Increased Monocyte Inflammatory Responses to Oxidized LDL Are Associated with Insulin Resistance in HIV-Infected Individuals on Suppressive Antiretroviral Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Study Design
2.2. Assessment of Monocyte Intracellular Cytokine Production
2.3. Statistical Analysis
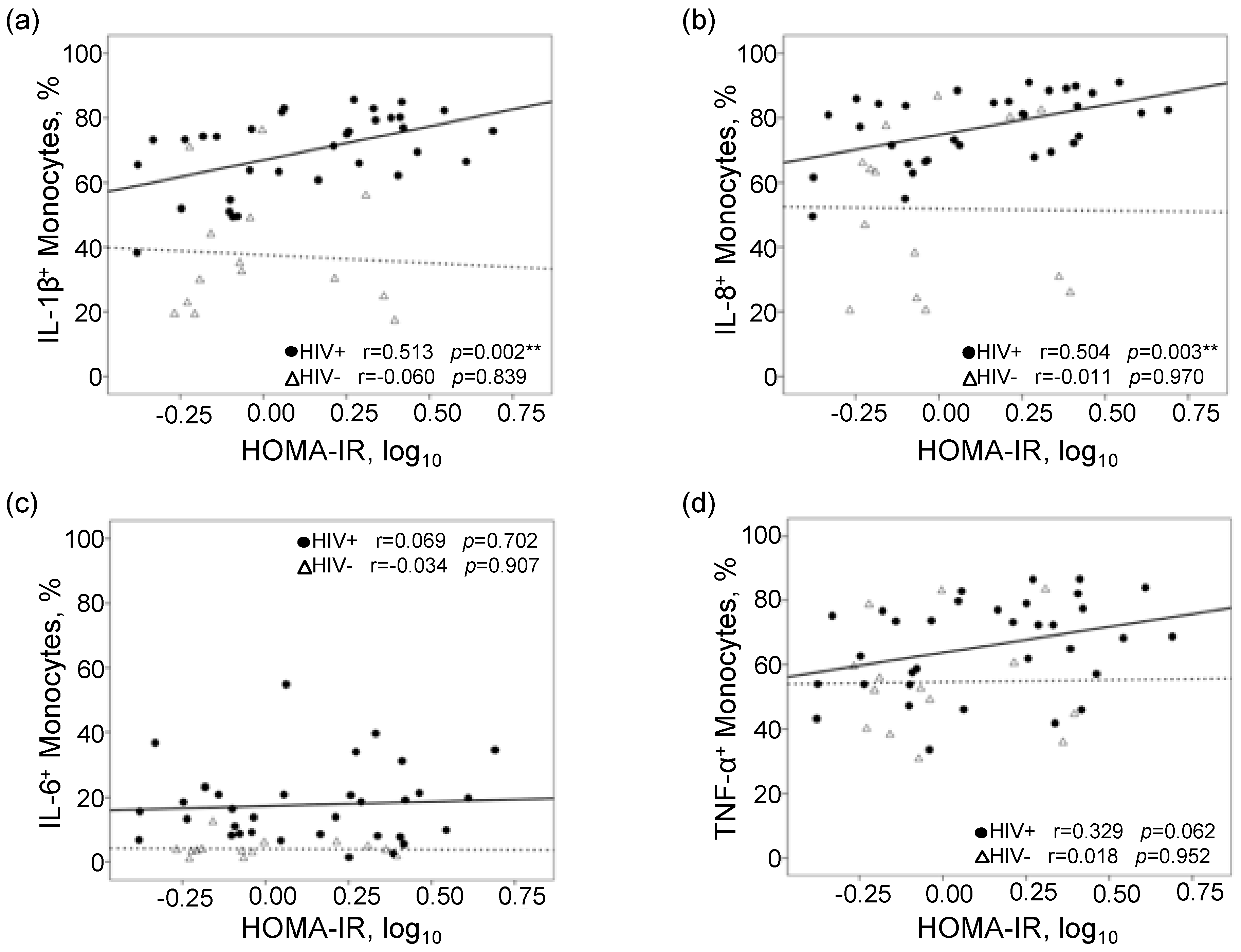
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef]
- Gutierrez, A.D.; Balasubramanyam, A. Dysregulation of glucose metabolism in HIV patients: Epidemiology, mechanisms, and management. Endocrine 2011, 41, 1–10. [Google Scholar] [CrossRef]
- Mondy, K.E.; Fuentes, L.D.L.; Waggoner, A.; Önen, N.F.; Bopp, C.S.; Lassa-Claxton, S.; Powderly, W.G.; Dávila-Román, V.; Yarasheski, K.E. Insulin resistance predicts endothelial dysfunction and cardiovascular risk in HIV-infected persons on long-term highly active antiretroviral therapy. AIDS 2008, 22, 849–856. [Google Scholar] [CrossRef]
- Nix, L.M.; Tien, P.C. Metabolic Syndrome, Diabetes, and Cardiovascular Risk in HIV. Curr. HIV/AIDS Rep. 2014, 11, 271–278. [Google Scholar] [CrossRef]
- Triant, V.A.; Lee, H.; Hadigan, C.; Grinspoon, S.K. Increased Acute Myocardial Infarction Rates and Cardiovascular Risk Factors among Patients with Human Immunodeficiency Virus Disease. J. Clin. Endocrinol. Metab. 2007, 92, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.; Martinez, E. Update on metabolic issues in HIV patients. Curr. Opin. HIV AIDS 2014, 9, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Paik, I.J.; Kotler, D.P. The prevalence and pathogenesis of diabetes mellitus in treated HIV-infection. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Tracy, R.; Douek, D.C. Systemic Effects of Inflammation on Health during Chronic HIV Infection. Immunity 2013, 39, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.; Tassiopoulos, K.; Bosch, R.J.; Shikuma, C.; Mccomsey, G.A. Association between Systemic Inflammation and Incident Diabetes in HIV-Infected Patients after Initiation of Antiretroviral Therapy. Diabetes Care 2010, 33, 2244–2249. [Google Scholar] [CrossRef]
- Shikuma, C.M.; Chow, M.C.; Gangcuangco, L.M.A.; Zhang, G.; Keating, S.M.; Norris, P.J.; Seto, T.B.; Parikh, N.; Kallianpur, K.J.; Nakamoto, B.K.; et al. Monocytes Expand with Immune Dysregulation and Is Associated with Insulin Resistance in Older Individuals with Chronic HIV. PLoS ONE 2014, 9, e90330. [Google Scholar] [CrossRef]
