Non-Coding RNAs and SARS-Related Coronaviruses
Abstract
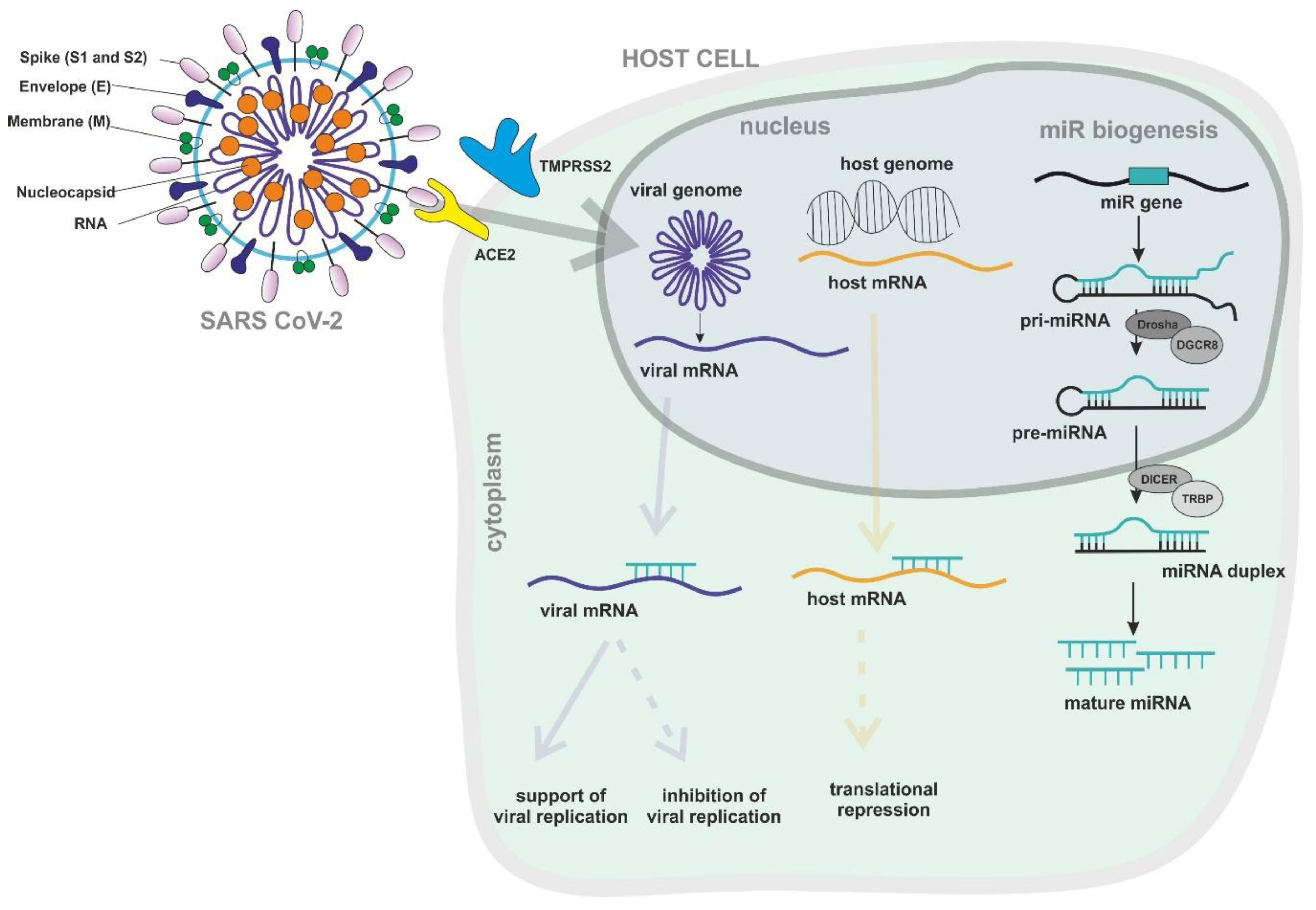
:1. Introduction
2. Methods
3. RNA Interference
3.1. Antiviral Interactions between Host miRNAs and Viral RNA
3.2. Evasion of miRNA-Mediated RNAi in the Untranslated Regions
4. Factors Enhancing Viral Pathogenicity
4.1. Mutations in the Viral Genome
4.2. Influence of the Host miRNA Expression on Viral Pathogenicity
4.2.1. Regulation of Receptor Expression
4.2.2. Other Ways miRNAs Influence Susceptibility to Virus Infection
4.3. Virus-Induced Alterations in the Transcriptome of the Host Cell
4.3.1. Virus-Induced Changes of miRNA Expression
4.3.2. The Role of lncRNA in the Cellular Response to Virus Infection
4.3.3. lncRNA Expression in SARS-CoV-2-Infected Cells
4.3.4. Expression of Viral miRNAs Mirroring Human miRNAs
5. Non-Coding RNAs in Therapeutic Approaches
5.1. RNA Interference Using Artificial siRNAs
5.2. Administration of siRNAs
5.3. Targeted Sequences in SARS-CoV
5.4. Attributes of Potential RNAi Targets in SARS-CoV-2
5.5. Viral Suppression Strategies of RNAi
5.6. miRNA-Related Approaches
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pontecorvi, G.; Bellenghi, M.; Ortona, E.; Care, A. microRNAs as New Possible Actors in Gender Disparities of Covid-19 Pandemic. Acta Physiol. (Oxf.) 2020, 230, e13538. [Google Scholar]
- Rabaan, A.A.; Al-Ahmed, S.H.; Haque, S.; Sah, R.; Tiwari, R.; Malik, Y.S.; Dhama, K.; Yatoo, M.I.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. SARS-CoV-2, SARS-CoV, and MERS-COV: A Comparative Overview. Infez Med. 2020, 28, 174–184. [Google Scholar] [PubMed]
- Mukherjee, M.; Goswami, S. Global Cataloguing of Variations in Untranslated Regions of Viral Genome and Prediction of Key Host RNA Binding Protein-microRNA Interactions Modulating Genome Stability in SARS-CoV-2. PLoS ONE 2020, 15, e0237559. [Google Scholar]
- Marra, M.A.; Jones, S.J.; Astell, C.R.; Holt, R.A.; Brooks-Wilson, A.; Butterfield, Y.S.; Khattra, J.; Asano, J.K.; Barber, S.A.; Chan, S.Y.; et al. The Genome Sequence of the SARS-Associated Coronavirus. Science 2003, 300, 1399–1404. [Google Scholar] [PubMed] [Green Version]
- Liu, D.X.; Fung, T.S.; Chong, K.K.; Shukla, A.; Hilgenfeld, R. Accessory Proteins of SARS-CoV and Other Coronaviruses. Antivir. Res. 2014, 109, 97–109. [Google Scholar] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell Entry Mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [PubMed]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pohlmann, S. TMPRSS2 and ADAM17 Cleave ACE2 Differentially and Only Proteolysis by TMPRSS2 Augments Entry Driven by the Severe Acute Respiratory Syndrome Coronavirus Spike Protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [Green Version]
- Coutard, B.; Valle, C.; de Lamballerie, X.; Canard, B.; Seidah, N.G.; Decroly, E. The Spike Glycoprotein of the New Coronavirus 2019-nCoV Contains a Furin-Like Cleavage Site Absent in CoV of the Same Clade. Antivir. Res. 2020, 176, 104742. [Google Scholar]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar]
