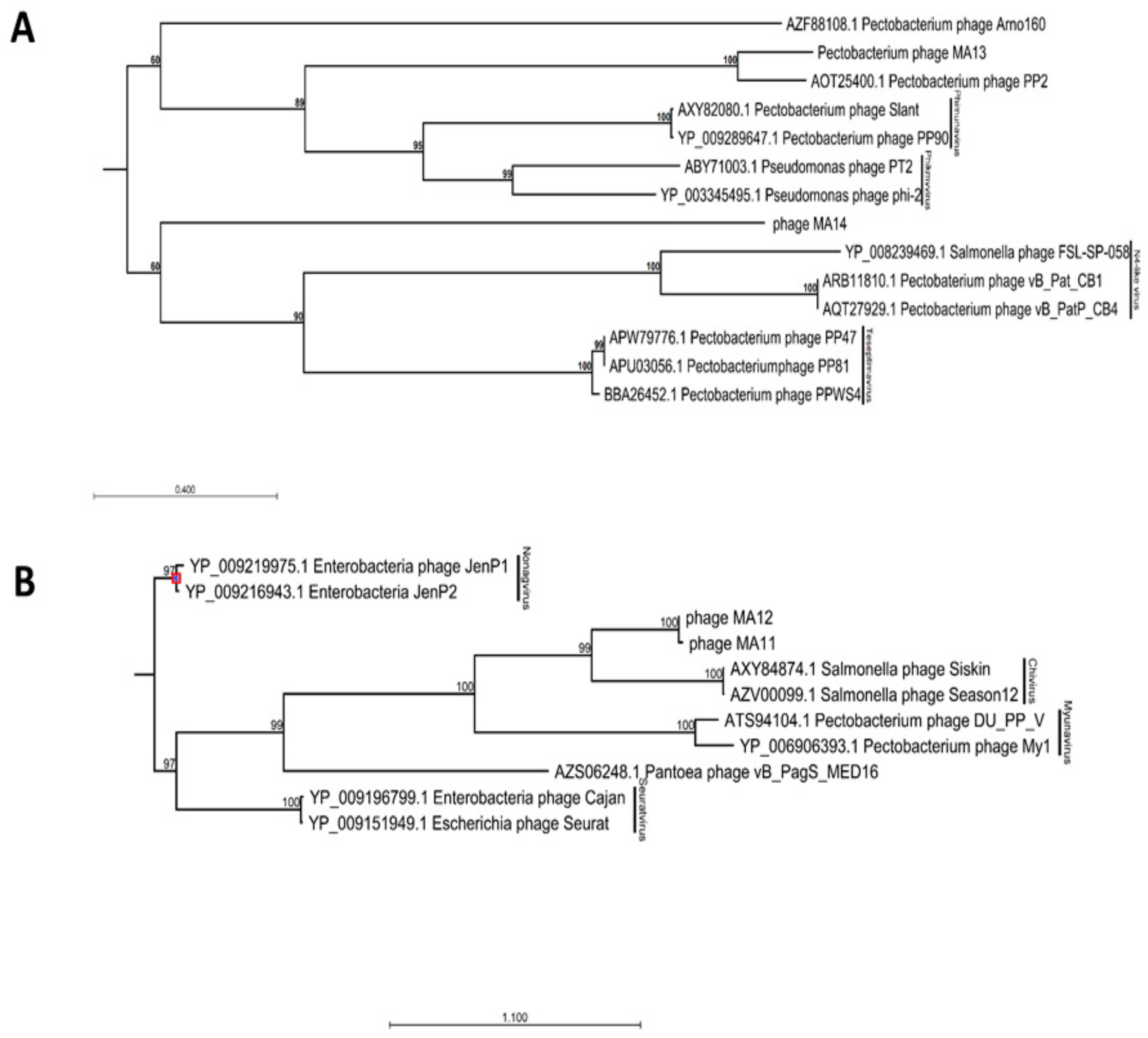
2.6. Genomic and Phylogenetic Analysis
2.7. Phage Cocktail Used in Field Trials
2.8. Planting Material
2.9. Treatments and Evaluation Methods Used in Field Trials
2.10. Persistence of Phage Cocktail Treated Tubers in Field Trial 2016
2.11. Statistical Analysis
3. Results
3.1. Isolation, Purification and Identification of Bacteriophages
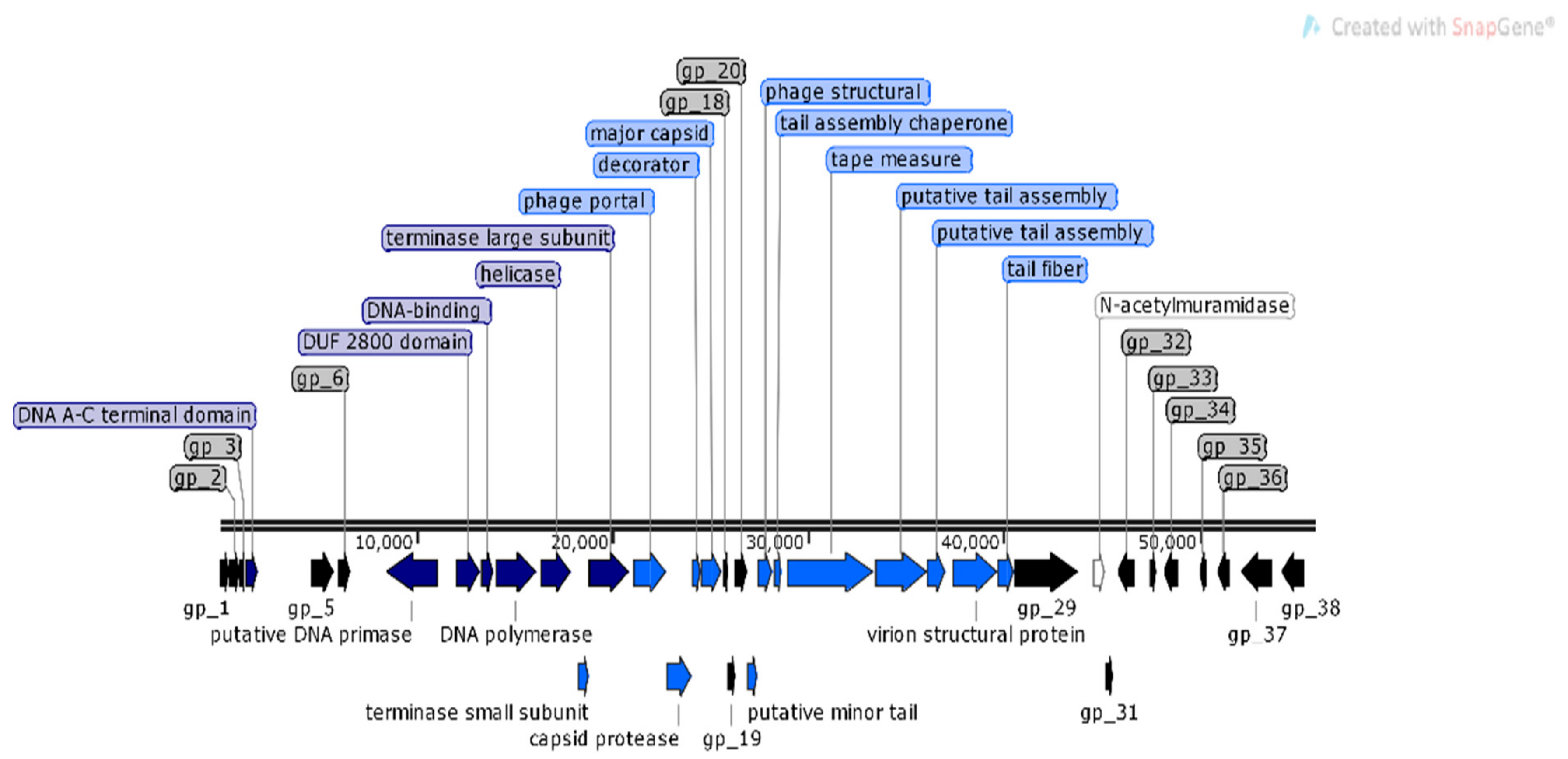
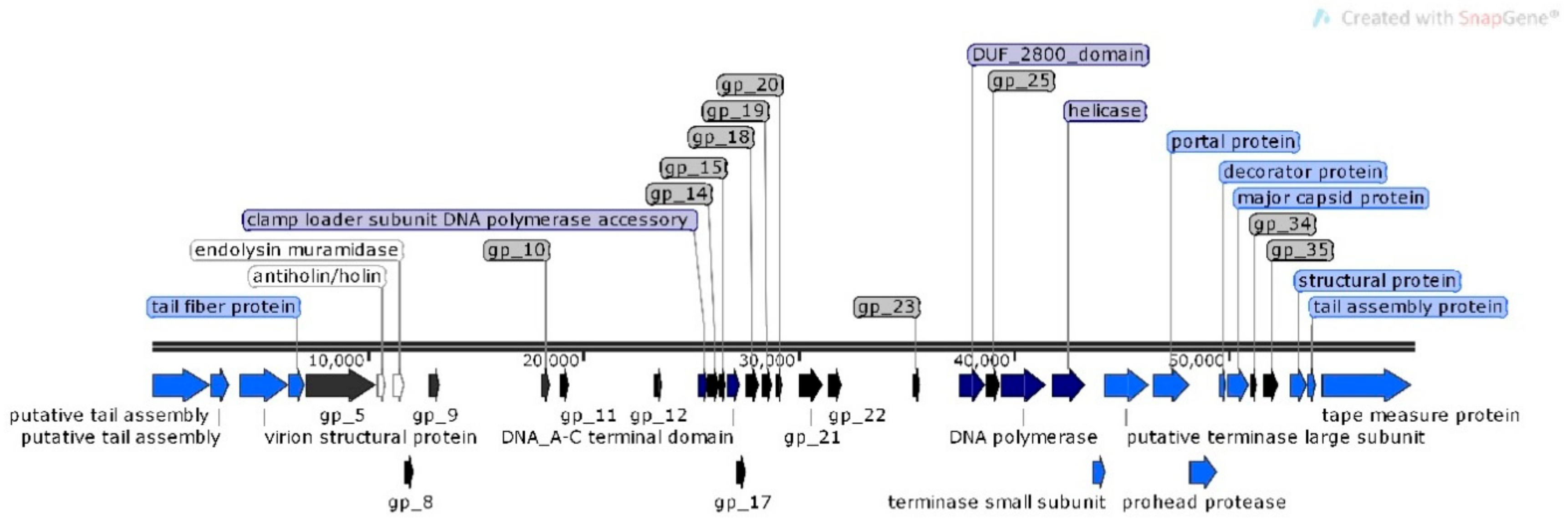
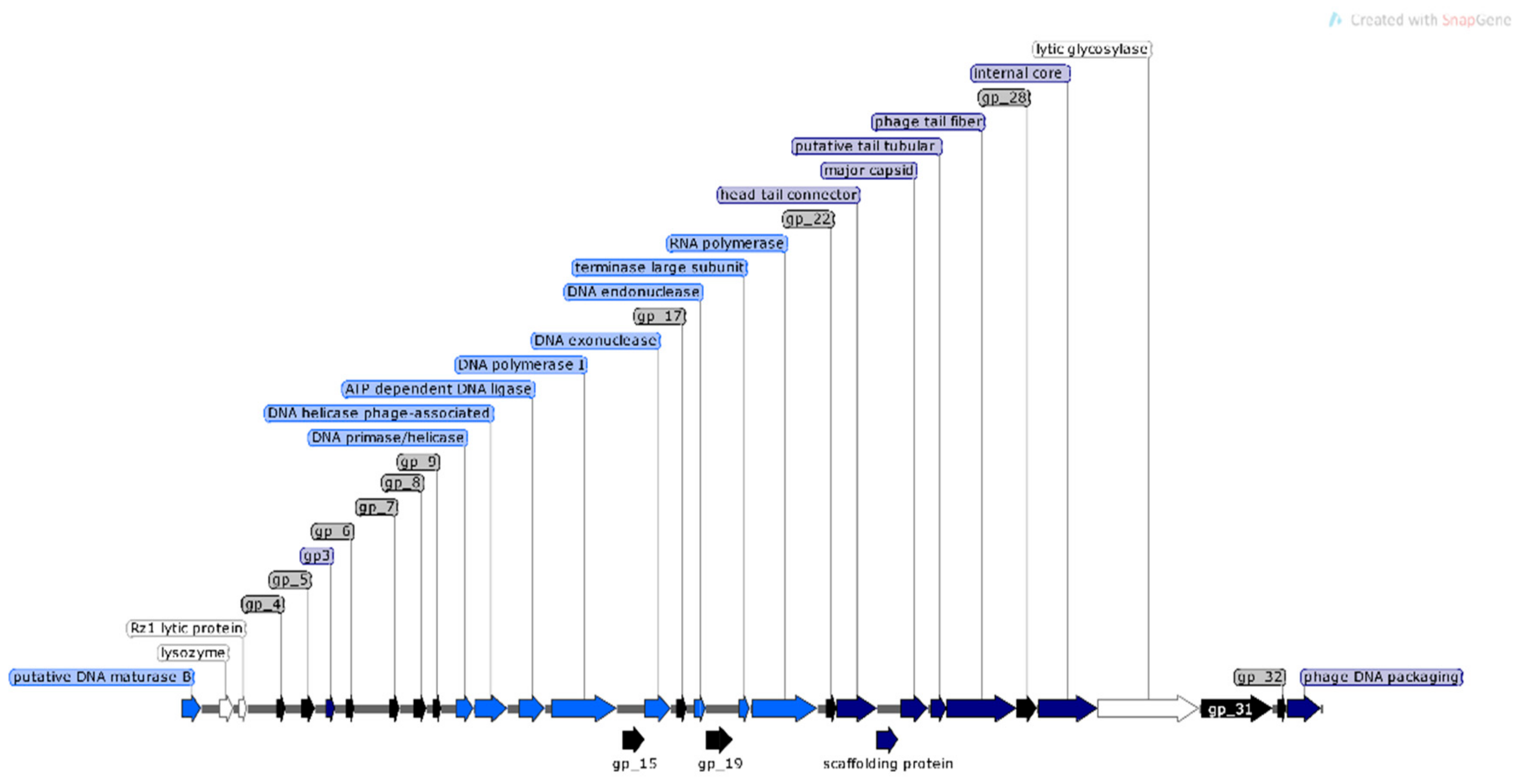
3.2. Genomic Characterization of Bacteriophages
3.3. Phage Cocktail Formulation
3.4. Evaluation of Efficacy of the Phage Cocktail in Vivo
3.5. Persistence Trial of Bacteriophages in Phage Cocktail Treated Onions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture: Trends and Challenges. Available online: http://www.fao.org/3/a-i6583e.pdf (accessed on 4 November 2017).
- Parnell, J.J.; Berka, R.; Young, H.A.; Sturino, J.M.; Kang, Y.; Barnhart, D.M.; DiLeo, M.V. From the lab to the farm: an industrial perspective of plant beneficial microorganisms. Front. Plant Sci. 2016, 7, 1110. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 18 January 2019).