- Ellery, P.J.; Tippett, E.; Chiu, Y.-L.; Paukovics, G.; Cameron, P.U.; Solomon, A.; Lewin, S.R.; Gorry, P.R.; Jaworowski, A.; Greene, W.C.; et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J. Immunol. 2007, 178, 6581–6589. [Google Scholar] [CrossRef] [PubMed]
- Jaworowski, A.; Ellery, P.; Sonza, S.; Mwapasa, V.; Tadesse, E.; Kamwendo, D.D.; Molyneux, M.E.; Rogerson, S.J.; Meshnick, S.R.; Crowe, S.M. CD16 + Monocyte Subset Preferentially Harbors HIV?1 and Is Expanded in Pregnant Malawian Women with Plasmodium falciparum Malaria and HIV?1 Infection. J. Infect. Dis. 2007, 196, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Westhorpe, C.L.; Maisa, A.; Spelman, T.; Hoy, J.; Dewar, E.M.; Karapanagiotidis, S.; Hearps, A.C.; Cheng, W.-J.; Trevillyan, J.M.; Lewin, S.R.; et al. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol. Cell Biol. 2013, 92, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Ghattas, A.; Griffiths, H.R.; Devitt, A.; Lip, G.Y.; Shantsila, E. Monocytes in Coronary Artery Disease and Atherosclerosis. J. Am. Coll. Cardiol. 2013, 62, 1541–1551. [Google Scholar] [CrossRef]
- Llodrá, J.; Angeli, V.; Liu, J.; Trogan, E.; Fisher, E.A.; Randolph, G.J. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl. Acad. Sci. USA 2004, 101, 11779–11784. [Google Scholar] [CrossRef]
- Kusao, I.; Shiramizu, B.; Liang, C.-Y.; Grove, J.; Agsalda, M.; Troelstrup, D.; Velasco, V.-N.; Marshall, A.; Whitenack, N.; Shikuma, C.; et al. Cognitive performance related to HIV-1-infected monocytes. J. Neuropsychiatr. Clin. Neurosci. 2012, 24, 71–80. [Google Scholar] [CrossRef]
- Shikuma, C.M.; Nakamoto, B.; Shiramizu, B.; Liang, C.-Y.; DeGruttola, V.; Bennett, K.; Paul, R.; Kallianpur, K.; Chow, D.; Gavegnano, C.; et al. Antiretroviral monocyte efficacy score linked to cognitive impairment in HIV. Antivir. Ther. 2012, 17, 1233–1242. [Google Scholar] [CrossRef]
- Wang, H.; Sun, J.; Goldstein, H. Human Immunodeficiency Virus Type 1 Infection Increases the In Vivo Capacity of Peripheral Monocytes To Cross the Blood-Brain Barrier into the Brain and the In Vivo Sensitivity of the Blood-Brain Barrier to Disruption by Lipopolysaccharide. J. Virol. 2008, 82, 7591–7600. [Google Scholar] [CrossRef]
- Persidsky, Y.; Stins, M.; Way, D.; Witte, M.H.; Weinand, M.; Kim, K.S.; Bock, P.; Gendelman, H.E.; Fiala, M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J. Immunol. 1997, 158, 3499–3510. [Google Scholar]
- Burdo, T.H.; Lackner, A.; Williams, K.C. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol. Rev. 2013, 254, 102–113. [Google Scholar] [CrossRef]
