A Survey of Mycoviral Infection in Fusarium spp. Isolated from Maize and Sorghum in Argentina Identifies the First Mycovirus from Fusarium verticillioides
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
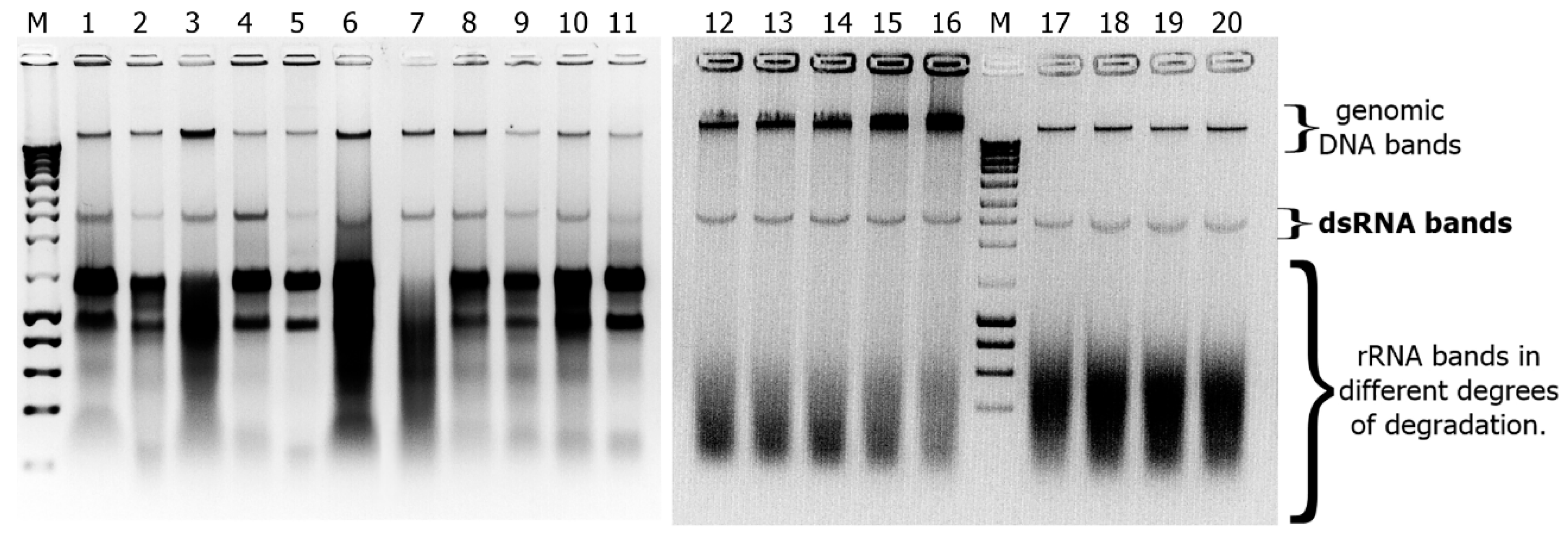
2.2. Detection of dsRNAs in Fusarium Isolates
2.3. Next Generation Sequencing of dsRNAs and Data Analysis
2.4. Phylogenetic Analyses
2.5. Analysis of Fungal Vegetative Growth and Mycovirus Transmission to Conidia
2.6. Fumonisins B Production
2.7. Phytopathogenicity Assay
2.8. Statistical Analyses
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hollings, M. Viruses associated with a die-back disease of cultivated mushroom. Nature 1962, 196, 962. [Google Scholar] [CrossRef]
- Hollings, M. Mycoviruses: Viruses that infect fungi. In Advances in Virus Research; Lauffer, M., Bang, F., Maramorosch, F., Smith, K., Eds.; Academic Press: Cambridge, MA, USA, 1978; Volume 22, pp. 1–53. [Google Scholar]
- Son, M.; Yu, J.; Kim, K.H. Five questions about mycoviruses. PLoS Pathog. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Ghabrial, S.A.; Castón, J.R.; Jiang, D.; Nibert, M.L.; Suzuki, N. 50-plus years of fungal viruses. Virology 2015, 479, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, K.B.; Holcomb, E.E.; Allscheid, R.L.; Carrington, J.C. Hiding in plain sight: New virus genomes discovered via a systematic analysis of fungal public transcriptomes. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Sato, Y.; Castón, J.R.; Suzuki, N. The biological attributes, genome architecture and packaging of diverse multi-component fungal viruses. Curr. Opin. Virol. 2018, 33, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Luque, D.; Mata, C.P.; Suzuki, N.; Ghabrial, S.A.; Castón, J.R. Capsid structure of dsRNA fungal viruses. Viruses 2018, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Krupovic, M.; Ghabrial, S.A.; Jiang, D.; Varsani, A. Genomoviridae: A new family of widespread single-stranded DNA viruses. Arch. Virol. 2016, 161, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, S.; Chiba, S.; Kondo, H.; Kanematsu, S.; Suzuki, N. A novel single-stranded RNA virus isolated from a phytopathogenic filamentous fungus, Rosellinia necatrix, with similarity to hypo-like viruses. Front. Microbiol. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Liu, L.; Xie, J.; Cheng, J.; Fu, Y.; Li, G.; Yi, X.; Jiang, D. Fungal negative-stranded RNA virus that is related to bornaviruses and nyaviruses. Proc. Natl. Acad. Sci. USA 2014, 111, 12205–12210. [Google Scholar] [CrossRef]
- Kanhayuwa, L.; Kotta-Loizou, I.; Özkan, S.; Gunning, A.P.; Coutts, R.H. A novel mycovirus from Aspergillus fumigatus contains four unique dsRNAs as its genome and is infectious as dsRNA. Proc. Natl. Acad. Sci. USA 2015, 112, 9100–9105. [Google Scholar] [CrossRef]
- Kotta-Loizou, I.; Coutts, R.H. Studies on the virome of the entomopathogenic fungus Beauveria bassiana reveal novel dsRNA elements and mild hypervirulence. PLoS Pathog. 2017, 13, e1006183. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, X.; Li, P.; Qiu, D.; Guo, L. Complete genome sequence of a Fusarium graminearum double-stranded RNA virus in a newly proposed family, Alternaviridae. Genome Announc. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Hisano, S.; Zhang, R.; Faruk, M.I.; Kondo, H.; Suzuki, N. A neo-virus lifestyle exhibited by a (+) ssRNA virus hosted in an unrelated dsRNA virus: Taxonomic and evolutionary considerations. Virus Res. 2018, 244, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Nuss, D.L. Hypovirulence: Mycoviruses at the fungal–plant interface. Nat. Rev. Microbiol. 2005, 3, 632. [Google Scholar] [CrossRef] [PubMed]
- Ghabrial, S.A.; Suzuki, N. Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 2009, 47, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.N.; Beever, R.E.; Boine, B.; Arthur, K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 2009, 10, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Picarelli, M.; Gobatto, D.; Patrício, F.; Rivas, E.B.; Colariccio, A. Vírus que infectam fungos fitopatogênicos. Arq. Inst. Biológico 2017, 84. [Google Scholar] [CrossRef][Green Version]
- García-Pedrajas, M.D.; Cañizares, M.C.; Sarmiento-Villamil, J.L.; Jacquat, A.G.; Dambolena, J.S. Mycoviruses in biological control: From basic research to field implementation. Phytopathology 2019, 109, 1828–1839. [Google Scholar] [CrossRef]
- Yaegashi, H.; Sawahata, T.; Ito, T.; Kanematsu, S. A novel colony-print immunoassay reveals differential patterns of distribution and horizontal transmission of four unrelated mycoviruses in Rosellinia necatrix. Virology 2011, 409, 280–289. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 2014, 52, 45–68. [Google Scholar] [CrossRef]
- Fulbright, D.W. Effect of eliminating dsRNA in hypovirulent Endothia parasitica. Phytopathology 1984, 74, 722–724. [Google Scholar] [CrossRef]