- Vahling-Armstrong, C.; Dung, J.K.S.; Humann, J.L. Effects of postharvest onion curing parameters on bulb rot caused by Pantoea agglomerans, Pantoea ananatis and Pantoea allii in storage. Plant Pathol. 2015, 65, 536–544. [Google Scholar] [CrossRef]
- Conn, E.K.; Lutton, J.S.; Rosenberger, S.A. Onion Disease Guide. Plant Health 2012, 72. [Google Scholar]
- Hadizadeh, I.; Peivastegan, B.; Hannukkala, A.; van der Wolf, J.M.; Nissinen, R. Biological control of potato soft rot caused by Dickeya solani and the survival of bacterial antagonists under cold storage conditions. Plant Pathol. 2018, 68, 297–311. [Google Scholar] [CrossRef]
- Lee, C.J.; Lee, A.J.T.; Kwon, A.J.H.; Kim, B.B.C.; Park, C.; Park, W. Occurrence of bacterial soft rot of onion plants caused by Burkholderia gladioli pv. alliicola in Korea. Australas. Plant Pathol. 2005, 34, 287–292. [Google Scholar] [CrossRef]
- Waleron, M.; Waleron, K.; Łojkowska, E. First report of Pectobacterium carotovorum subsp. brasiliense causing soft rot on potato and other vegetables in Poland. Plant Dis. 2015, 99, 1271. [Google Scholar] [CrossRef]
- Palacio-Bielsa, A.; Cambra, M.A.; Lopez, M.M. First report of bacterial soft rot on onion caused by Dickeya sp. (ex Pectobacterium chrysanthemi) in Spain. Plant Pathol. 2006, 56, 722. [Google Scholar] [CrossRef]