- Jalbert, E.; Crawford, T.Q.; D’Antoni, M.L.; Keating, S.M.; Norris, P.J.; Nakamoto, B.K.; Seto, T.; Parikh, N.I.; Shikuma, C.M.; Ndhlovu, L.C.; et al. IL-1B enriched monocytes mount massive IL-6 responses to common inflammatory triggers among chronically HIV-1 infected adults on stable anti-retroviral therapy at risk for cardiovascular disease. PLoS ONE 2013, 8, e75500. [Google Scholar] [CrossRef] [PubMed]
- Shikuma, C.M.; Seto, T.; Liang, C.-Y.; Bennett, K.; DeGruttola, V.; Gerschenson, M.; Stein, J.H.; Budoff, M.; Hodis, H.N.; Delaney, J.A.; et al. Vitamin D Levels and Markers of Arterial Dysfunction in HIV. AIDS Res. Hum. Retroviruses 2012, 28, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.; Færch, K. Estimating insulin sensitivity and beta cell function: Perspectives from the modern pandemics of obesity and type 2 diabetes. Diabetologia 2012, 55, 2863–2867. [Google Scholar] [CrossRef] [PubMed]
- Executive Summary: Standards of Medical Care in Diabetes--2014. Diabetes Care 2013, 37, 4–10. [CrossRef]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C.; American Heart Association; National Heart, Lung, Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Jager, J.; Grémeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.-F. Interleukin-1β-Induced Insulin Resistance in Adipocytes through Down-Regulation of Insulin Receptor Substrate-1 Expression. Endocrinology 2007, 148, 241–251. [Google Scholar] [CrossRef]
- Kobashi, C.; Asamizu, S.; Ishiki, M.; Iwata, M.; Usui, I.; Yamazaki, K.; Tobe, K.; Kobayashi, M.; Urakaze, M. Inhibitory effect of IL-8 on insulin action in human adipocytes via MAP kinase pathway. J. Inflamm. (Lond. Engl.) 2009, 6, 25. [Google Scholar] [CrossRef]
- Rotter, V.; Nagaev, I.; Smith, U. Interleukin-6 (IL-6) Induces Insulin Resistance in 3T3-L1 Adipocytes and Is, Like IL-8 and Tumor Necrosis Factor-α, Overexpressed in Human Fat Cells from Insulin-resistant Subjects. J. Boil. Chem. 2003, 278, 45777–45784. [Google Scholar] [CrossRef]
- Stephens, J.M.; Pekala, P.H. Transcriptional repression of the C/EBP-alpha and GLUT4 genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. Regulations is coordinate and independent of protein synthesis. J. Biol. Chem. 1992, 267, 13580–13584. [Google Scholar]
- Rui, L.; Aguirre, V.; Kim, J.K.; Shulman, G.I.; Lee, A.; Corbould, A.; Dunaif, A.; White, M.F. Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Investig. 2001, 107, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Flower, L.; Gray, R.; Pinkney, J.; Mohamed-Ali, V. Stimulation of interleukin-6 release by interleukin-1beta from isolated human adipocytes. Cytokine 2003, 21, 32–37. [Google Scholar] [CrossRef]