- Choi, G.H.; Nuss, D.L. A viral gene confers hypovirulence-associated traits to the chestnut blight fungus. EMBO J. 1992, 11, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakis, S.L. Biological control of chestnut blight. Science 1982, 215, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Heiniger, U.; Rigling, D. Biological control of chestnut blight in Europe. Annu. Rev. Phytopathol. 1994, 32, 581–599. [Google Scholar] [CrossRef]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef]
- Jian, J.; Lakshman, D.K.; Tavantzis, S.M. A virulence-associated, 6.4-kb, double-stranded RNA from Rhizoctonia solani is phylogenetically related to plant bromoviruses and electron transport enzymes. Mol. Plant-Microbe Interact. 1998, 11, 601–609. [Google Scholar] [CrossRef]
- Okada, R.; Ichinose, S.; Takeshita, K.; Urayama, S.-I.; Fukuhara, T.; Komatsu, K.; Arie, T.; Ishihara, A.; Egusa, M.; Kodama, M.; et al. Molecular characterization of a novel mycovirus in Alternaria alternata manifesting two-sided effects: Down-regulation of host growth and up-regulation of host plant pathogenicity. Virology 2018, 519, 23–32. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Lo, G.C.S.; Chow, W.-N.; Fan, R.Y.Y.; Cai, J.J.; Yuen, K.-Y.; Woo, P.C.Y. Novel partitivirus enhances virulence of and causes aberrant gene expression in Talaromyces marneffei. mBio 2018, 9. [Google Scholar] [CrossRef]
- Rep, M.; Kistler, H.C. The genomic organization of plant pathogenicity in Fusarium species. Curr. Opin. Plant Biol. 2010, 13, 420–426. [Google Scholar] [CrossRef]
- Ma, L.-J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef]
- Herron, D.A.; Wingfield, M.J.; Wingfield, B.D.; Rodas, C.A.; Marincowitz, S.; Steenkamp, E.T. Novel taxa in the Fusarium fujikuroi species complex from Pinus spp. Stud. Mycol. 2015, 80, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C. Fusarium wilt of banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Nganje, W.E.; Bangsund, D.A.; Leistritz, F.L.; Wilson, W.W.; Tiapo, N.M. Regional economic impacts of Fusarium head blight in wheat and barley. Rev. Agric. Econ. 2004, 26, 332–347. [Google Scholar] [CrossRef]
- Wu, M.D.; Zhang, L.; Li, G.Q.; Jiang, D.H.; Hou, M.S.; Huang, H.C. Hypovirulence and double-stranded RNA in Botrytis cinerea. Phytopathology 2007, 97, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.E.; Proctor, R.H. Molecular biology of Fusarium mycotoxins. Int. J. Food Microbiol. 2007, 119, 47–50. [Google Scholar] [CrossRef]
- Nesic, K.; Ivanovic, S.; Nesic, V. Fusarial toxins: Secondary metabolites of Fusarium fungi. In Reviews of Environmental Contamination and Toxicology; Whitacre, D., Ed.; Springer: New York, NY, USA, 2014; pp. 101–120. [Google Scholar]
- Carmona, M.; Sautua, F. La problemática de la resistencia de hongos a fungicidas. Causas y efectos en cultivos extensivos. Agron. Ambiente Rev. Fac. Agron. UBA 2017, 37, 1–19. [Google Scholar]
- Carmona, M.; Sautua, F.; Pérez-Hérnandez, O.; Reis, E.M. Role of Fungicide Applications on the Integrated Management of Wheat Stripe Rust. Front. Plant Sci. 2020, 11, 733. [Google Scholar] [CrossRef]
- Mazzoni, E.; Scandolara, A.; Giorni, P.; Pietri, A.; Battilani, P. Field control of Fusarium ear rot, Ostrinia nubilalis (Hübner), and fumonisins in maize kernels. Pest Manag. Sci. 2011, 67, 458–465. [Google Scholar] [CrossRef]
- Reis, E.M.; Carmona, M.A. Integrated disease management of Fusarium head blight. In Fusarium Head Blight in Latin America; Alconada Magliano, T.M., Chulze, S.N., Eds.; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2013; pp. 159–173. [Google Scholar]
- Crini, G.; Saintemarie, A.E.; Rocchi, S.; Fourmentin, M.; Jeanvoine, A.; Millon, L.; Morin-Crini, N. Simultaneous removal of five triazole fungicides from synthetic solutions on activated carbons and cyclodextrin-based adsorbents. Heliyon 2017, 3, e00380. [Google Scholar] [CrossRef]
- Alonso, L.L.; Demetrio, P.M.; Etchegoyen, M.A.; Marino, D.J. Glyphosate and atrazine in rainfall and soils in agroproductive areas of the pampas region in Argentina. Sci. Total Environ. 2018, 645, 89–96. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, A.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- Gesesew, H.A.; Woldemichael, K.; Massa, D.; Mwanri, L. Farmers knowledge, attitudes, practices and health problems associated with pesticide use in rural irrigation villages, Southwest Ethiopia. PLoS ONE 2016, 11, e0162527. [Google Scholar] [CrossRef] [PubMed]
- Landini, F.; Beramendi, M.; Vargas, G.L. Uso y manejo de agroquímicos en agricultores familiares y trabajadores rurales de cinco provincias argentinas. Rev. Argent Salud Pública 2019, 10, 22–28. [Google Scholar]
- Nasreddine, L.; Parent-Massin, D. Food contamination by metals and pesticides in the European Union. Should we worry? Toxicol. Lett. 2002, 127, 29–41. [Google Scholar] [CrossRef]
- Rezg, R.; Mornagui, B.; El-Fazaa, S.; Gharbi, N. Organophosphorus pesticides as food chain contaminants and type 2 diabetes: A review. Trends Food Sci. Technol. 2010, 21, 345–357. [Google Scholar] [CrossRef]
