Parenterally Administered Porcine Epidemic Diarrhea Virus-Like Particle-Based Vaccine Formulated with CCL25/28 Chemokines Induces Systemic and Mucosal Immune Protectivity in Pigs
Abstract
1. Introduction
2. Materials and Methods
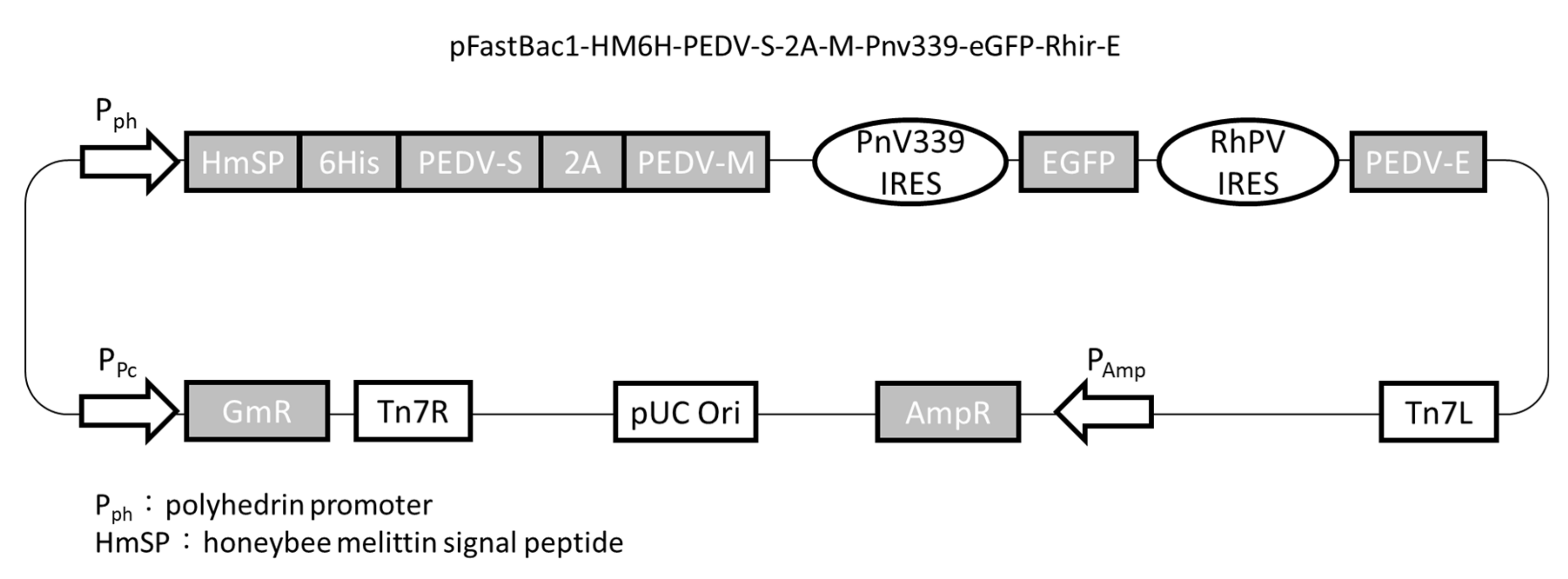
2.1. Plasmid Construction
2.2. Generation of VLPs
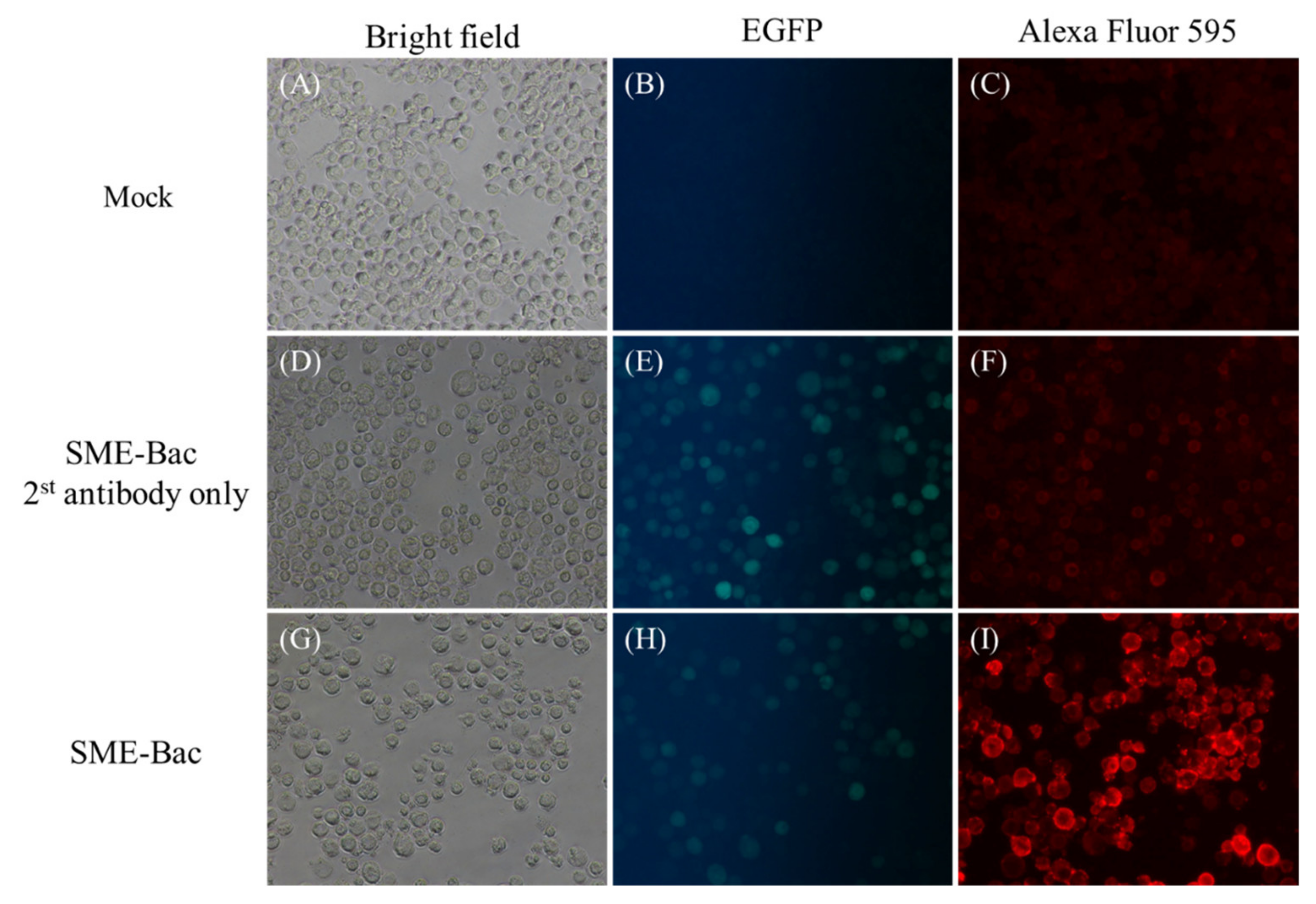
2.3. Indirect Fluorescent Antibody Test
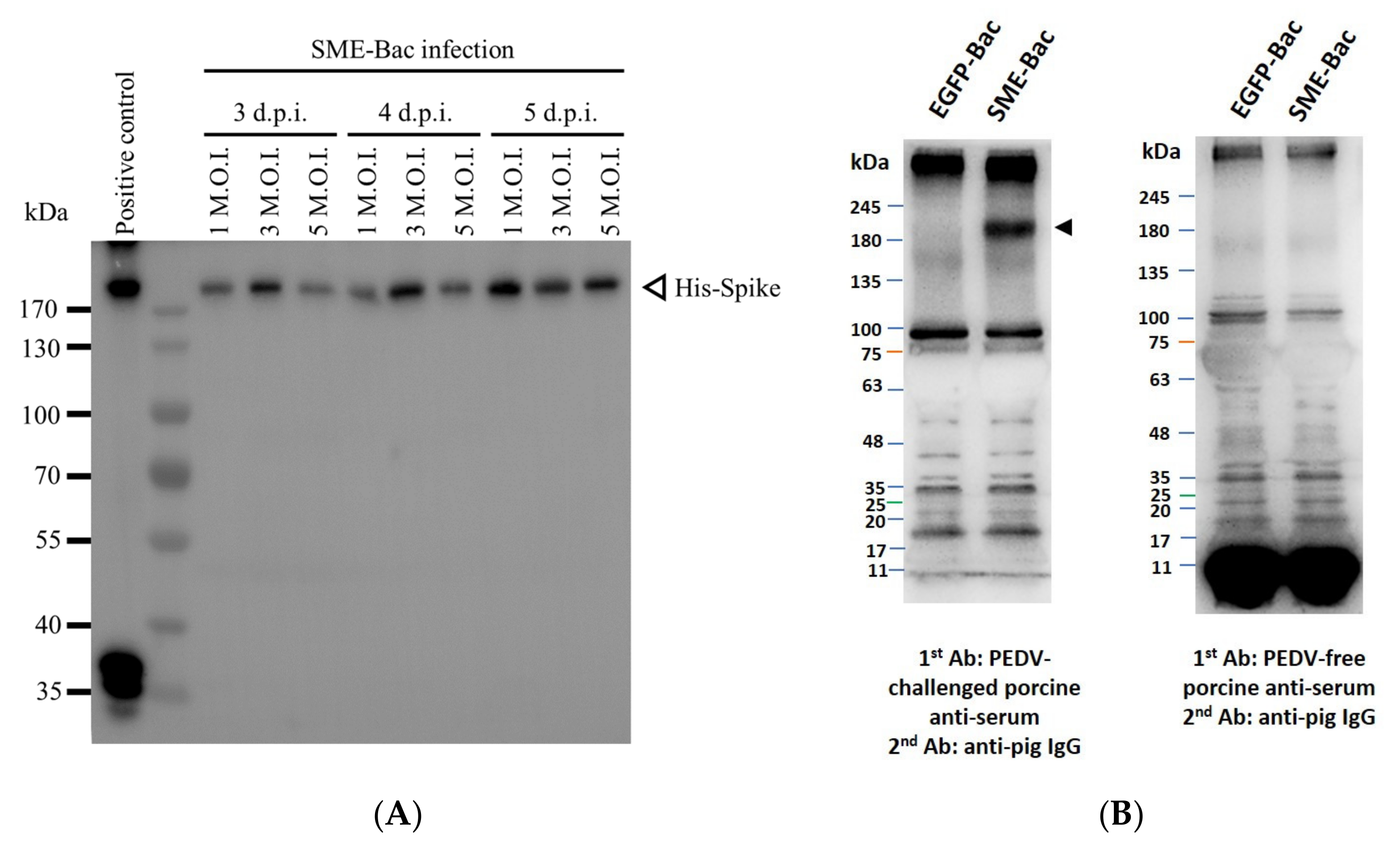
2.4. Western Blotting
2.5. Characterization of VLPs Using Electron Microscopy
2.6. Expression and Purification of CC Chemokines
2.7. Cell Lines and Viruses