- Arisan, E.D.; Dart, A.; Grant, G.H.; Arisan, S.; Cuhadaroglu, S.; Lange, S.; Uysal-Onganer, P. The Prediction of miRNAs in SARS-CoV-2 Genomes: Hsa-miR Databases Identify 7 Key miRs Linked to Host Responses and Virus Pathogenicity-Related KEGG Pathways Significant for Comorbidities. Viruses 2020, 12, 614. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Liu, G.; Luo, W.; Xia, N. COVID-19: Progress in Diagnostics, Therapy and Vaccination. Theranostics 2020, 10, 7821–7835. [Google Scholar] [PubMed]
- Cui, L.; Wang, H.; Ji, Y.; Yang, J.; Xu, S.; Huang, X.; Wang, Z.; Qin, L.; Tien, P.; Zhou, X.; et al. The Nucleocapsid Protein of Coronaviruses Acts as a Viral Suppressor of RNA Silencing in Mammalian Cells. J. Virol. 2015, 89, 9029–9043. [Google Scholar] [PubMed] [Green Version]
- Zhang, X.; Ma, X.; Jing, S.; Zhang, H.; Zhang, Y. Non-Coding RNAs and Retroviruses. Retrovirology 2018, 15, 1–14. [Google Scholar]
- Maillard, P.V.; Ciaudo, C.; Marchais, A.; Li, Y.; Jay, F.; Ding, S.W.; Voinnet, O. Antiviral RNA Interference in Mammalian Cells. Science 2013, 342, 235–238. [Google Scholar]
- Trobaugh, D.W.; Klimstra, W.B. MicroRNA Regulation of RNA Virus Replication and Pathogenesis. Trends Mol. Med. 2017, 23, 80–93. [Google Scholar]
- Agrawal, N.; Dasaradhi, P.V.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA Interference: Biology, Mechanism, and Applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar]
- Ghosh, Z.; Mallick, B.; Chakrabarti, J. Cellular Versus Viral microRNAs in Host-Virus Interaction. Nucleic Acids Res. 2009, 37, 1035–1048. [Google Scholar]
- Song, X.; Zhao, X.; Huang, Y.; Xiang, H.; Zhang, W.; Tong, D. Transmissible Gastroenteritis Virus (TGEV) Infection Alters the Expression of Cellular microRNA Species that Affect Transcription of TGEV Gene 7. Int. J. Biol. Sci. 2015, 11, 913–922. [Google Scholar]
- Gu, S.; Kay, M.A. How do miRNAs Mediate Translational Repression? Silence 2010, 1, 11. [Google Scholar]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target mRNAs are Repressed as Efficiently by microRNA-Binding Sites in the 5’ UTR as in the 3’ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Liu, H.; Gao, S.; Jiang, W.; Huang, W. Cellular microRNAs Inhibit Replication of the H1N1 Influenza A Virus in Infected Cells. J. Virol. 2010, 84, 8849–8860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Ke, X.; Wang, M.; He, S.; Li, Q.; Zheng, C.; Zhang, Z.; Liu, Y.; Wang, H. Human microRNA Hsa-miR-296-5p Suppresses Enterovirus 71 Replication by Targeting the Viral Genome. J. Virol. 2013, 87, 5645–5656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rad, A.H.; McLellan, A.D. Implications of SARS-CoV-2 Mutations for Genomic RNA Structure and Host microRNA Targeting. Int. J. Mol. Sci. 2020, 21, 4807. [Google Scholar] [CrossRef]