- Rodríguez, L.C.; Niemeyer, H.M. Integrated pest management, semiochemicals and microbial pest-control agents in Latin American agriculture. Crop Prot. 2005, 24, 615–623. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Sharma, M.; Guleria, S.; Singh, K.; Chauhan, A.; Kulshrestha, S. Mycovirus associated hypovirulence, a potential method for biological control of Fusarium species. Virus Dis. 2018, 29, 134–140. [Google Scholar] [CrossRef]
- Li, P.; Bhattacharjee, P.; Wang, S.; Zhang, L.; Ahmed, I.; Guo, L. Mycoviruses in Fusarium species: An updating review. Frontiers in Cellular and Infection Microbiology 2019, 9, 257. [Google Scholar] [CrossRef]
- Darissa, O.; Adam, G.; Schafer, W. A dsRNA mycovirus causes hypovirulence of Fusarium graminearum to wheat and maize. Eur. J. Plant Pathol. 2012, 134, 181–189. [Google Scholar] [CrossRef]
- Lemus-Minor, C.G.; Cañizares, M.C.; García-Pedrajas, M.D.; Pérez-Artés, E. Horizontal and vertical transmission of the hypovirulence-associated mycovirus Fusarium oxysporum f. sp. dianthi virus 1. Eur. J. Plant Pathol. 2019, 153, 645–650. [Google Scholar] [CrossRef]
- Lemus-Minor, C.G.; Cañizares, M.C.; García-Pedrajas, M.D.; Pérez-Artés, E. Fusarium oxysporum f. sp. dianthi virus 1 accumulation is correlated with changes in virulence and other phenotypic traits of its fungal host. Phytopathology 2018, 108, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, H.; Chen, X.; Qiu, D.; Guo, L. Molecular characterization of a novel hypovirus from the plant pathogenic fungus Fusarium graminearum. Virology 2015, 481, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-M.; Jeon, J.-J.; Yea, S.-J.; Kim, Y.-H.; Yun, S.-H.; Lee, Y.-W.; Kim, K.-H. Double-stranded RNA mycovirus from Fusarium graminearum. Appl. Environ. Microbiol. 2002, 68, 2529–2534. [Google Scholar] [CrossRef]
- Cho, W.K.; Lee, K.M.; Yu, J.; Son, M.; Kim, K.H. Insight into mycoviruses infecting Fusarium species. In Advances in Virus Research; Ghabrial, S.A., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 86, pp. 273–288. [Google Scholar]
- Yu, J.; Kim, K.H. Exploration of the interactions between mycoviruses and Fusarium graminearum. In Advances in Virus Research; Kielian, M., Mettenleiter, T.C., Roossinck, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 106, pp. 123–144. [Google Scholar]
- Zabalgogeazcoa, I.; Alvarez, A.; Herrero, N.; Vazquez-de-Aldana, B.R. Production of fumonisins by endophytic strains of Tolypocladium cylindrosporum and its relation to fungal virus infection. Mycotoxin Res. 2018, 34, 49–57. [Google Scholar] [CrossRef]
- Nerva, L.; Chitarra, W.; Siciliano, I.; Gaiotti, F.; Ciuffo, M.; Forgia, M.; Varese, G.C.; Turina, M. Mycoviruses mediate mycotoxin regulation in Aspergillus ochraceus. Environ. Microbiol. 2019, 21, 1957–1968. [Google Scholar] [CrossRef]
- Duan, C.; Qin, Z.; Yang, Z.; Li, W.; Sun, S.; Zhu, Z.; Wang, X. Identification of pathogenic Fusarium spp. causing maize ear rot and potential mycotoxin production in China. Toxins 2016, 8, 186. [Google Scholar] [CrossRef]
- Blacutt, A.A.; Gold, S.E.; Voss, K.A.; Gao, M.; Glenn, A.E. Fusarium verticillioides: Advancements in understanding the toxicity, virulence, and niche adaptations of a model mycotoxigenic pathogen of maize. Phytopathology 2018, 108, 312–326. [Google Scholar] [CrossRef]
- Gai, X.; Dong, H.; Wang, S.; Liu, B.; Zhang, Z.; Li, X.; Gao, Z. Infection cycle of maize stalk rot and ear rot caused by Fusarium verticillioides. PLoS ONE 2018, 13, e0201588. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M. Symptomless endophytic colonization of maize by Fusarium moniliforme. Can. J. Bot. 1996, 74, 1195–1202. [Google Scholar] [CrossRef]
- Martínez, M.; Moschini, R.; Barreto, D.; Bodega, J.; Comerio, R.; Forjan, H.; Piatti, F.; Presello, D.; Valentinuz, O. Environmental factors that affect the fumonisin content in maize grain. Trop. Plant Pathol. 2010, 35, 277–284. [Google Scholar]
- Proctor, R.H.; Plattner, R.D.; Desjardins, A.E.; Busman, M.; Butchko, R.A. Fumonisin production in the maize pathogen Fusarium verticillioides: Genetic basis of naturally occurring chemical variation. J. Agric. Food Chem. 2006, 54, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Ed.; IARC Press: Lyon, France, 2002; Volume 82. [Google Scholar]
- Glenn, A.E. Mycotoxigenic Fusarium species in animal feed. Anim. Feed Sci. Technol. 2007, 137, 213–240. [Google Scholar] [CrossRef]
- Theumer, M.G.; Cánepa, M.C.; Lopez, A.G.; Mary, V.S.; Dambolena, J.S.; Rubinstein, H.R. Subchronic mycotoxicoses in Wistar rats: Assessment of the in vivo and in vitro genotoxicity induced by fumonisins and aflatoxin B1, and oxidative stress biomarkers status. Toxicology 2010, 268, 104–110. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Pub: New York, NY, USA, 2006. [Google Scholar]
- Sampietro, D.A.; Marín, P.; Iglesias, J.; Presello, D.A.; Vattuone, M.A.; Catalán, C.A.; Jaen, M.G. A molecular based strategy for rapid diagnosis of toxigenic Fusarium species associated to cereal grains from Argentina. Fungal Biol. 2010, 114, 74–81. [Google Scholar] [CrossRef]