2.8. Immunization Program of Pigs
2.9. Evaluation of Systemic IgG and Mucosal IgA Levels
2.10. Neutralizing Antibody Assay
2.11. Isolation of Peripheral Blood Mononuclear Cells
2.12. Enzyme-Linked Immunospot Assay of PEDV S-Specific IFN-γ
2.13. Stool Consistency Scoring and Body Weight Measurement
2.14. RNA Extraction, Complementary DNA Synthesis, and Probe-Based Quantitative Real-Time PCR
2.15. Statistical Analysis
3. Results
3.1. Preparation and Characterization of PEDV VLPs Expressed Using Recombinant Baculovirus, SME-Bac
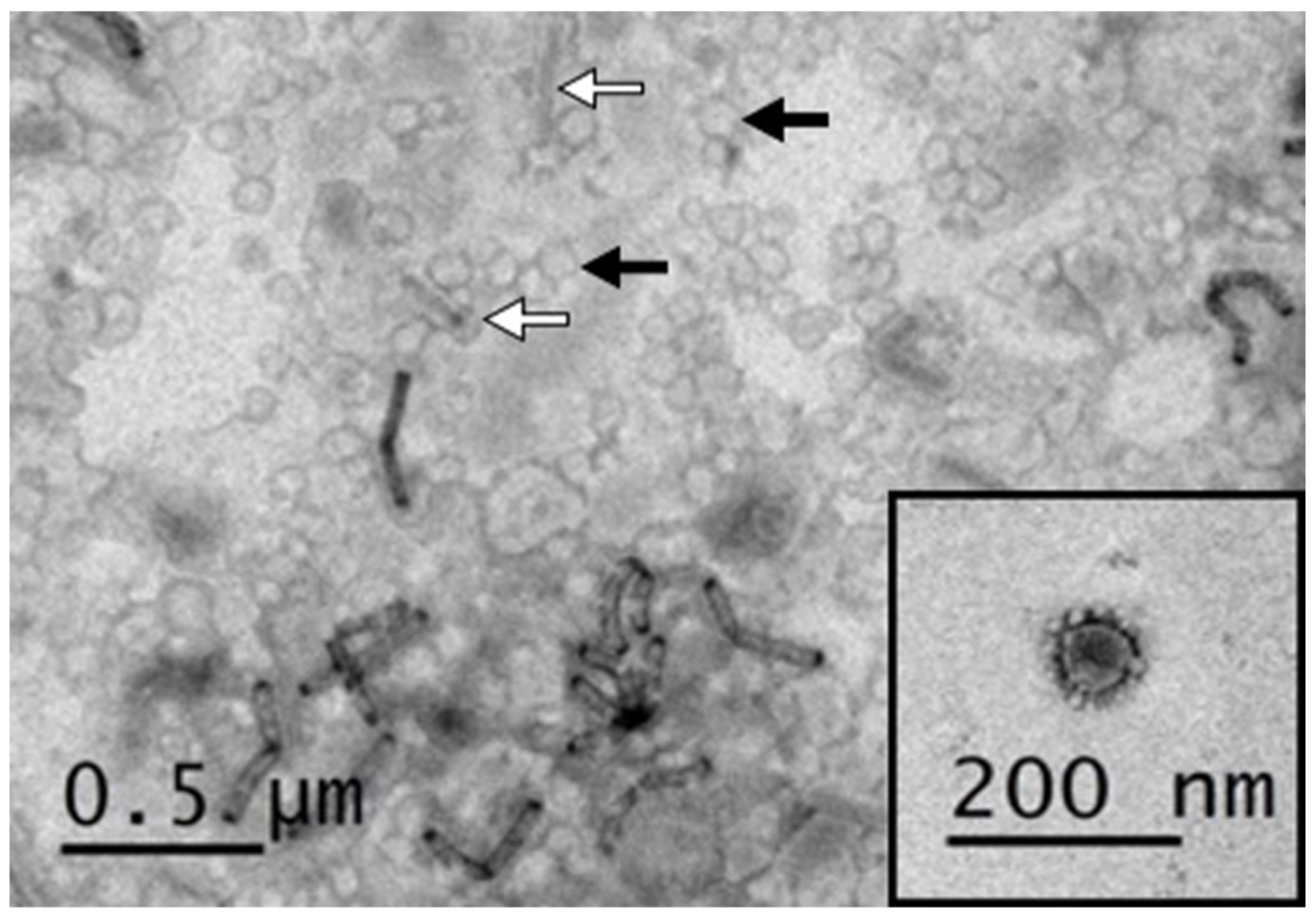
3.2. Negative Staining Electron Microscopy of PEDV VLPs
