The Potentials and Pitfalls of a Human Cervical Organoid Model Including Langerhans Cells
Abstract
:1. Introduction
2. Materials and Methods
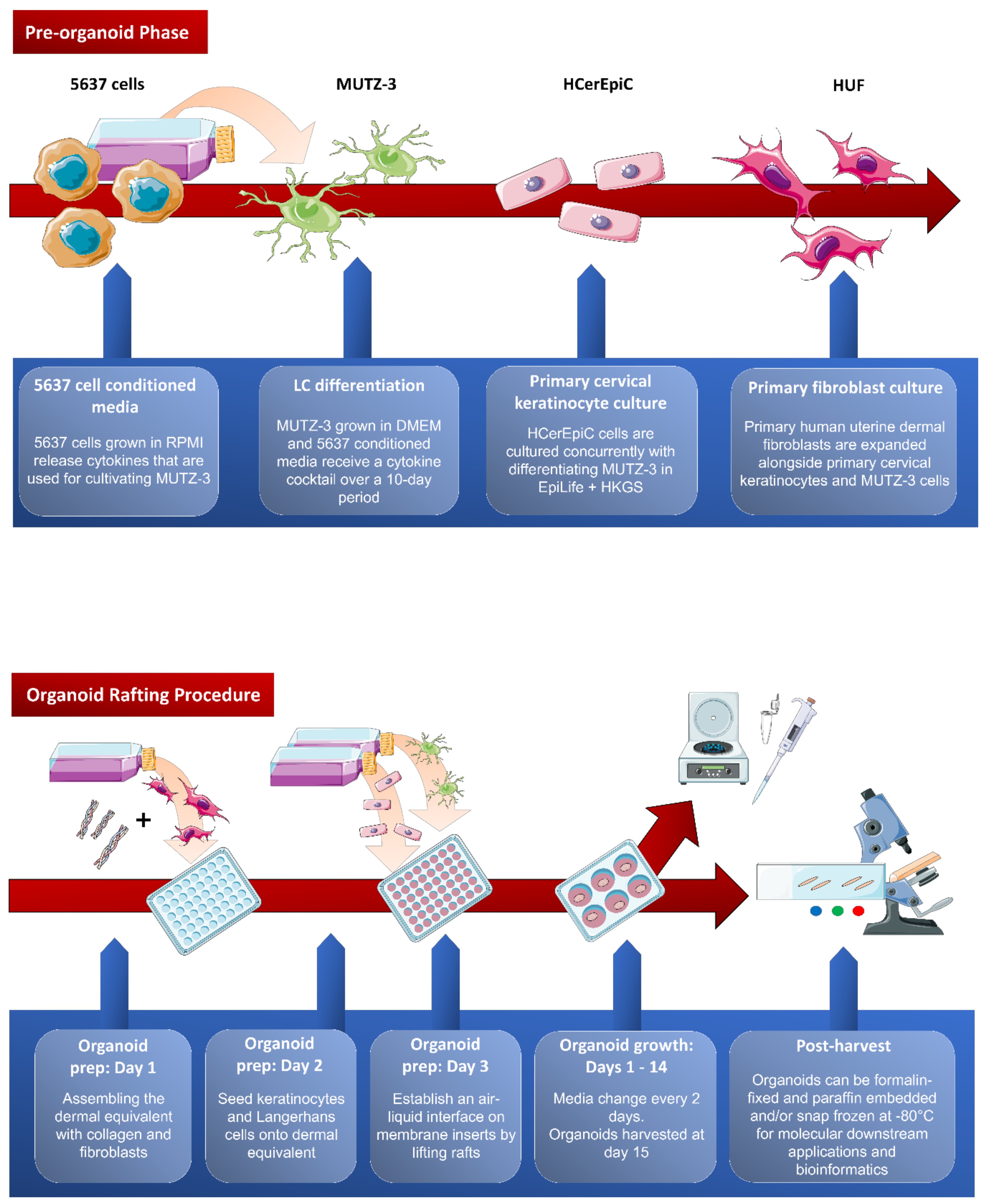
2.1. Pre-Organoid Phase: Culture Concomitantly Two Primary Cell Types and Two Cell Lines
2.2. Organoid Rafting Procedure
2.3. Organoid Harvest
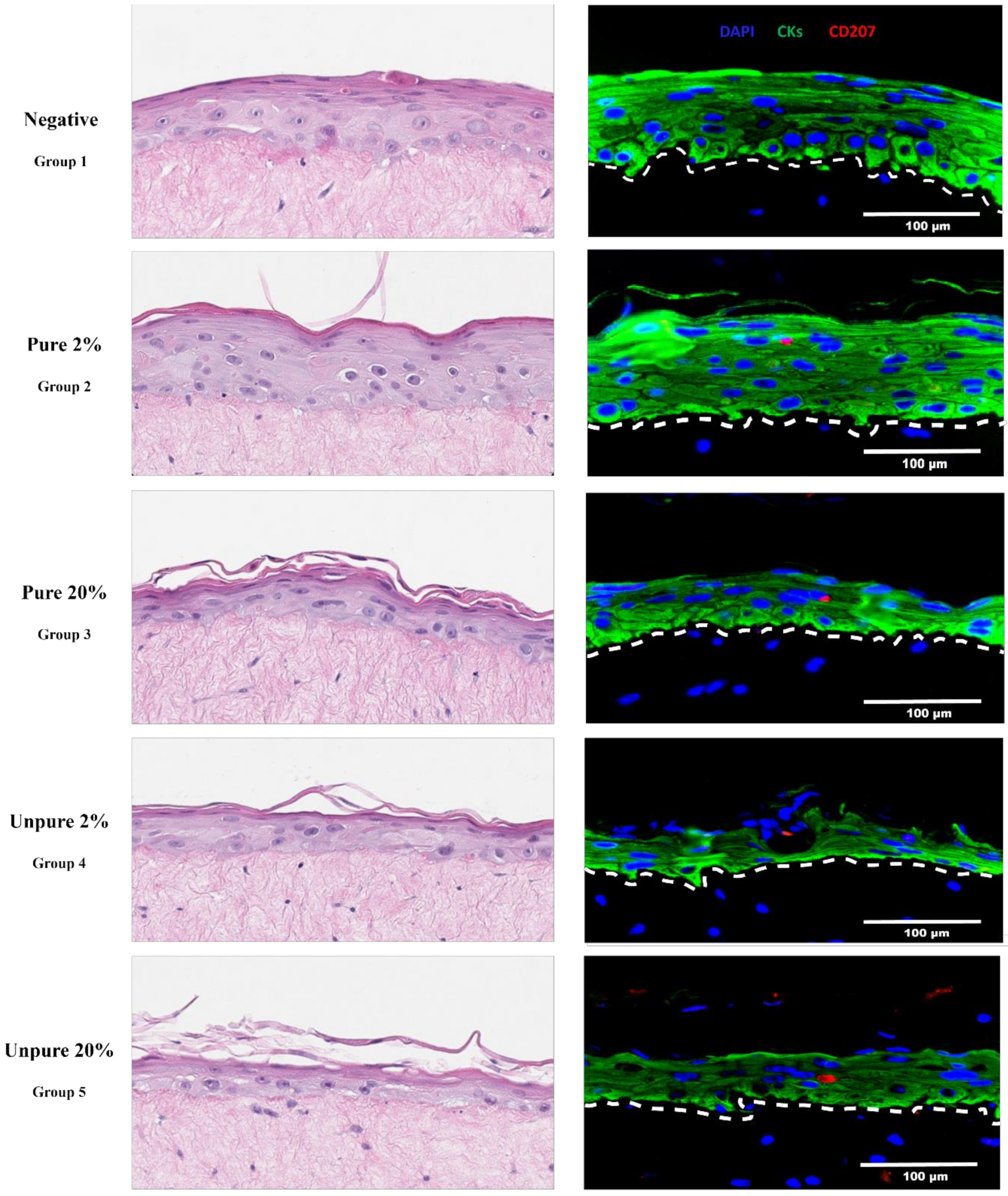
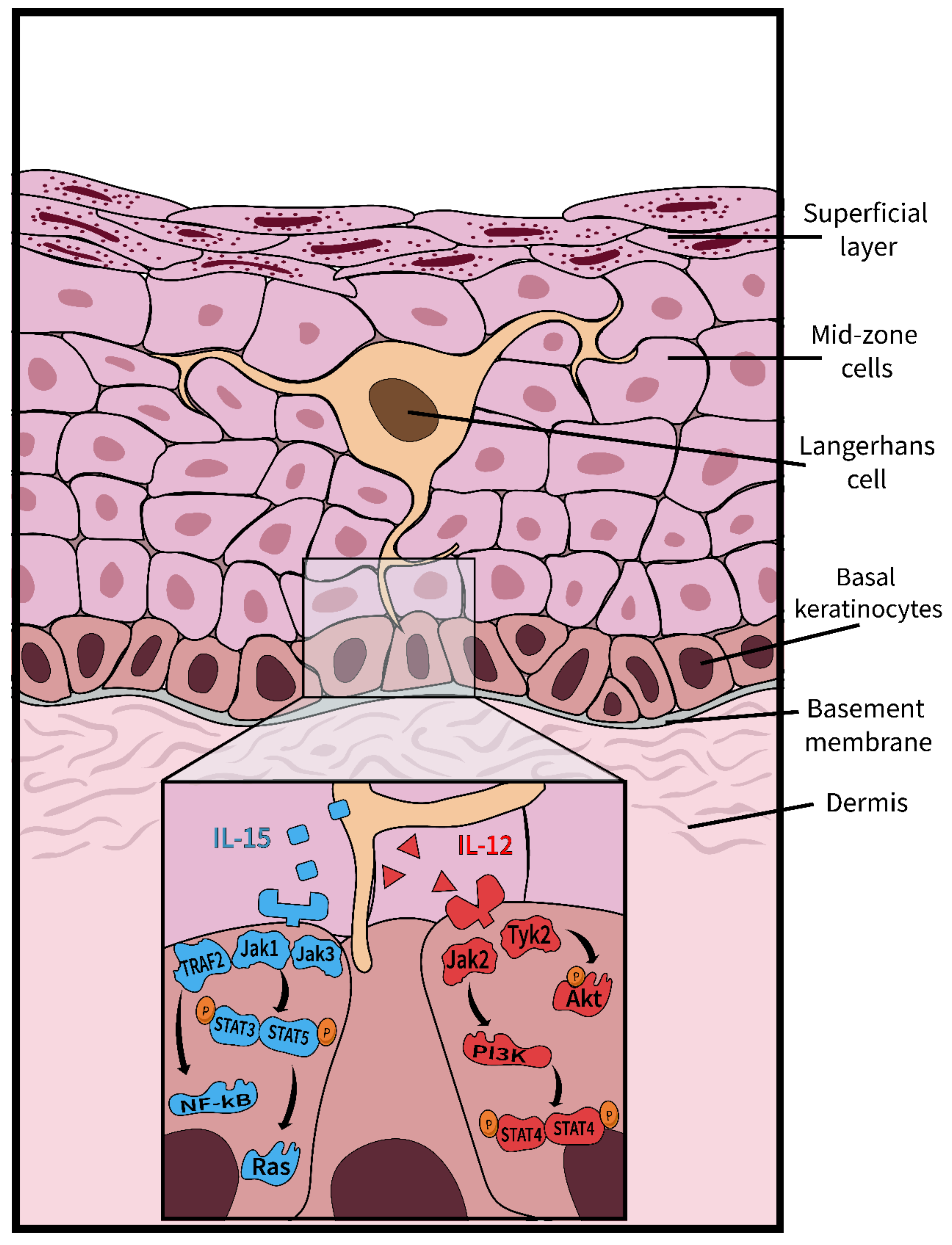
3. Results & Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Jackson, R.; Eade, S.; Zehbe, I. An epithelial organoid model with Langerhans cells for assessing virus-host interactions. Philos. T. R. Soc. B. 2019, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, N.; Moutschen, M.P.; Franzen-Detrooz, E.; Boniver, V.; Boniver, J.; Delvenne, P. Organotypic culture of HPV-transformed keratinocytes: A model for testing lymphocyte infiltration of (pre)neoplastic lesions of the uterine cervix. Virchows Arch. 1998, 432, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.; van den Brûle, F.; Giannini, S.L.; Franzen-Detrooz, E.; Boniver, J.; Delvenne, P. Colonization of in vitro-formed cervical human papillomavirus-associated (pre)neoplastic lesions with dendritic cells: Role of granulocyte/macrophage colonystimulating factor. Am. J. Pathol. 1999, 154, 775–784. [Google Scholar] [CrossRef]