- Leslie, J.F.; Pearson, C.A.; Nelson, P.E.; Toussoun, T. Fusarium spp. from corn, sorghum, and soybean fields in the central and eastern United States. Ecol. Stud. 1990, 44, 66. [Google Scholar]
- Valverde, R.A.; Nameth, S.T.; Jordan, R.L. Analysis of doublestranded—RNA for plant-virus diagnosis. Plant Dis. 1990, 74, 255–258. [Google Scholar] [CrossRef]
- Nibert, M.L.; Vong, M.; Fugate, K.K.; Debat, H.J. Evidence for contemporary plant mitoviruses. Virology 2018, 518, 14–24. [Google Scholar] [CrossRef]
- Yao, Z.; Zou, C.; Peng, N.; Zhu, Y.; Bao, Y.; Zhou, Q.; Wu, Q.; Chen, B.; Zhang, M. Virome Identification and Characterization of Fusarium sacchari and F. andiyazi: Causative Agents of Pokkah Boeng Disease in Sugarcane. Front. Microbiol. 2020, 11, 240. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Dambolena, J.S.; López, A.G.; Cánepa, M.C.; Theumer, M.G.; Zygadlo, J.A.; Rubinstein, H.R. Inhibitory effect of cyclic terpenes (limonene, menthol, menthone and thymol) on Fusarium verticillioides MRC 826 growth and fumonisin B1 biosynthesis. Toxicon 2008, 51, 37–44. [Google Scholar] [CrossRef]
- Shephard, G.S.; Sydenham, E.W.; Thiel, P.G.; Gelderblom, W.C.A. Quantitative determination of fumonisins B1 and B2 by high-performance liquid chromatography with fluorescence detection. J. Liq. Chromatogr. 1990, 13, 2077–2087. [Google Scholar] [CrossRef]
- Arias, S.L.; Mary, V.S.; Otaiza, S.N.; Wunderlin, D.A.; Rubinstein, H.R.; Theumer, M.G. Toxin distribution and sphingoid base imbalances in Fusarium verticillioides-infected and fumonisin B1-watered maize seedlings. Phytochemistry 2016, 125, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Geilfus, C.M.; Mühling, K.H.; Ludwig-Müller, J. The influence of salt stress on ABA and auxin concentrations in two maize cultivars differing in salt resistance. J. Plant Physiol. 2013, 170, 220–224. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020; Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba: Cordoba, Argentina, 2020; Available online: http://www.infostat.com.ar (accessed on 1 July 2020).
- Mahillon, M.; Romay, G.; Liénard, C.; Legrève, A.; Bragard, C. Description of a Novel Mycovirus in the Phytopathogen Fusarium culmorum and a Related EVE in the Yeast Lipomyces starkeyi. Viruses 2020, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Chulze, S.; Ramirez, M.L.; Farnochi, M.; Pascale, M.; Visconti, A.; March, G. Fusarium and fumonisins occurrence in Argentinian corn at different ear maturity stages. J. Agric. Food Chem. 1996, 2797–2801. [Google Scholar] [CrossRef]
- Castañares, E.; Martínez, M.; Cristos, D.; Rojas, D.; Lara, B.; Stenglein, S.; Dinolfo, M.I. Fusarium species and mycotoxin contamination in maize in Buenos Aires province, Argentina. Eur. J. Plant Pathol. 2019, 155, 1265–1275. [Google Scholar] [CrossRef]
- Marasas, W.F.; Rheeder, J.P.; Lamprecht, S.C.; Zeller, K.A.; Leslie, J.F. Fusarium andiyazi sp. nov., a new species from sorghum. Mycologia 2001, 93, 1203–1210. [Google Scholar] [CrossRef]
- Leslie, J.F.; Zeller, K.A.; Lamprecht, S.C.; Rheeder, J.P.; Marasas, W.F. Toxicity, pathogenicity, and genetic differentiation of five species of Fusarium from sorghum and millet. Phytopathology 2005, 95, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, F.E.; Ruiz-Vázquez, R.M. Functional diversity of RNAi-associated sRNAs in fungi. Int. J. Mol. Sci. 2013, 14, 15348–15360. [Google Scholar] [CrossRef] [PubMed]
- Sesma, A. RNA metabolism and regulation of virulence programs in fungi. In Seminars in Cell & Developmental Biology; Nóbrega, M.A., Gómez-Skarmeta, J.L., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 57, pp. 120–127. [Google Scholar]
- Wolf, Y.I.; Kazlauskas, D.; Iranzo, J.; Lucía-Sanz, A.; Kuhn, J.H.; Krupovic, M.; Dolja, V.V.; Koonin, E.V. Origins and evolution of the global RNA virome. mBio 2018, 9, e02329-18. [Google Scholar] [CrossRef]
- Xie, J.; Ghabrial, S.A. Molecular characterizations of two mitoviruses co-infecting a hyovirulent isolate of the plant pathogenic fungus Sclerotinia sclerotiorum. Virology 2012, 428, 77–85. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Vainio, E.J.; Botella, L.; Hantula, J.; Diez, J.J. Three mitovirus strains infecting a single isolate of Fusarium circinatum are the first putative members of the family Narnaviridae detected in a fungus of the genus Fusarium. Arch. Virol. 2014, 159, 2153–2155. [Google Scholar] [CrossRef]
- Vainio, E.J.; Hakanpää, J.; Dai, Y.C.; Hansen, E.; Korhonen, K.; Hantula, J. Species of Heterobasidion host a diverse pool of partitiviruses with global distribution and interspecies transmission. Fungal Biol. 2011, 115, 1234–1243. [Google Scholar] [CrossRef]
- Hillman, B.I.; Cai, G. The family Narnaviridae: Simplest of RNA viruses. In Advances in Virus Research; Ghabrial, S.A., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 86, pp. 149–176. [Google Scholar]
- Elzanowski, A.; Ostell, J. The Genetic Codes. National Center for Biotechnology. 2019. Available online: http://www.ncbi.nlm.nih.gov/Taxonomy/Utils/wprintgc.cgi?mode=c (accessed on 14 April 2020).