- Tsai, W.A.; Lin, P.R.; Huang, C.J. First report of Dickeya fangzhongdai causing soft rot disease of Welsh onion in Taiwan. J. Plant Pathol. 2019, 101, 797–798. [Google Scholar] [CrossRef]
- Adriaenssens, E.M.; van Vaerenbergh, J.; Vandenheuvel, D.; Dunon, V.; Ceyssens, P.J.; de Proft, M.; Kropinski, A.M.; Noben, J.P.; Maes, M.; Lavigne, R. T4-related bacteriophage LIMEstone isolates for the control of soft rot on potato caused by ‘Dickeya solani’. PLoS ONE 2012, 7, e33227. [Google Scholar] [CrossRef]
- Lim, J.A.; Jee, S.; Lee, D.H.; Roh, E.; Jung, K.; Oh, C.; Heu, S. Biocontrol of Pectobacterium carotovorum subsp. carotovorum using bacteriophage PP1. J. Microbiol. Biotechnol. 2013, 23, 1147–1153. [Google Scholar] [CrossRef]
- Czajkowski, R.; Smolarska, A.; Ozymko, Z. The viability of lytic bacteriophage ΦD5 in potato-associated environments and its effect on Dickeya solani in potato (Solanum tuberosum L.) plants. PLoS ONE 2017, 12, e0183200. [Google Scholar] [CrossRef]
- Svircev, A.; Roach, D.; Castle, A. Framing the future with bacteriophages in agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef]