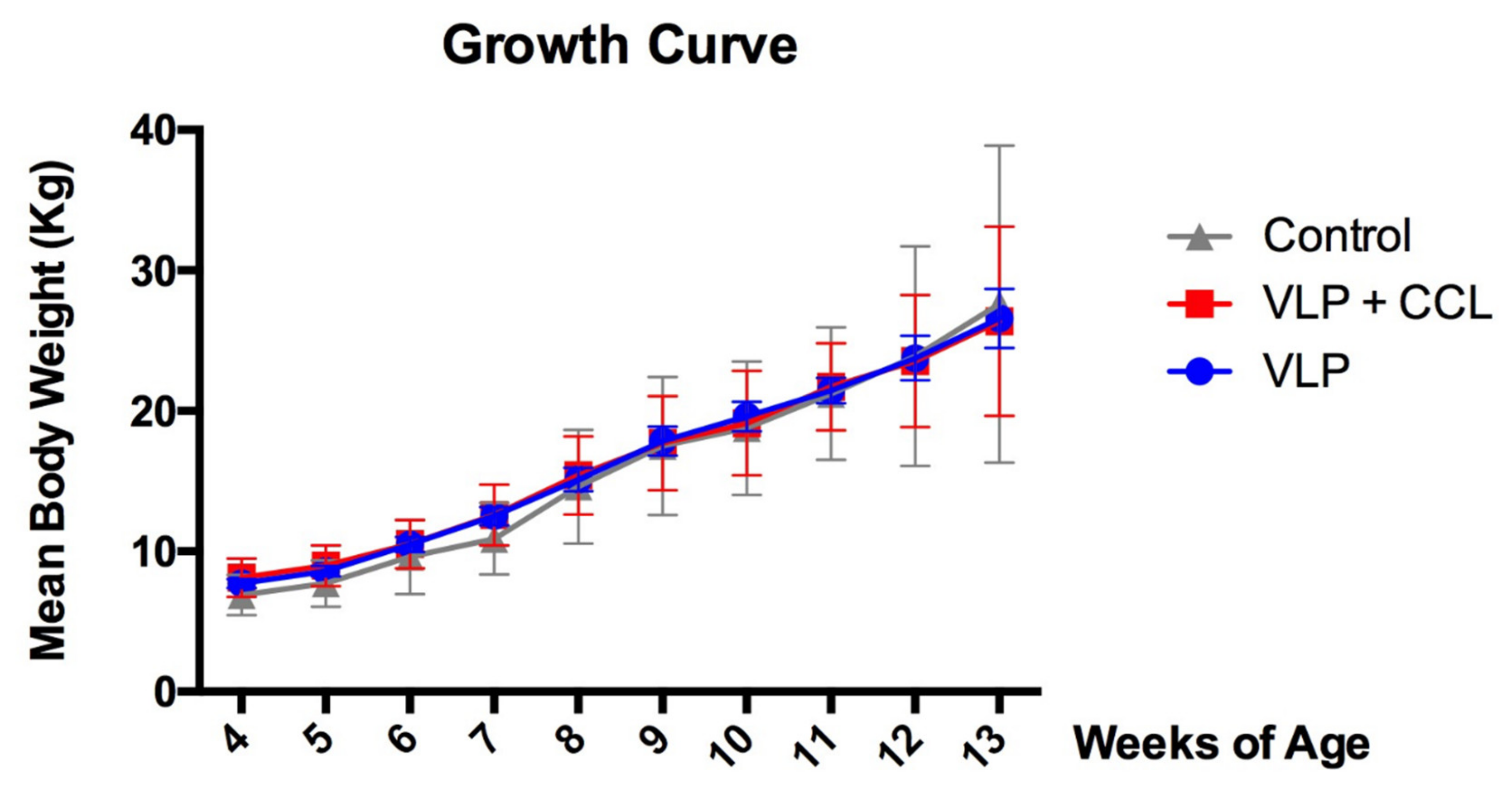
3.3. Changes in Body Weight
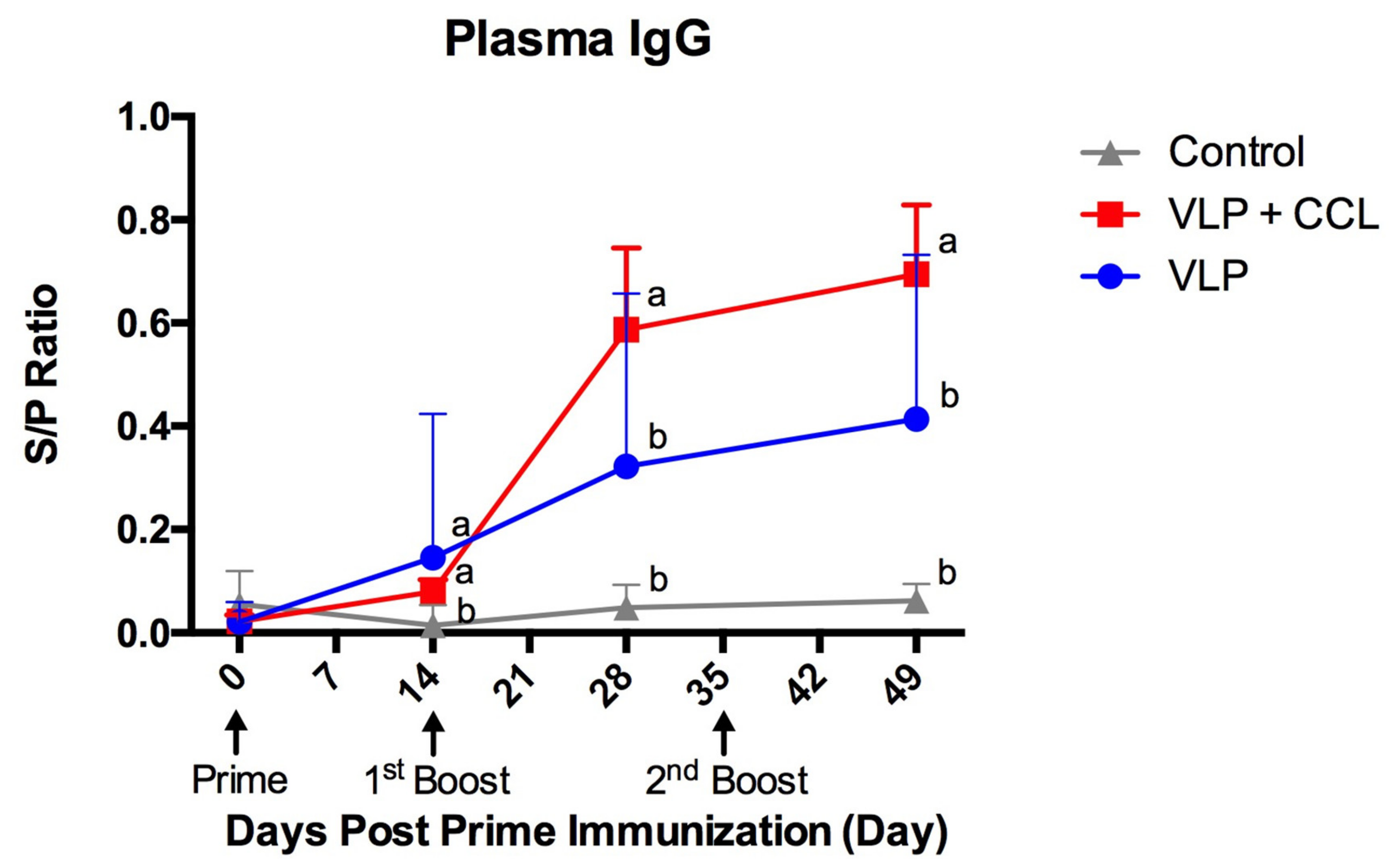
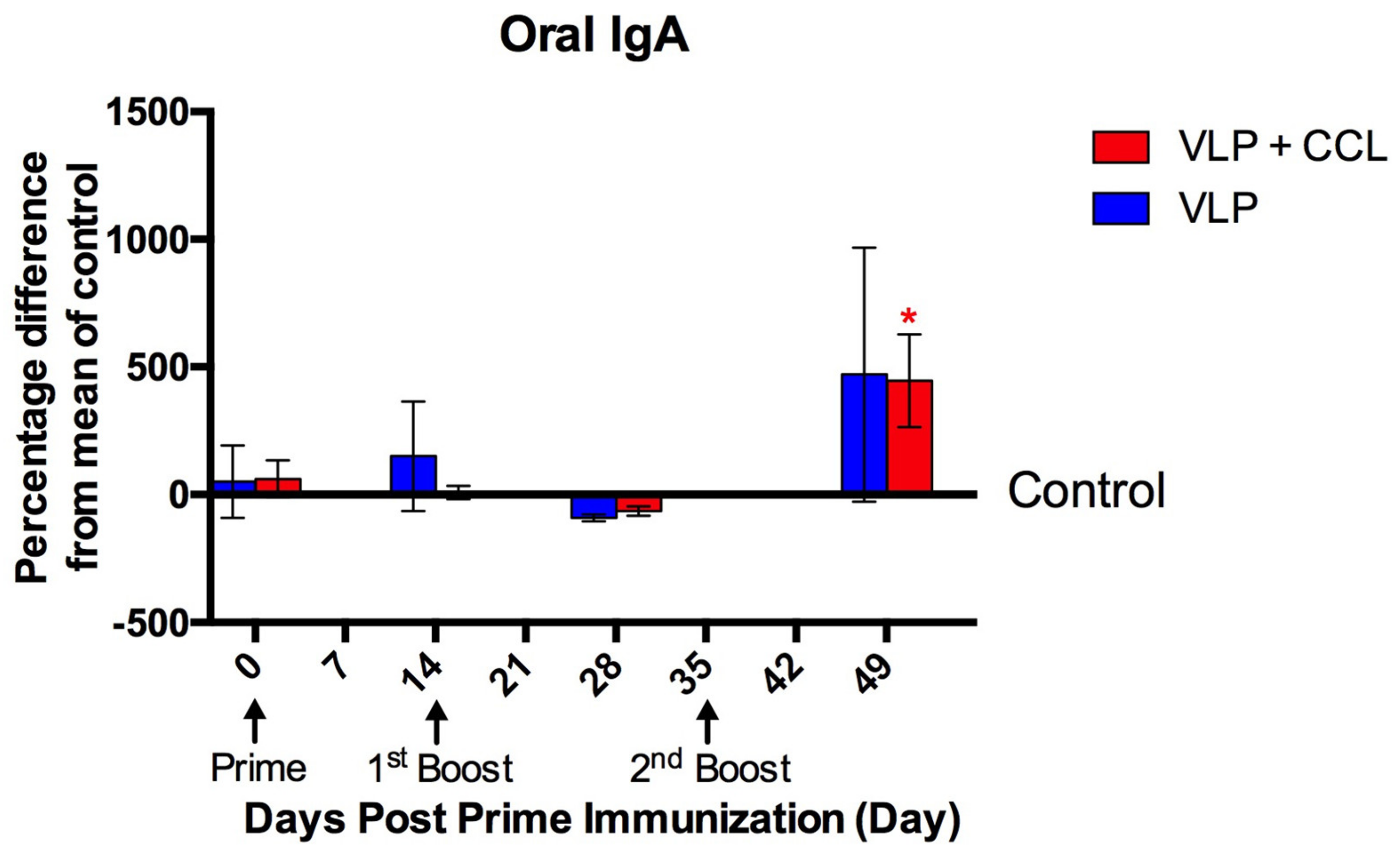
3.4. Detection of Systemic and Mucosal S-Specific Antibody Titers
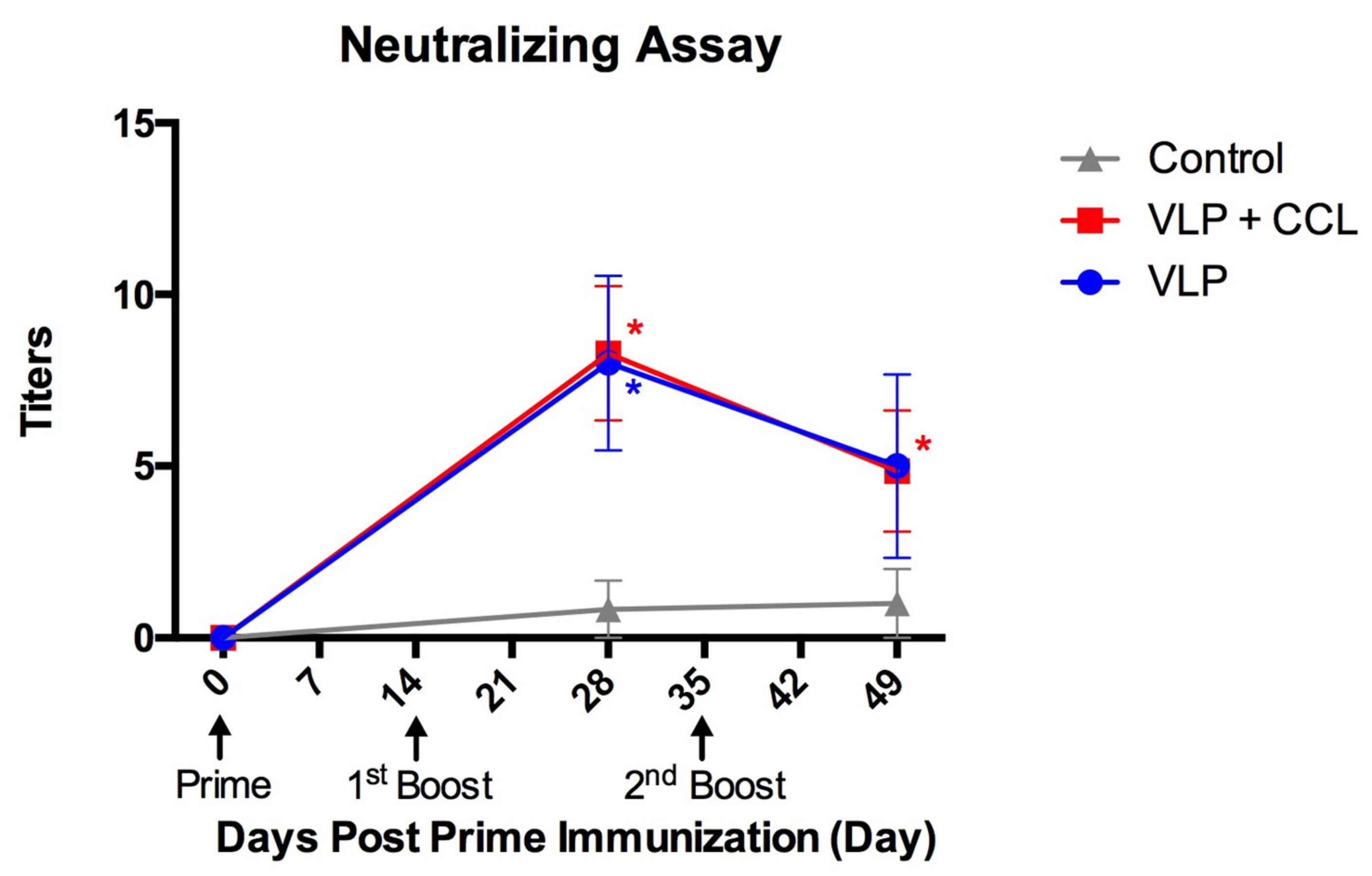
3.5. Evaluation of Neutralizing Antibody Titers in the Blood
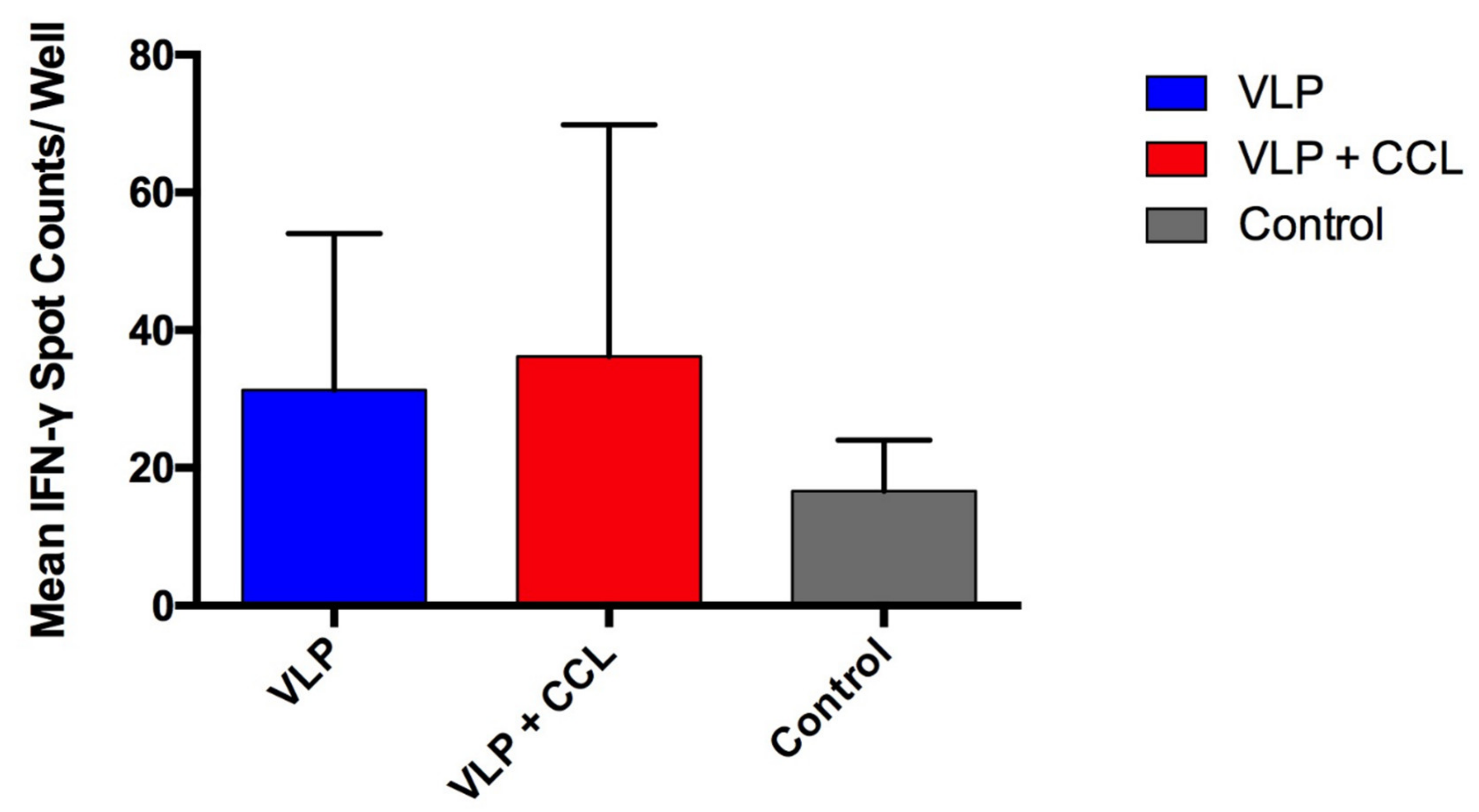
3.6. Assessment of S-Specific Interferon-γ-Secreting Cells in the PBMCs
3.7. Evaluation of the Protection Provided by the VLP Adjuvant with/without CCL25 and CCL28 against Virulent PEDV Challenge