- Nibert, M.L. Mitovirus UGA (Trp) codon usage parallels that of host mitochondria. Virology 2017, 507, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Chiapello, M.; Rodríguez-Romero, J.; Nerva, L.; Forgia, M.; Chitarra, W.; Ayllón, M.A.; Turina, M. Putative new plant viruses associated with Plasmopara viticola-infected grapevine samples. Ann. Appl. Biol. 2020, 176, 180–191. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.-D.; Tian, J.-H.; Chen, L.-J.; Chen, X.; Li, C.-X.; Qin, X.-C.; Li, J.; Cao, J.-P.; Eden, J.-S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, L.; Li, G.; Jiang, D.; Ghabrial, S.A. Genome characterization of a debilitation-associated mitovirus infecting the phytopathogenic fungus Botrytis cinerea. Virology 2010, 406, 117–126. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, S.; Liu, L.; Cheng, J.; Fu, Y.; Jiang, D.; Xie, J. A mitovirus related to plant mitochondrial gene confers hypovirulence on the phytopathogenic fungus Sclerotinia sclerotiorum. Virus Res. 2015, 197, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Chen, X.; Punja, Z.K. Molecular and biological characterization of a mitovirus in Chalara elegans (Thielaviopsis basicola). Phytopathology 2006, 96, 468–479. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glenn, A.E.; Zitomer, N.C.; Zimeri, A.M.; Williams, L.D.; Riley, R.T.; Proctor, R.H. Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Mol. Plant-Microbe Interact. 