- Lang, J.M.; Gent, D.H.; Schwartz, H.F. Management of Xanthomonas leaf blight of onion with bacteriophages and a plant activator. Plant Dis. 2007, 91, 871–878. [Google Scholar] [CrossRef]
- Czajkowski, R.; Ozymko, Z.; de Jager, V.; Siwinska, J.; Smolarska, A.; Ossowicki, A.; Narajczyk, M.; Lojkowska, E. Genomic, proteomic and morphological characterization of two novel broad host lytic bacteriophages ΦPD10.3 and ΦPD23.1 infecting pectinolytic Pectobacterium spp. and Dickeya spp. PLoS ONE 2015, 10, e0119812. [Google Scholar] [CrossRef]
- Buttimer, C.; Hendrix, H.; Lucid, A.; Neve, H.; Noben, J.P.; Franz, C.; O’Mahony, J.; Lavigne, R.; Coffey, A. Novel N4-Like Bacteriophages of Pectobacterium atrosepticum. Pharmaceuticals 2018, 11, 45. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA) Home Page. Available online: https://echa.europa.eu/regulations/biocidal-products-regulation/authorisation-of-biocidal-products (accessed on 22 May 2012).
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Zaczek-Moczydłowska, M.A.; Fleming, C.C.; Young, G.K.; Campbell, K.; O’Hanlon, R. Pectobacterium and Dickeya species detected in vegetables in Northern Ireland. Eur. J. Plant Pathol. 2019, 154, 635–647. [Google Scholar] [CrossRef]
- Fortier, L.C.; Moineau, S. Morphological and genetic diversity of temperate phages. Appl. Environ. Microbiol. 2007, 73, 7358–7366. [Google Scholar] [CrossRef]
- Kutter, E. Phage Host Range and Efficiency of Plating. In Bacteriophages; Humana Press: Totowa, NJ, USA, 2009; Volume 501, pp. 141–149. [Google Scholar]
- Devaney, R.; Trudgett, J.; Trudgett, A.; Meharg, C.; Smyth, V. A metagenomic comparison of endemic viruses from broiler chickens with runting-stunting syndrome and from normal birds. Avian Pathol. 2016, 45, 616–629. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 10, 8365. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, 200–203. [Google Scholar] [CrossRef]
- Center for Genomic Epidemiology. ResFinder 3.2. Available online: https://cge.cbs.dtu.dk/services/ResFinder/ (accessed on 16 October 2019).
- Center for Genomic Epidemiology. Virulence Finder 2.0. Available online: https://cge.cbs.dtu.dk/services/VirulenceFinder/ (accessed on 16 October 2019).
- Center for Genomic Epidemiology. ToxFinder 1.0. Available online: https://cge.cbs.dtu.dk/services/ToxFinder/ (accessed on 16 October 2019).