- Fulzele, S.; Sahay, B.; Yusufu, I.; Lee, T.J.; Sharma, A.; Kolhe, R.; Isales, C.M. COVID-19 Virulence in Aged Patients might be Impacted by the Host Cellular MicroRNAs Abundance/Profile. Aging Dis. 2020, 11, 509–522. [Google Scholar] [CrossRef]
- Pinzon, N.; Li, B.; Martinez, L.; Sergeeva, A.; Presumey, J.; Apparailly, F.; Seitz, H. microRNA Target Prediction Programs Predict Many False Positives. Genome Res. 2017, 27, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, X. Prediction of Functional microRNA Targets by Integrative Modeling of microRNA Binding and Target Expression Data. Genome Biol. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Mockly, S.; Seitz, H. Inconsistencies and Limitations of Current MicroRNA Target Identification Methods. Methods Mol. Biol. 2019, 1970, 291–314. [Google Scholar]
- Ritchie, W. microRNA Target Prediction. Methods Mol. Biol. 2017, 1513, 193–200. [Google Scholar]
- Williams, G.D.; Chang, R.Y.; Brian, D.A. A Phylogenetically Conserved Hairpin-Type 3’ Untranslated Region Pseudoknot Functions in Coronavirus RNA Replication. J. Virol. 1999, 73, 8349–8355. [Google Scholar] [CrossRef] [Green Version]
- Yin, C. Genotyping Coronavirus SARS-CoV-2: Methods and Implications. Genomics 2020, 112, 3588–3596. [Google Scholar] [CrossRef] [PubMed]
- Nersisyan, S.; Shkurnikov, M.; Turchinovich, A.; Knyazev, E.; Tonevitsky, A. Integrative Analysis of miRNA and mRNA Sequencing Data Reveals Potential Regulatory Mechanisms of ACE2 and TMPRSS2. PLoS ONE 2020, 15, e0235987. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Das, P.M.; Huynh, F.C.; Jones, F.E. Jumonji/ARID1 B (JARID1B) Protein Promotes Breast Tumor Cell Cycle Progression through Epigenetic Repression of microRNA Let-7e. J. Biol. Chem. 2011, 286, 40531–40535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enkhbaatar, Z.; Terashima, M.; Oktyabri, D.; Tange, S.; Ishimura, A.; Yano, S.; Suzuki, T. KDM5B Histone Demethylase Controls Epithelial-Mesenchymal Transition of Cancer Cells by Regulating the Expression of the microRNA-200 Family. Cell. Cycle 2013, 12, 2100–2112. [Google Scholar] [CrossRef] [PubMed]
- Howard, E.W.; Yang, X. microRNA Regulation in Estrogen Receptor-Positive Breast Cancer and Endocrine Therapy. Biol. Proced. Online 2018, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Segal, C.V.; Koufaris, C.; Powell, C.; Gooderham, N.J. Effects of Treatment with Androgen Receptor Ligands on microRNA Expression of Prostate Cancer Cells. Toxicology 2015, 333, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients with COVID-19: Focus on Severity and Mortality. Front. Public. Health 2020, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Bhat-Nakshatri, P.; Wang, G.; Collins, N.R.; Thomson, M.J.; Geistlinger, T.R.; Carroll, J.S.; Brown, M.; Hammond, S.; Srour, E.F.; Liu, Y.; et al. Estradiol-Regulated microRNAs Control Estradiol Response in Breast Cancer Cells. Nucleic Acids Res. 2009, 37, 