2008, 21, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Busman, M.; Proctor, R.H. Fusarium verticillioides SGE1 is required for full virulence and regulates expression of protein effector and secondary metabolite biosynthetic genes. Mol. Plant-Microbe Interact. 2014, 27, 809–823. [Google Scholar] [CrossRef]
- Marvelli, R.A.; Hobbs, H.A.; Li, S.; McCoppin, N.K.; Domier, L.L.; Hartman, G.L.; Eastburn, D.M. Identification of novel double-stranded RNA mycoviruses of Fusarium virguliforme and evidence of their effects on virulence. Arch. Virol. 2014, 159, 349–352. [Google Scholar] [CrossRef]
- Paoletti, M. Vegetative incompatibility in fungi: From recognition to cell death, whatever does the trick. Fungal Biol. Rev. 2016, 30, 152–162. [Google Scholar] [CrossRef]
- Chulze, S.N.; Ramirez, M.L.; Torres, A.; Leslie, J.F. Genetic Variation in Fusarium SectionLiseola from No-Till Maize in Argentina. Appl. Environ. Microbiol. 2000, 66, 5312–5315. [Google Scholar] [CrossRef]
- Caten, C.E. Vegetative incompatibility and cytoplasmic infection in fungi. Microbiology 1972, 72, 221–229. [Google Scholar] [CrossRef]
- Biella, S.; Smith, M.L.; Aist, J.R.; Cortesi, P.; Milgroom, M.G. Programmed cell death correlates with virus transmission in a filamentous fungus. Proc. R. Soc. Lond. B 2002, 269, 2269–2276. [Google Scholar] [CrossRef]
- Pearson, M.N.; Bailey, A.M. Viruses of botrytis. In Advances in Virus Research; Ghabrial, S.A., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 86, pp. 249–272. [Google Scholar]