- Bdliya, B.S.; Langerfeld, E.; Rudolph, K. A modified crystal violet pectate (CVP) medium for detection and isolation of soft rot Erwinia spp. from plant materials. J. Plant Dis. Protect. 2004, 111, 506–515. [Google Scholar]
- Czajkowski, R.; Pérombelon, M.C.M.; Jafra, S.; Lojkowska, E.; Potrykus, M.; van der Wolf, J.M.; Sledz, W. Detection, identification and differentiation of Pectobacterium and Dickeya species causing potato blackleg and tuber soft rot: A review. Ann. Appl. Biol. 2015, 166, 18–38. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Mackiewicz, B.; Lemieszek, M.K.; Golec, M.; Skórska, C.; Góra-Florek, A.; Malinowski, J. Pantoea agglomerans: A mysterious bacterium of evil and good. Part II. Deleterious effects: Dust-borne endotoxins and allergens—Focus on grain dust, other agricultural dusts and wood dust. Ann. Agric. Environ. Med. 2016, 23, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Tho, K.E.; Wiriyajitsomboon, P.; Hausbeck, M.K. First report of Pantoea agglomerans causing onion leaf blight and bulb rot in Michigan. Plant Dis. 2015, 99, 1034. [Google Scholar] [CrossRef]
- Hu, J. First report of onion bulb rot caused by Pantoea agglomerans in Arizona. Dis. Notes. 2019, 103, 1408. [Google Scholar] [CrossRef]
- Czajkowski, R. Bacteriophages of soft rot Enterobacteriaceae—A minireview. FEMS Microbiol. Lett. 2016, 363, fnv230. [Google Scholar] [CrossRef]
- Carstens, A.B.; Djurhuus, A.M.; Kot, W.; Hansen, L.H. A novel six-phage cocktail reduces Pectobacterium atrosepticum soft rot infection in potato tubers under simulated storage conditions. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef]
- Smolarska, A.; Rabalski, L.; Narajczyk, M.; Czajkowski, R. Isolation and phenotypic and morphological characterization of the first Podoviridae lytic bacteriophages ϕA38 and ϕA41 infecting Pectobacterium parmentieri (former Pectobacterium wasabiae). Eur. J. Plant. Pathol. 2018, 150, 413–425. [Google Scholar] [CrossRef]
- Kabanova, A.; Shneider, M.; Bugaeva, E.; Ha, V.T.N.; Miroshnikov, K.; Korzhenkov, A.; Kulikov, E.; Toschakov, S.; Ignatov, A.; Miroshnikov, K. Genomic characteristics of vB_PpaP_PP74, a T7-like Autographivirinae bacteriophage infecting a potato pathogen of the newly proposed species Pectobacterium parmentieri. Arch. Virol. 2018, 163, 1691–1694. [Google Scholar] [CrossRef]
- Alič, Š.; Naglič, T.; Tušek-Žnidarič, M.; Ravnikar, M.; Rački, N.; Peterka, M.; Dreo, T. Newly isolated bacteriophages from the Podoviridae, Siphoviridae, and Myoviridae families have variable effects on putative novel Dickeya spp. Front. Microbiol. 2017, 8, 1870. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Ceyssens, P.J.; Dunon, V.; Ackermann, H.W.; Van Vaerenbergh, J.; Maes, M.; De Proft, M.; Lavigne, R. Bacteriophages LIMElight and LIMEzero of Pantoea agglomerans, belonging to the “phiKMV-like viruses”. Appl. Environ. Microbiol. 2011, 77, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Šimoliūnas, E.; Šimoliūnienė, M.; Kaliniene, L.; Zajančkauskaitė, A.; Skapas, M.; Meškys, R.; Truncaitė, L. Pantoea Bacteriophage vB_PagS_Vid5: A low-temperature Siphovirus that harbors a cluster of genes involved in the biosynthesis of archaeosine. Viruses 2018, 10, 583. [Google Scholar]
- Lim, J.A.; Heu, S.; Park, J.; Roh, E. Genomic characterization of bacteriophage vB_PcaP_PP2 infecting Pectobacterium carotovorum subsp. carotovorum, a new member of a proposed genus in the subfamily Autographivirinae. Arch. Virol. 2017, 162, 2441–2444. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, J.H.; Shin, H.; Ji, S.; Roh, E.; Jung, K.; Ryu, S.; Choi, J.; Heu, S. Complete genome sequence of Pectobacterium carotovorum subsp. carotovorum bacteriophage My1. J. Virol. 2012, 86, 11410–11411. [Google Scholar] [CrossRef]
- Park, T.H. The National Center for Biotechnology Information Home Page. Complete genome sequence of bacteriophage (DU_PP_V) infecting Pectobacterium spp. Available online: https://www.ncbi.nlm.nih.gov/nuccore/1275231159 (accessed on 12 November 2017).