4850–4861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Shen, A.; Liu, A. Long Non-Coding RNA H19 and Cancer: A Competing Endogenous RNA. Bull. Cancer 2019, 106, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Momi, N.; Kaur, S.; Rachagani, S.; Ganti, A.K.; Batra, S.K. Smoking and microRNA Dysregulation: A Cancerous Combination. Trends Mol. Med. 2014, 20, 36–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.; Gao, L.; Yang, Y.; Tong, D.; Guo, B.; Liu, L.; Li, Z.; Song, T.; Huang, C. miR-145 Mediates the Antiproliferative and Gene Regulatory Effects of Vitamin D3 by Directly Targeting E2F3 in Gastric Cancer Cells. Oncotarget 2015, 6, 7675–7685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daneshkhah, A.; Agrawal, V.; Eshein, A.; Subramanian, H.; Roy, H.K.; Backman, V. The Possible Role of Vitamin D in Suppressing Cytokine Storm and Associated Mortality in COVID-19 Patients. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Usul Afsar, C. 2019-nCoV-SARS-CoV-2 (COVID-19) Infection: Cruciality of Furin and Relevance with Cancer. Med. Hypotheses 2020, 140, 109770. [Google Scholar] [CrossRef] [PubMed]
- Mitash, N.; Donovan, J.E.; Swiatecka-Urban, A. The Role of MicroRNA in the Airway Surface Liquid Homeostasis. Int. J. Mol. Sci. 2020, 21, 3848. [Google Scholar] [CrossRef]
- Berkebile, A.R.; Bartlett, J.A.; Abou Alaiwa, M.; Varga, S.M.; Power, U.F.; McCray, P.B., Jr. Airway Surface Liquid has Innate Antiviral Activity that is Reduced in Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2020, 62, 104–111. [Google Scholar] [CrossRef]
- Peckham, D.; McDermott, M.F.; Savic, S.; Mehta, A. COVID-19 Meets Cystic Fibrosis: For Better or Worse? Genes Immun. 2020, 21, 260–262. [Google Scholar] [CrossRef]
- Karjee, S.; Mukherjee, S.K. RNAi Suppressor: The Hidden Weapon of SARS-CoV. J. Biosci. 2020, 45, 1–6. [Google Scholar] [CrossRef]
- Mallick, B.; Ghosh, Z.; Chakrabarti, J. MicroRNome Analysis Unravels the Molecular Basis of SARS Infection in Bronchoalveolar Stem Cells. PLoS ONE 2009, 4, e7837. [Google Scholar] [CrossRef]
- Bertrams, W.; Griss, K.; Han, M.; Seidel, K.; Klemmer, A.; Sittka-Stark, A.; Hippenstiel, S.; Suttorp, N.; Finkernagel, F.; Wilhelm, J.; et al. Transcriptional Analysis Identifies Potential Biomarkers and Molecular Regulators in Pneumonia and COPD Exacerbation. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Guterres, A.; de Azeredo Lima, C.H.; Miranda, R.L.; Gadelha, M.R. What is the Potential Function of microRNAs as Biomarkers and Therapeutic Targets in COVID-19? Infect. Genet. Evol. 2020, 85, 104417. [Google Scholar] [CrossRef]