- Juroszek, P.; Von Tiedemann, A. Climatic changes and the potential future importance of maize diseases: A short review. J. Plant Dis. Prot. 2013, 120, 49–56. [Google Scholar] [CrossRef]
- Elad, Y.; Pertot, I. Climate change impacts on plant pathogens and plant diseases. J. Crop Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
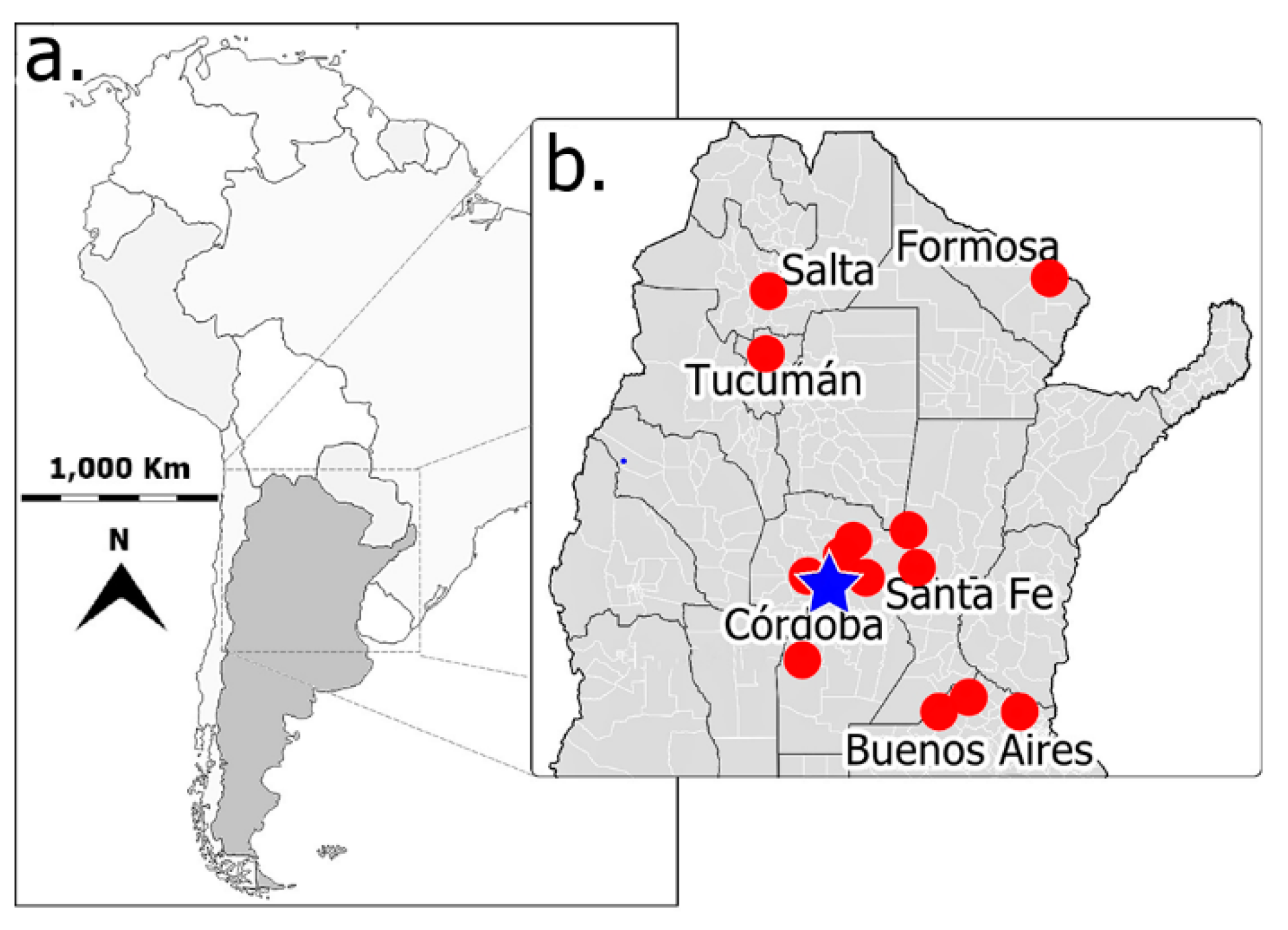
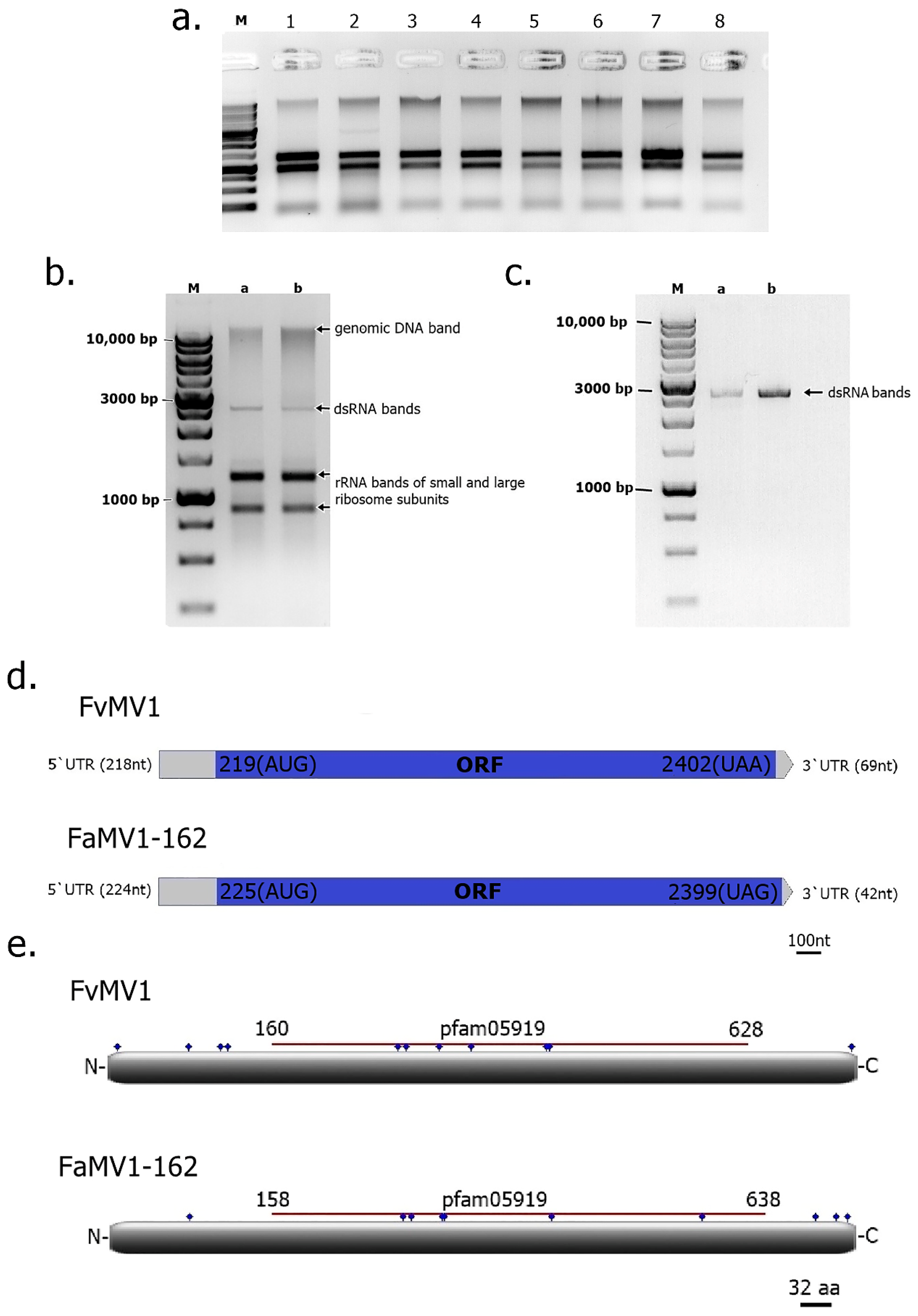
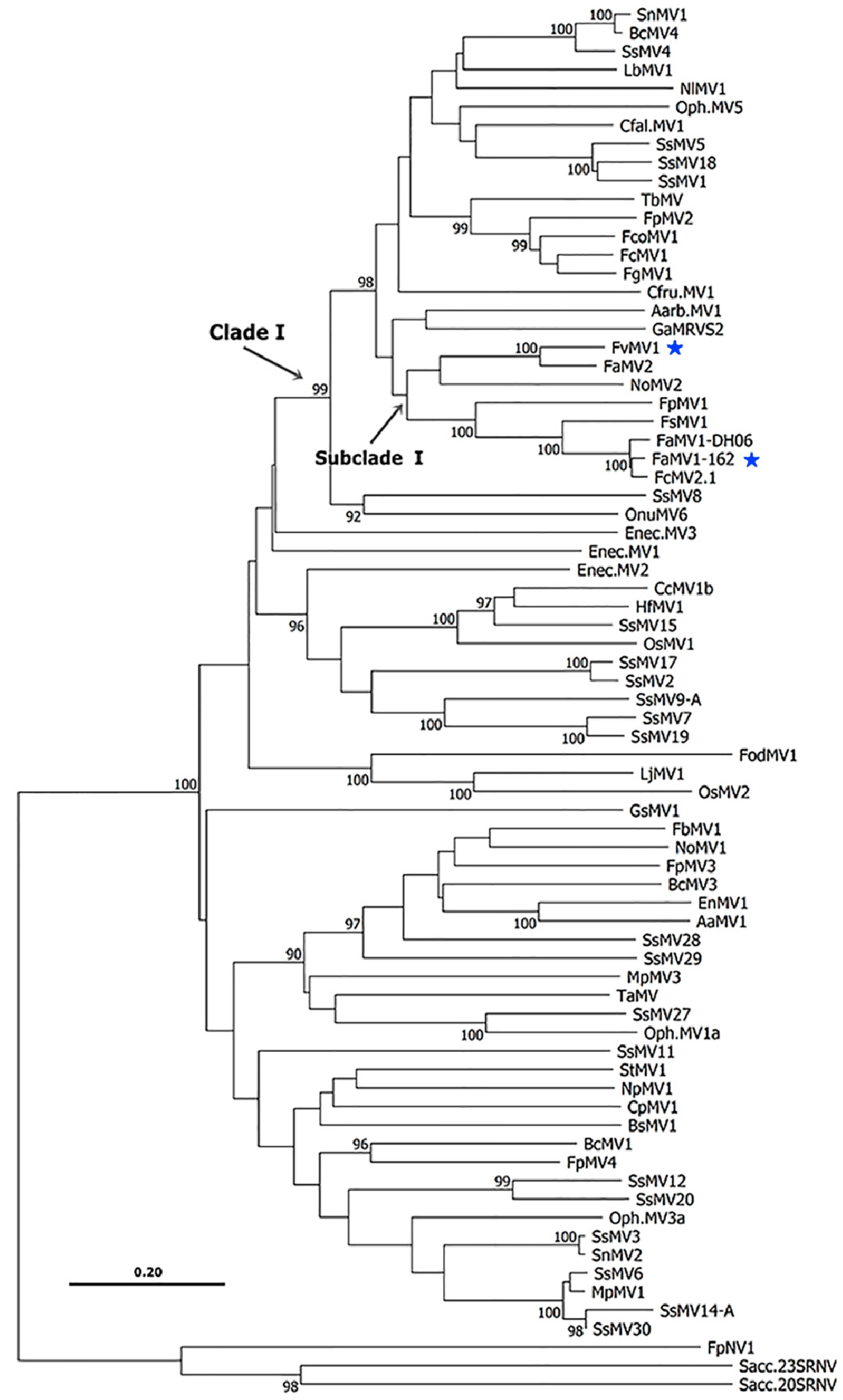
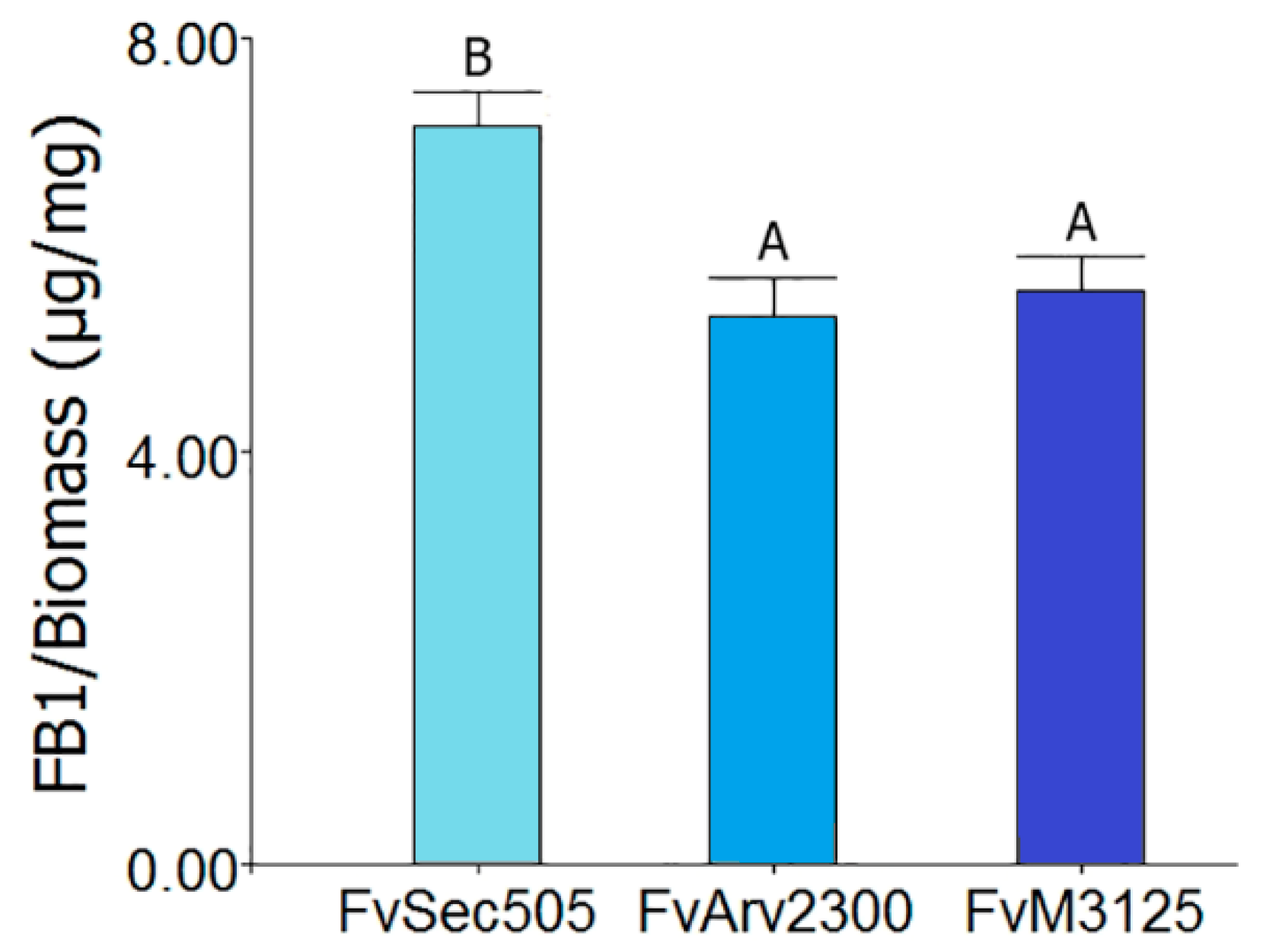
| Abbreviations | Meaning |
|---|---|
| FB | B-series fumonisins |
| (+)ssRNA | positive sense single-stranded RNA |
| dsRNA | double-stranded RNA |
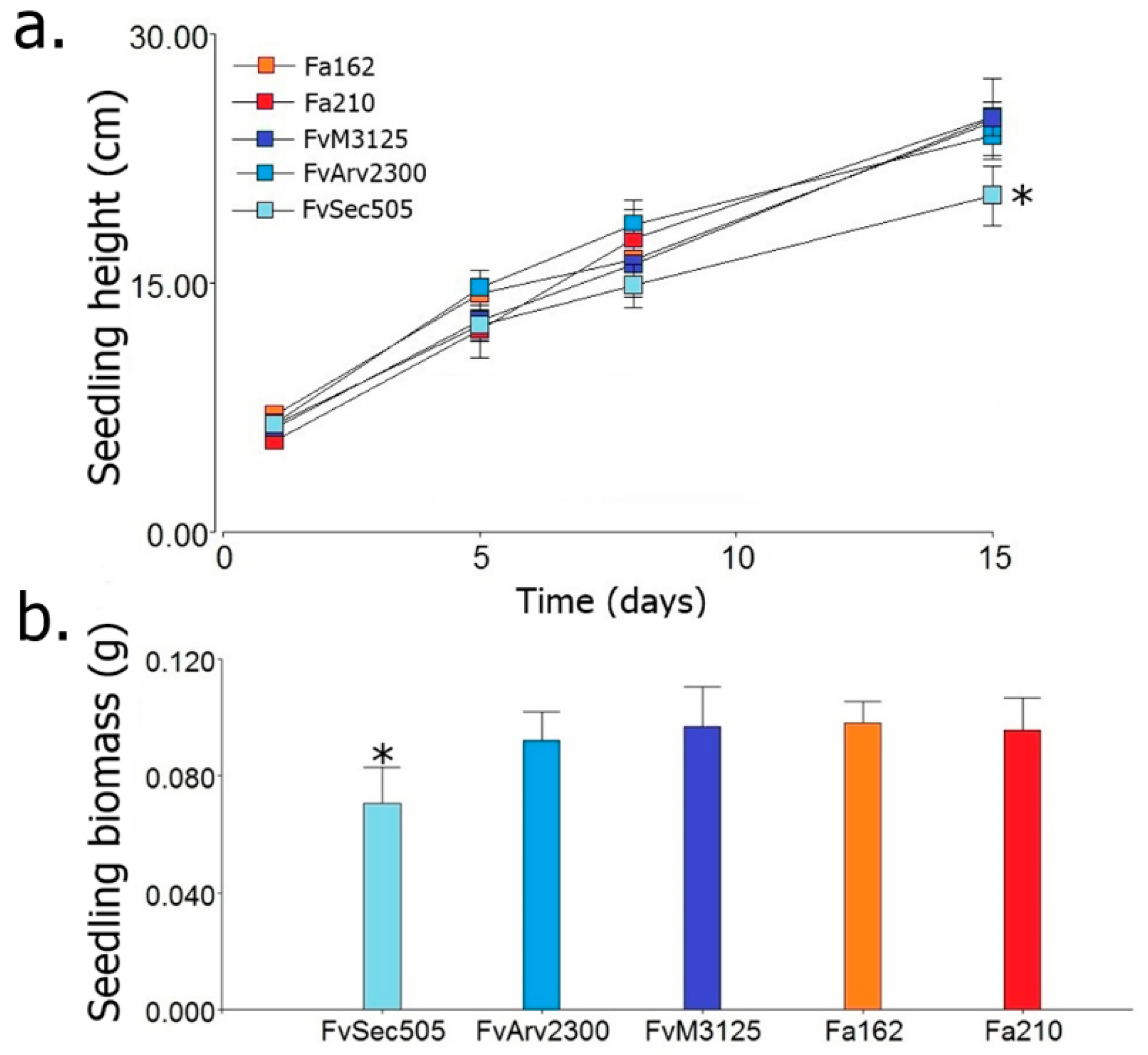
| FvSec505 | F. verticillioides strain Sec505 (infected with FvMV1) |
| FvM3125 | Virus-free strain F. verticillioides M3125 |
| FvArv2300 | Virus-free strain F. verticillioides Arv2300 |
| Fa162 | F. andiyazi strain 162 (infected with FaMV1-162) |
| Fa210 | Virus-free strain F. andiyazi 210 |
| FvMV1 | Fusarium verticillioides mitovirus 1 |
| FaMV1-162 | F. andiyazi mitovirus 1 strain 162 |
| Fusarium Isolate | Growth Rate (mm/Day) | Lag Phase (Hour) | Conidia/mL/mm2 | ||
|---|---|---|---|---|---|
| PDA | CDA | PDA | CDA | ||
| FvM3125 | 4.89 ± 0.09a | 6.06 ± 0.10a | 46.58 ± 1.13b | 31.58 ± 0.85a | 3303.85 ± 463.13a |
| FvSec505 | 6.53 ± 0.09b | 7.09 ± 0.0c | 35.40 ± 1.04a | 31.38 ± 0.95a | 5346.24 ± 463.14b |
| FvArv2300 | 6.97 ± 0.08c | 6.65 ± 0.10b | 37.73 ± 0.90a | 28.88 ± 0.95a | 3797.20 ± 463.14a |
| Fa162 | 6.20 ± 0.08y | 8.18 ± 0.09z | 32.88 ± 0.96z | 31.35 ± 0.62z | 4472.34 ± 401.94z |