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lee, C. Porcine Epidemic Diarrhea Virus: An Emerging and Re-Emerging Epizootic Swine Virus. Virol. J. 2015, 12. [Google Scholar] [CrossRef]
- Li, W.; van Kuppeveld, F.J.M.; He, Q.; Rottier, P.J.M.; Bosch, B.J. Cellular Entry of the Porcine Epidemic Diarrhea Virus. Virus Res. 2016, 226. [Google Scholar] [CrossRef] [PubMed]
- Nam, E.; Lee, C. Contribution of the Porcine Aminopeptidase N (CD13) Receptor Density to Porcine Epidemic Diarrhea Virus Infection. Vet. Microbiol. 2010, 144. [Google Scholar] [CrossRef] [PubMed]
- Costantini, V.; Lewis, P.; Alsop, J.; Templeton, C.; Saif, L.J. Respiratory and Fecal Shedding of Porcine Respiratory Coronavirus (PRCV) in Sentinel Weaned Pigs and Sequence of the Partial S-Gene of the PRCV Isolates. Arch. Virol. 2004, 149. [Google Scholar] [CrossRef] [PubMed]
- Masters, P.S. The Molecular Biology of Coronaviruses. Adv. Virus Res. 2006, 66. [Google Scholar] [CrossRef]
- Venkatagopalan, P.; Daskalova, S.M.; Lopez, L.A.; Dolezal, K.A.; Hogue, B.G. Coronavirus Envelope (E) Protein Remains at the Site of Assembly. Virology 2015, 478. [Google Scholar] [CrossRef] [PubMed]