- Peng, X.; Gralinski, L.; Armour, C.D.; Ferris, M.T.; Thomas, M.J.; Proll, S.; Bradel-Tretheway, B.G.; Korth, M.J.; Castle, J.C.; Biery, M.C.; et al. Unique Signatures of Long Noncoding RNA Expression in Response to Virus Infection and Altered Innate Immune Signaling. mBio 2010, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josset, L.; Tchitchek, N.; Gralinski, L.E.; Ferris, M.T.; Eisfeld, A.J.; Green, R.R.; Thomas, M.J.; Tisoncik-Go, J.; Schroth, G.P.; Kawaoka, Y.; et al. Annotation of Long Non-Coding RNAs Expressed in Collaborative Cross Founder Mice in Response to Respiratory Virus Infection Reveals a New Class of Interferon-Stimulated Transcripts. RNA Biol. 2014, 11, 875–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishnubalaji, R.; Shaath, H.; Alajez, N.M. Protein Coding and Long Noncoding RNA (lncRNA) Transcriptional Landscape in SARS-CoV-2 Infected Bronchial Epithelial Cells Highlight a Role for Interferon and Inflammatory Response. Genes 2020, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, J.; Han, Z.; Chen, Z.; Zhang, Q. Silencing of lncRNA MALAT1 Prevents Inflammatory Injury After Lung Transplant Ischemia-Reperfusion by Downregulation of IL-8 Via p300. Mol. Ther. Nucleic Acids 2019, 18, 285–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, K.J.; Blobe, G.C. Role of Transforming Growth Factor-Beta Superfamily Signaling Pathways in Human Disease. Biochim. Biophys. Acta 2008, 1782, 197–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voss, D.; Pfefferle, S.; Drosten, C.; Stevermann, L.; Traggiai, E.; Lanzavecchia, A.; Becker, S. Studies on Membrane Topology, N-Glycosylation and Functionality of SARS-CoV Membrane Protein. Virol. J. 2009, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta, L.; Yousefi, B.; Damania, B.; Khan, A.A. Endosomal TLR-8 Senses microRNA-1294 Resulting in the Production of NFkB Dependent Cytokines. Front. Immunol. 2019, 10, 2860. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef]
- Schubert, S.; Kurreck, J. Oligonucleotide-Based Antiviral Strategies. Handb. Exp. Pharmacol. 2006, 173, 261–287. [Google Scholar]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-Nucleotide RNAs Mediate RNA Interference in Cultured Mammalian Cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Semizarov, D.; Frost, L.; Sarthy, A.; Kroeger, P.; Halbert, D.N.; Fesik, S.W. Specificity of Short Interfering RNA Determined through Gene Expression Signatures. Proc. Natl. Acad. Sci. USA 2003, 100, 6347–6352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doench, J.G.; Sharp, P.A. Specificity of microRNA Target Selection in Translational Repression. Genes Dev. 2004, 18, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Li, B.; Woodle, M.; Lu, P.Y. Application of siRNA Against SARS in the Rhesus Macaque Model. Methods Mol. Biol. 2008, 442, 139–158. [Google Scholar] [PubMed]
- Hu, B.; Weng, Y.; Xia, X.H.; Liang, X.J.; Huang, Y. Clinical Advances of siRNA Therapeutics. J. Gene Med. 2019, 21, e3097. [Google Scholar] [CrossRef]