| Fa210 | 6.79 ± 0.08z | 7.15 ± 0.10y | 32.31 ± 0.90z | 31.22 ± 0.60z | 5006.96 ± 401.94z |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacquat, A.G.; Theumer, M.G.; Cañizares, M.C.; Debat, H.J.; Iglesias, J.; García Pedrajas, M.D.; Dambolena, J.S. A Survey of Mycoviral Infection in Fusarium spp. Isolated from Maize and Sorghum in Argentina Identifies the First Mycovirus from Fusarium verticillioides. Viruses 2020, 12, 1161. https://doi.org/10.3390/v12101161
Jacquat AG, Theumer MG, Cañizares MC, Debat HJ, Iglesias J, García Pedrajas MD, Dambolena JS. A Survey of Mycoviral Infection in Fusarium spp. Isolated from Maize and Sorghum in Argentina Identifies the First Mycovirus from Fusarium verticillioides. Viruses. 2020; 12(10):1161. https://doi.org/10.3390/v12101161
Chicago/Turabian StyleJacquat, Andrés Gustavo, Martín Gustavo Theumer, María Carmen Cañizares, Humberto Julio Debat, Juliana Iglesias, María Dolores García Pedrajas, and José Sebastián Dambolena. 2020. "A Survey of Mycoviral Infection in Fusarium spp. Isolated from Maize and Sorghum in Argentina Identifies the First Mycovirus from Fusarium verticillioides" Viruses 12, no. 10: 1161. https://doi.org/10.3390/v12101161
APA StyleJacquat, A. G., Theumer, M. G., Cañizares, M. C., Debat, H. J., Iglesias, J., García Pedrajas, M. D., & Dambolena, J. S. (2020). A Survey of Mycoviral Infection in Fusarium spp. Isolated from Maize and Sorghum in Argentina Identifies the First Mycovirus from Fusarium verticillioides. Viruses, 12(10), 1161. https://doi.org/10.3390/v12101161