- Sloan-Lancaster, J.; Abu-Raddad, E.; Polzer, J.; Miller, J.W.; Scherer, J.C.; De Gaetano, A.; Berg, J.K.; Landschulz, W.H. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1beta antibody, in patients with type 2 diabetes. Diabetes Care 2013, 3, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Weder, C.; Babians-Brunner, A.; Keller, C.; Stahel, M.A.; Kurz-Levin, M.; Zayed, H.; Solinger, A.M.; Mandrup-Poulsen, T.; Dinarello, C.A.; Donath, M.Y. Effects of Gevokizumab on Glycemia and Inflammatory Markers in Type 2 Diabetes. Diabetes Care 2012, 35, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.M.; Faulenbach, M.; Vaag, A.; Vølund, A.; Ehses, J.A.; Seifert, B.; Mandrup-Poulsen, T.; Donath, M.Y. Interleukin-1–Receptor Antagonist in Type 2 Diabetes Mellitus. N. Engl. J. Med. 2007, 356, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Skov, L.; Beurskens, F.J.; Zachariae, C.; Reitamo, S.; Teeling, J.L.; Satijn, D.; Knudsen, K.M.; Boot, E.P.J.; Hudson, D.; Baadsgaard, O.; et al. IL-8 as antibody therapeutic target in inflammatory diseases: Reduction of clinical activity in palmoplantar pustulosis. J. Immunol. 2008, 181, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Seitz, M.; Dewald, B.; Gerber, N.; Baggiolini, M. Enhanced production of neutrophil-activating peptide-1/interleukin-8 in rheumatoid arthritis. J. Clin. Investig. 1991, 87, 463–469. [Google Scholar] [CrossRef]
- Grimm, M.C.; Elsbury, S.K.; Pavli, P.; Doe, W.F. Interleukin 8: Cells of origin in inflammatory bowel disease. Gut 1996, 38, 90–98. [Google Scholar] [CrossRef]
- Sharabiani, M.T.A.; Vermeulen, R.C.H.; Scoccianti, C.; Hosnijeh, F.S.; Minelli, L.; Sacerdote, C.; Palli, D.; Krogh, V.; Tumino, R.; Chiodini, P.; et al. Immunologic profile of excessive body weight. Biomarkers 2011, 16, 243–251. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Sainz, T.; Lee, S.A.; Hunt, P.W.; Sinclair, E.; Shacklett, B.L.; Ferre, A.L.; Hayes, T.L.; Somsouk, M.; Hsue, P.Y.; et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014, 10, e1004078. [Google Scholar] [CrossRef]
- Merino, A.; Buendia, P.; Martin-Malo, A.; Aljama, P.; Ramirez, R.; Carracedo, J. Senescent CD14+CD16+Monocytes Exhibit Proinflammatory and Proatherosclerotic Activity. J. Immunol. 2010, 186, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
| Baseline Measures | PLWHA n = 33 | Controls n = 14 | p-Value |