- Day, A.; Ahn, J.; Fang, X.; Salmond, G.P.C. Environmental bacteriophages of the emerging Enterobacterial phytopathogen, Dickeya solani, show genomic conservation and capacity for horizontal gene transfer between their bacterial hosts. Front. Microbiol. 2017, 8, 165. [Google Scholar] [CrossRef]
- Fillol-Salom, A.; Alsaadi, A.; Sousa, J.A.M.; Zhong, L.; Foster, K.R.; Rocha, E.P.C.; Penadés, J.R.; Ingmer, H.; Haaber, J. Bacteriophages benefit from generalized transduction. PLoS Pathog. 2019, 15, e1007888. [Google Scholar] [CrossRef]
- Zhang, F.; Song, G.; Tian, Y. Anti- CRISPRs: The natural inhibitors for CRISPR-Cas systems. Anim. Model. Exp. Med. 2019, 29, 69–75. [Google Scholar] [CrossRef]
- Maxwell, K.L. Phages Fight Back: Inactivation of the CRISPR-Cas bacterial immune system by anti-CRISPR proteins. PLoS Pathog. 2016, 12, e1005282. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, F.; Huang, Z. Structural insights into the inactivation of CRISPR-Cas systems by diverse anti-CRISPR proteins. BMC Biol. 2018, 16, 32. [Google Scholar] [CrossRef]
- Dupuis, B. The movement of potato virus Y (PVY) in the vascular system of potato plants. Eur. J. Plant Pathol. 2017, 147, 365–373. [Google Scholar] [CrossRef]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and bacterial plant diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Schmerer, M.; Molineux, I.J.; Bull, J.J. Synergy as a rationale for phage therapy using phage cocktails. Peer J. 2014, 2, e590. [Google Scholar] [CrossRef]
| Year | Location | Number of Applications per Treatment × Volume [L] /Plot | |||||
|---|---|---|---|---|---|---|---|
| Spraying (T1) 1 | Spraying (T2) 2 | Immersion (T3) 3 | Untreated (C1) 4 | Negative (C2) 5 | Positive (C3) 6 | ||
| 2016 | Belfast, Co. Antrim, NI | 4 × 1 | 4 × 1 | 1 × 2 | NT | 1 × 2 | 1 × 2 |
| 2017 | Crossnacreevy, Co. Down, NI | 4 × 1 | 4 × 1 | 1 × 2 | NT | 1 × 2 | 1 × 2 |
| 2018 | Loughgall, Co. Armagh, NI | 4 × 1 | 4 × 1 | 1 × 2 | NT | 1 × 2 | 1 × 2 |
| Accession No. | Phage ID | Morphological Characteristics a | Order a,b | Family a,b | Subfamily/Genus b | Id b (%) | Genome Size (bp) | |
|---|---|---|---|---|---|---|---|---|
| Head (nm) | Tail (nm) | |||||||
| MN518139 | φMA11 | 50.1 × 42.1 | 223.4 | Caudovirales | Siphoviridae | Chivirus | 70 | 55,830 |
| MN692199 | φMA12 | 58.7 × 48.7 | 227.9 | Caudovirales | Siphoviridae | Chivirus | 65 | 58,573 |
| MN509793 | φMA13 | 67.1 × 72.1 | nm | Caudovirales | Podoviridae | Autographivirinae | 76 | 42,464 |
| MN692200 | φMA14 | 57.3 × 55.1 | 11.22 | Caudovirales | Podoviridae | Autographivirinae | 71 | 10,019 |
| Isolate Id | County Location | Year of Isolation | EOP of Bacteriophages 1 | |||
|---|---|---|---|---|---|---|
| φMA11 | φMA12 | φMA13 | φMA14 | |||
| Pectobacterium spp. | ||||||
| OM/Z-1/15 | Armagh | 2015 | (1) | (1) | (1) | - |
| OM/Z-4/15 | Armagh | 2015 | (1) | - | - | - |
| OM/Z-5/15 | Armagh | 2015 | 1 * | 0.01 | 0.001 * | 0.04 |
| OM/Z-1/10/15 | Armagh | 2015 | - | - | - | 0.5 |
| OM/Z-2/10/15 | Armagh | 2015 | - | - | - | 0.1 |
| 05A/16 | Down | 2016 | (1) | - | (1) | (1) |
| 05B/16 | Down | 2016 | - | - | (1) | (1) |