- Haussecker, D. RNAi Arrives at the Bedside After a Mere Two Decades. Mol. Ther. 2018, 26, 2533–2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wooddell, C.I.; Yuen, M.F.; Chan, H.L.; Gish, R.G.; Locarnini, S.A.; Chavez, D.; Ferrari, C.; Given, B.D.; Hamilton, J.; Kanner, S.B.; et al. RNAi-Based Treatment of Chronically Infected Patients and Chimpanzees Reveals that Integrated Hepatitis B Virus DNA is a Source of HBsAg. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Chang, Z.; Babiuk, L.A.; Hu, J. Therapeutic and Prophylactic Potential of Small Interfering RNAs Against Severe Acute Respiratory Syndrome: Progress to Date. BioDrugs 2007, 21, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.L.; Wang, Y.; Guo, R.; Yang, F.; Zhang, Y.; Wang, S.H.; Liu, L. Identification and Characterization of Three Novel Small Interference RNAs that Effectively Down-Regulate the Isolated Nucleocapsid Gene Expression of SARS Coronavirus. Molecules 2011, 16, 1544–1558. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cao, Y.L.; Yang, F.; Zhang, Y.; Wang, S.H.; Liu, L. Small Interfering RNA Effectively Inhibits the Expression of SARS Coronavirus Membrane Gene at Two Novel Targeting Sites. Molecules 2010, 15, 7197–7207. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.L.; Zhao, P.; Cao, M.M.; Qi, Z.T. siRNAs Targeting Terminal Sequences of the SARS-Associated Coronavirus Membrane Gene Inhibit M Protein Expression through Degradation of M mRNA. J. Virol. Methods 2007, 145, 146–154. [Google Scholar] [CrossRef]
- Li, F.; Berardi, M.; Li, W.; Farzan, M.; Dormitzer, P.R.; Harrison, S.C. Conformational States of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein Ectodomain. J. Virol. 2006, 80, 6794–6800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.Y.; Kong, W.P.; Huang, Y.; Roberts, A.; Murphy, B.R.; Subbarao, K.; Nabel, G.J. A DNA Vaccine Induces SARS Coronavirus Neutralization and Protective Immunity in Mice. Nature 2004, 428, 561–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, T.; Fu, L.; Yu, C.; Li, Y.; Xu, X.; Wang, Y.; Ning, H.; Zhang, S.; Chen, W.; et al. Silencing SARS-CoV Spike Protein Expression in Cultured Cells by RNA Interference. FEBS Lett. 2004, 560, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.L.; Zhao, P.; Zhang, X.L.; Yu, J.G.; Cao, M.M.; Zhao, L.J.; Luan, J.; Qi, Z.T. Silencing of SARS-CoV Spike Gene by Small Interfering RNA in HEK 293T Cells. Biochem. Biophys. Res. Commun. 2004, 324, 1186–1193. [Google Scholar] [CrossRef]
- Li, B.J.; Tang, Q.; Cheng, D.; Qin, C.; Xie, F.Y.; Wei, Q.; Xu, J.; Liu, Y.; Zheng, B.J.; Woodle, M.C.; et al. Using siRNA in Prophylactic and Therapeutic Regimens Against SARS Coronavirus in Rhesus Macaque. Nat. Med. 2005, 11, 944–951. [Google Scholar] [CrossRef]
- Wu, C.J.; Huang, H.W.; Liu, C.Y.; Hong, C.F.; Chan, Y.L. Inhibition of SARS-CoV Replication by siRNA. Antivir. Res. 2005, 65, 45–48. [Google Scholar] [CrossRef]
- Meng, B.; Lui, Y.W.; Meng, S.; Cao, C.; Hu, Y. Identification of Effective siRNA Blocking the Expression of SARS Viral Envelope E and RDRP Genes. Mol. Biotechnol. 2006, 33, 141–148. [Google Scholar] [CrossRef]