|---|---|---|---|
| Age, years | 53 (49, 56) | 51 (46, 60) | 0.552 |
| Male, n (%) | 29 (88%) | 14 (100%) | 0.302 |
| Caucasian, n (%) | 22 (67%) | 9 (64%) | 1.000 |
| Body mass index, kg/m2 | 26 (23, 27) | 24 (23, 27) | 0.601 |
| History of smoking, n (%) | 22 (67%) | 11 (79%) | 0.724 |
| History of hypertension, n (%) | 10 (30%) | 4 (29%) | 0.516 |
| HOMA-IR | 1.46 (0.79, 2.48) | 0.85 (0.62, 1.74) | 0.129 |
| Metabolic syndrome, n (%) | 7 (21%) | 1 (7%) | 0.405 |
| Type 2 Diabetes Mellitus, n (%) | 4 (12%) | 0 | 0.302 |
| Total cholesterol, mg/dL | 175 (146, 189) | 173 (151, 192) | 0.658 |
| HDL cholesterol, mg/dL | 36 (30, 45) | 55 (46, 64) | 0.001 |
| LDL cholesterol, mg/dL | 101 (81, 122) | 107 (86, 114) | 0.585 |
| Triglycerides, mg/dL | 125 (83, 161) | 78 (56, 140) | 0.076 |
| Hepatitis C infection, n (%) | 5 (15%) | 0 | 0.303 |
| Nadir CD4+ T cells, cells/μL | 181 (63, 275) | - | - |
| CD4+ T cells, cells/μL | 574 (450, 713) | - | - |
| CD4+ T cells, % | 33 (24, 37) | - | - |
| CD8+ T cells, cells/μL | 801 (594, 1087) | - | - |
| CD8+ T cells, % | 43 (35, 50) | - | - |
| Activated CD8+ T cells (CD38+HLA-DR+), cells/μL | 83 (56, 161) | - | - |
| Activated CD8+ T cells (CD38+HLA-DR+), % | 12 (9, 17) | - | - |
| HIV RNA < 50 copies/mL, n (%) | 28 (85%) | - | - |
| Duration since HIV diagnosis, years | 16 (8, 23) | - | - |
| Duration since ART initiation, years | 12 (6, 15) | - | - |
| History of NRTI use, n (%) | 33 (100%) | - | - |
| History of NNRTI use, n (%) | 23 (70%) | - | - |
| History of Protease Inhibitor use, n (%) | 21 (64%) | - | - |
| Variable | Unstimulated (Basal) Monocyte Response | Oxidized LDL-Stimulated Monocyte Response | ||||||
|---|---|---|---|---|---|---|---|---|
| IL-1β+ | IL-8+ | IL-6+ | TNF-α+ | IL-1β+ | IL-8+ | IL-6+ | TNF-α+ | |
| Total cholesterol | r = −0.238 | r= −0.017 | r= 0.180 | r = −0.344 | r = −0.240 | r = 0.017 | r = −0.174 | r = 0.167 |
| HDL cholesterol | r = 0.233 | r = 0.125 | r = −0.024 | r = −0.167 | r = −0.284 | r = −0.223 | r = −0.002 | r = 0.042 |
| LDL cholesterol | r = −0.227 | r = −0.102 | r = 0.145 | r = −0.166 | r = −0.270 | r = 0.013 | r = −0.280 | r = 0.197 |
| Triglycerides | r = −0.168 | r = 0.060 | r = 0.232 | r = −0.167 | r = 0.489 * | r = 0.414 * | r = 0.235 | r = −0.152 |
| Nadir CD4 T cells | r = −0.027 | r = −0.037 | r = −0.291 | r = −0.196 | r = 0.209 | r = 0.021 | r = 0.240 | r = 0.033 |
| CD4+ T cells | r = 0.279 | r = 0.241 | r = 0.013 | r = 0.227 | r = 0.100 | r = −0.054 | r = 0.267 | r = −0.181 |