| 06/16-1 | Down | 2016 | (1) | (1) | (1) | (1) |
| O7/16 | Antrim | 2016 | - | - | - | - |
| O7B/16 | Antrim | 2016 | - | - | - | - |
| O17A/16 | Antrim | 2016 | - | - | - | - |
| O12/16 | Antrim | 2016 | - | - | - | - |
| O17B/16 | Antrim | 2016 | 0.003 | - | - | - |
| O7C/16 | Antrim | 2016 | 0.01 | 0.01 | - | - |
| 015/16 | Antrim | 2016 | - | 0.1 | - | - |
| Pantoea spp. | ||||||
| O21/16 | Antrim | 2016 | - | 1* | - | - |
| O21B/16 | Antrim | 2016 | - | - | - | 1* |
| Treatment | Emergence 4 (%) | Bulbs Mass 5 (g) | Foliage Mass 6 (g) | Disease 7 (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2016 1 | 2017 2 | 2018 3 | 2016 1 | 2017 2 | 2018 3 | 2016 1 | 2017 2 | 2018 3 | 2016 1 | 2017 2 | 2018 3 |
| Means | ||||||||||||
| Spraying (T1) 8 | 54.2a | 91.7a | 86.7a | 642a | 2270b | 2040a | 282a | 1312b | 881ab | 3.3a | 0a | 1.7b |
| Spraying (T2) 9 | 72.5a | 95.0a | 70.8b | 819a | 1988bc | 1242b | 481a | 1526ab | 458bc | 3.3a | 0a | 0c |
| Immersion (T3) 10 | 63.3a | 90.8a | 82.5ab | 466a | 3056a | 1399b | 222a | 1696a | 585ab | 3.3a | 0a | 2.5b |
| Untreated (C1) 11 | 54.2a | 85.0a | 55.0c | 642a | 2383b | 702c | 245a | 624c | 301c | 18.3b | 10.0b | 5.0b |
| Negative (C2) 12 | 59.2a | 90.0a | 78.3ab | 561a | 1608c | 1587ab | 342a | 795c | 900a | 15.8b | 12.5b | 10.8a |
| Positive (C3) 13 | NT | 39.2b | 25.3d | NT | 324d | 313d | NT | 249d | 203c | NT | 13.3b | 1.8b |
| Treatment | Emergence 5 (%) | Soft Rot 6 (%) | Mass of Bulbs 7 (g) | Mass of Foliage 8 (g) |
|---|---|---|---|---|
| Means | ||||
| Spraying (T1) 1 | 93.3 ** | 3.3 * | 749 * | 807 * |
| Spraying (T2) 2 | 80.0 ** | 3.3 * | 541 * | 666 * |
| Immersion (T3) 3 | 30 | 3.3 * | 346 | 314 |
| Control (Untreated) 4 | 36.7 | 30 | 314 | 288 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaczek-Moczydłowska, M.A.; Young, G.K.; Trudgett, J.; Fleming, C.C.; Campbell, K.; O'Hanlon, R. Genomic Characterization, Formulation and Efficacy in Planta of a Siphoviridae and Podoviridae Protection Cocktail against the Bacterial Plant Pathogens Pectobacterium spp. Viruses 2020, 12, 150. https://doi.org/10.3390/v12020150
Zaczek-Moczydłowska MA, Young GK, Trudgett J, Fleming CC, Campbell K, O'Hanlon R. Genomic Characterization, Formulation and Efficacy in Planta of a Siphoviridae and Podoviridae Protection Cocktail against the Bacterial Plant Pathogens Pectobacterium spp. Viruses. 2020; 12(2):150. https://doi.org/10.3390/v12020150
Chicago/Turabian StyleZaczek-Moczydłowska, Maja A., Gillian K. Young, James Trudgett, Colin C. Fleming, Katrina Campbell, and Richard O'Hanlon. 2020. "Genomic Characterization, Formulation and Efficacy in Planta of a Siphoviridae and Podoviridae Protection Cocktail against the Bacterial Plant Pathogens Pectobacterium spp." Viruses 12, no. 2: 150. https://doi.org/10.3390/v12020150
APA StyleZaczek-Moczydłowska, M. A., Young, G. K., Trudgett, J., Fleming, C. C., Campbell, K., & O'Hanlon, R. (2020). Genomic Characterization, Formulation and Efficacy in Planta of a Siphoviridae and Podoviridae Protection Cocktail against the Bacterial Plant Pathogens Pectobacterium spp. Viruses, 12(2), 150. https://doi.org/10.3390/v12020150