- Masterson, A.J.; Sombroek, C.C.; de Gruijl, T.D.; Graus, Y.M.; van der Vliet, H.J.; Lougheed, S.M.; van den Eertwegh, A.J.; Pinedo, H.M.; Scheper, R.J. MUTZ-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from CD34+ precursors. Blood 2002, 100, 701–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosten, I.J.; Spiekstra, S.W.; de Gruijl, T.D.; Gibbs, S. MUTZ-3 derived Langerhans cells in human skin equivalents show differential migration and phenotypic plasticity after allergen or irritant exposure. Toxicol. Appl. Pharm. 2015, 287, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romani, N.; Holzmann, S.; Tripp, C.H.; Koch, F.; Stoitzner, P. Langerhans cells-dendritic cells of the epidermis. APMIS 2003, 111, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Bahmer, F.A.; Wörl, J.; Neuhuber, W.; Schuler, G.; Fartasch, M.A. Strikingly constant ratio exists between Langerhans cells and other epidermal cells in human skin. A stereologic study using the Optical Disector methods and the confocal laser scanning microscope. J. Investig. Dermatol. 2001, 116, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Togtema, M.; Lambert, P.F.; Zehbe, I. Tumourigenesis driven by the human papillomavirus type 16 Asian-American E6 variant in a three-dimensional keratinocyte model. PLoS ONE 2014, 9, e101540. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Rosa, B.A.; Lameiras, S.; Cuninghame, S.; Bernard, J.; Floriano, W.B.; Lambert, P.F.; Nicolas, A.; Zehbe, I. Functional variants of human papillomavirus type 16 demonstrate host genome integration and transcriptional alterations corresponding to their unique cancer epidemiology. BMC Genom. 2016, 17, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santegoets, S.J.A.M.; Masterson, A.J.; van der Sluis, P.C.; Lougheed, S.M.; Fluitsma, D.M.; van den Eertwegh, A.J.M.; Pinedo, H.M.; Scheper, R.J.; de Gruijl, T.D. A CD34+ human cell line model of myeloid dendritic cell differentiation: Evidence for a CD14+CD11b+ Langerhans cell precursor. J. Leukoc. Biol. 2006, 80, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.L.; Jackson, R.; Eade, S.; Escott, N.; Zehbe, I. Isolation of biopsy-derived, human cervical keratinocytes propagated as monolayer and organoid cultures. Sci. Rep. 2018, 8, 17869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, M.; Gantchev, J.; Ghazawi, F.M.; Litvinov, I.V. Protocol for adhesion and immunostaining of lymphocytes and other non-adherent cells in culture. Biotechniques 2018, 63, 230–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.M.; Griffiths, J.L.; Sanders, A.J.; Owen, S.; Ruge, F.; Harding, K.G.; Jiang, W.G. The clinical significance and impact of interleukin 15 on keratinocyte cell growth and