| CD4+ T cells (%) | r = 0.062 | r = −0.095 | r = −0.389 * | r = 0.142 | r = −0.223 | r = −0.523 * | r = 0.069 | r = −0.410 * |
| CD8+ T cells | r = 0.191 | r = 0.241 | r = 0.368 * | r = 0.054 | r = 0.297 | r = 0.460 * | r = 0.245 | r = 0.142 |
| CD8* T cells (%) | r = −0.032 | r = 0.040 | r = 0.244 | r = 0.114 | r = −0.041 | r = 0.261 | r = −0.021 | r = 0.150 |
| CD4/CD8 T cell ratio | r = 0.079 | r = −0.011 | r = −0.295 | r = 0.057 | r = −0.180 | r = −0.501 * | r = 0.146 | r = −0.417 * |
| Activated CD8+ T cells | r = 0.224 | r = 0.292 | r = 0.513 * | r = 0.026 | r = 0.113 | r = 0.190 | r = 0.266 | r = −0.004 |
| Activated CD8+ T cells (%) | r = 0.072 | r = 0.051 | r = 0.211 | r = 0.080 | r = −0.075 | r = −0.130 | r = 0.103 | r = −0.188 |
| Duration since HIV diagnosis | r = 0.345 * | r = 0.161 | r = 0.410 * | r = 0.403 * | r = 0.092 | r = 0.181 | r = −0.132 | r = −0.238 |
| Duration since ART initiation | r = 0.178 | r = 0.229 | r = 0.503 * | r = 0.133 | r = 0.136 | r = 0.311 | r = −0.004 | r = −0.043 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitchell, B.I.; Laws, E.I.; Chow, D.C.; Sah Bandar, I.N.; Gangcuangco, L.M.A.; Shikuma, C.M.; Ndhlovu, L.C. Increased Monocyte Inflammatory Responses to Oxidized LDL Are Associated with Insulin Resistance in HIV-Infected Individuals on Suppressive Antiretroviral Therapy. Viruses 2020, 12, 1129. https://doi.org/10.3390/v12101129
Mitchell BI, Laws EI, Chow DC, Sah Bandar IN, Gangcuangco LMA, Shikuma CM, Ndhlovu LC. Increased Monocyte Inflammatory Responses to Oxidized LDL Are Associated with Insulin Resistance in HIV-Infected Individuals on Suppressive Antiretroviral Therapy. Viruses. 2020; 12(10):1129. https://doi.org/10.3390/v12101129
Chicago/Turabian StyleMitchell, Brooks I., Elizabeth I. Laws, Dominic C. Chow, Ivo N. Sah Bandar, Louie Mar A. Gangcuangco, Cecilia M. Shikuma, and Lishomwa C. Ndhlovu. 2020. "Increased Monocyte Inflammatory Responses to Oxidized LDL Are Associated with Insulin Resistance in HIV-Infected Individuals on Suppressive Antiretroviral Therapy" Viruses 12, no. 10: 1129. https://doi.org/10.3390/v12101129
APA StyleMitchell, B. I., Laws, E. I., Chow, D. C., Sah Bandar, I. N., Gangcuangco, L. M. A., Shikuma, C. M., & Ndhlovu, L. C. (2020). Increased Monocyte Inflammatory Responses to Oxidized LDL Are Associated with Insulin Resistance in HIV-Infected Individuals on Suppressive Antiretroviral Therapy. Viruses, 12(10), 1129. https://doi.org/10.3390/v12101129