- He, M.L.; Zheng, B.J.; Chen, Y.; Wong, K.L.; Huang, J.D.; Lin, M.C.; Peng, Y.; Yuen, K.Y.; Sung, J.J.; Kung, H.F. Kinetics and Synergistic Effects of siRNAs Targeting Structural and Replicase Genes of SARS-Associated Coronavirus. FEBS Lett. 2006, 580, 2414–2420. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Yang, D.H.; Xiong, J.; Jia, J.; Huang, B.; Jin, Y.X. Inhibition of Genes Expression of SARS Coronavirus by Synthetic Small Interfering RNAs. Cell Res. 2005, 15, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Zhang, Y.; Fu, L.; Yu, C.; Li, X.; Li, Y.; Zhang, X.; Rong, Z.; Wang, Y.; Ning, H.; et al. siRNA Targeting the Leader Sequence of SARS-CoV Inhibits Virus Replication. Gene Ther. 2005, 12, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Akerstrom, S.; Mirazimi, A.; Tan, Y.J. Inhibition of SARS-CoV Replication Cycle by Small Interference RNAs Silencing Specific SARS Proteins, 7a/7b, 3a/3b and S. Antiviral Res. 2007, 73, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Elmen, J.; Thonberg, H.; Ljungberg, K.; Frieden, M.; Westergaard, M.; Xu, Y.; Wahren, B.; Liang, Z.; Orum, H.; Koch, T.; et al. Locked Nucleic Acid (LNA) Mediated Improvements in siRNA Stability and Functionality. Nucleic Acids Res. 2005, 33, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Torrecilla, J.; Rodriguez-Gascon, A.; Solinis, M.A.; del Pozo-Rodriguez, A. Lipid Nanoparticles as Carriers for RNAi Against Viral Infections: Current Status and Future Perspectives. Biomed. Res. Int. 2014, 2014, 161794. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery Materials for siRNA Therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; Tobaiqy, M.; Al Faraj, A. Optimizing use of Theranostic Nanoparticles as a Life-Saving Strategy for Treating COVID-19 Patients. Theranostics 2020, 10, 5932–5942. [Google Scholar] [CrossRef] [PubMed]
- Law, S.L.; Huang, K.J.; Chou, V.H.; Cherng, J.Y. Enhancement of Nasal Absorption of Calcitonin Loaded in Liposomes. J. Liposome Res. 2001, 11, 165–174. [Google Scholar] [CrossRef]
- Tan, S.; Wu, T.; Zhang, D.; Zhang, Z. Cell Or Cell Membrane-Based Drug Delivery Systems. Theranostics 2015, 5, 863–881. [Google Scholar] [CrossRef] [Green Version]
- Saw, P.E.; Song, E.W. siRNA Therapeutics: A Clinical Reality. Sci. China Life. Sci. 2020, 63, 485–500. [Google Scholar] [CrossRef]
- Dong, Y.; Siegwart, D.J.; Anderson, D.G. Strategies, Design, and Chemistry in siRNA Delivery Systems. Adv. Drug Deliv. Rev. 2019, 144, 133–147. [Google Scholar] [CrossRef]
- Jiang, Y.; Huo, S.; Hardie, J.; Liang, X.J.; Rotello, V.M. Progress and Perspective of Inorganic Nanoparticle-Based siRNA Delivery Systems. Expert Opin. Drug Deliv. 2016, 13, 547–559. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Ping, Y.H.; Lee, H.C.; Chen, K.H.; Lee, Y.M.; Chan, Y.J.; Lien, T.C.; Jap, T.S.; Lin, C.H.; Kao, L.S.; et al. Open Reading Frame 8a of the Human Severe Acute Respiratory Syndrome Coronavirus Not Only Promotes Viral Replication but also Induces Apoptosis. J. Infect. Dis. 2007, 196, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zheng, B.J.; Xu, K.; Schwarz, W.; Du, L.; Wong, C.K.; Chen, J.; Duan, S.; Deubel, V.; Sun, B. Severe Acute Respiratory Syndrome-Associated Coronavirus 3a Protein Forms an Ion Channel and Modulates Virus Release. Proc. Natl. Acad. Sci. USA 2006, 103, 12540–12545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Feng, P.; Liu, K.; Wu, M.; Lin, H. Computational Identification of Small Interfering RNA Targets in SARS-CoV-2. Virol. Sin. 2020, 35, 359–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.Y.; Huang, H.Y.; Yang, T.H.; Chang, L.Y.; Lee, C.Y.; Huang, L.M. siRNA Silencing of Angiotensin-Converting Enzyme 2 Reduced Severe Acute Respiratory Syndrome-Associated Coronavirus Replications in Vero E6 Cells. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Karjee, S.; Minhas, A.; Sood, V.; Ponia, S.S.; Banerjea, A.C.; Chow, V.T.; Mukherjee, S.K.; Lal, S.K. The 7a Accessory Protein of Severe Acute Respiratory Syndrome Coronavirus Acts as an RNA Silencing Suppressor. J. Virol. 2010, 84, 10395–10401. [Google Scholar] [CrossRef] [Green Version]
- Stalder, L.; Heusermann, W.; Sokol, L.; Trojer, D.; Wirz, J.; Hean, J.; Fritzsche, A.; Aeschimann, F.; Pfanzagl, V.; Basselet, P.; et al. The Rough Endoplasmatic Reticulum is a Central Nucleation Site of siRNA-Mediated RNA Silencing. EMBO J. 2013, 32, 1115–1127. [Google Scholar] [CrossRef] [Green Version]
- Ivashchenko, A.; Rakhmetullina, A.; Aisina, D. How miRNAs can Protect Humans from Coronaviruses COVID-19, SARS-CoV, and MERS-CoV. Res. Sq. 2020. [Google Scholar] [CrossRef] [Green Version]
- Kreis, N.N.; Ritter, A.; Louwen, F.; Yuan, J. A Message from the Human Placenta: Structural and Immunomodulatory Defense Against SARS-CoV-2. Cells 2020, 9, 1777. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Wang, X.; Rai, A.; Groot, M.; Jin, Y. Exosome-Mediated Small RNA Delivery: A Novel Therapeutic Approach for Inflammatory Lung Responses. Mol. Ther. 2018, 26, 2119–2130. [Google Scholar] [CrossRef] [Green Version]
- Chow, J.T.; Salmena, L. Prediction and Analysis of SARS-CoV-2-Targeting MicroRNA in Human Lung Epithelium. Genes 2020, 11, 1002. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henzinger, H.; Barth, D.A.; Klec, C.; Pichler, M. Non-Coding RNAs and SARS-Related Coronaviruses. Viruses 2020, 12, 1374. https://doi.org/10.3390/v12121374
Henzinger H, Barth DA, Klec C, Pichler M. Non-Coding RNAs and SARS-Related Coronaviruses. Viruses. 2020; 12(12):1374. https://doi.org/10.3390/v12121374
Chicago/Turabian StyleHenzinger, Hanna, Dominik A. Barth, Christiane Klec, and Martin Pichler. 2020. "Non-Coding RNAs and SARS-Related Coronaviruses" Viruses 12, no. 12: 1374. https://doi.org/10.3390/v12121374
APA StyleHenzinger, H., Barth, D. A., Klec, C., & Pichler, M. (2020). Non-Coding RNAs and SARS-Related Coronaviruses. Viruses, 12(12), 1374. https://doi.org/10.3390/v12121374