migration. Int. J. Mol. Med. 2016, 38, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potten, C.S.; Allen, T.D. A model implicating the Langerhans cell in keratinocyte proliferation control. Differentiation 1976, 5, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Komine, M.; Fujimoto, M.; Okochi, H.; Tamaki, K. Interleukin 15 induces the signals of epidermal proliferation through ERK and PI 3-kinase in a human epidermal keratinocyte cell line, HaCaT. Biochem. Biophys. Res. Commun. 2003, 301, 841–847. [Google Scholar] [CrossRef]
- Watford, W.T.; Hissong, B.D.; Bream, J.H.; Kanno, Y.; Muul, L.; O’Shea, J.J. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol. Rev. 2004, 202, 139–156. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, R.; Lukacs, J.D.; Zehbe, I. The Potentials and Pitfalls of a Human Cervical Organoid Model Including Langerhans Cells. Viruses 2020, 12, 1375. https://doi.org/10.3390/v12121375
Jackson R, Lukacs JD, Zehbe I. The Potentials and Pitfalls of a Human Cervical Organoid Model Including Langerhans Cells. Viruses. 2020; 12(12):1375. https://doi.org/10.3390/v12121375
Chicago/Turabian StyleJackson, Robert, Jordan D. Lukacs, and Ingeborg Zehbe. 2020. "The Potentials and Pitfalls of a Human Cervical Organoid Model Including Langerhans Cells" Viruses 12, no. 12: 1375. https://doi.org/10.3390/v12121375