- Debouck, P.; Pensaert, M.; Coussement, W. The Pathogenesis of an Enteric Infection in Pigs, Experimentally Induced by the Coronavirus-like Agent, CV 777. Vet. Microbiol. 1981, 6. [Google Scholar] [CrossRef]
- Li, B.X.; Ge, J.W.; Li, Y.J. Porcine Aminopeptidase N Is a Functional Receptor for the PEDV Coronavirus. Virology 2007, 365. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J. Porcine Epidemic Diarrhea Virus Infection: Etiology, Epidemiology, Pathogenesis and Immunoprophylaxis. Vet. J. 2015, 204. [Google Scholar] [CrossRef]
- Shibata, I.; Tsuda, T.; Mori, M.; Ono, M.; Sueyoshi, M.; Uruno, K. Isolation of Porcine Epidemic Diarrhea Virus in Porcine Cell Cultures and Experimental Infection of Pigs of Different Ages. Vet. Microbiol. 2000, 72. [Google Scholar] [CrossRef]
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J.; et al. Emergence of Porcine Epidemic Diarrhea Virus in the United States: Clinical Signs, Lesions, and Viral Genomic Sequences. J. Vet. Diagn. Investig. 2013, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, H.; Liu, Y.; Pan, Y.; Deng, F.; Song, Y.; Tang, X.; He, Q. New Variants of Porcine Epidemic Diarrhea Virus, China, 2011. Emerg. Infect. Dis. 2012, 18. [Google Scholar] [CrossRef]
- Song, D.; Park, B. Porcine Epidemic Diarrhoea Virus: A Comprehensive Review of Molecular Epidemiology, Diagnosis, and Vaccines. Virus Genes 2012, 44. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Moon, H.; Kang, B. Porcine Epidemic Diarrhea: A Review of Current Epidemiology and Available Vaccines. Clin. Exp. Vaccine Res. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Shi, D.; Shi, H.; Zhang, X.; Yuan, J.; Jiang, S.; Feng, L. Immunogenicity and Antigenic Relationships among Spike Proteins of Porcine Epidemic Diarrhea Virus Subtypes G1 and G2. Arch. Virol. 2016, 161. [Google Scholar] [CrossRef]
- Makadiya, N.; Brownlie, R.; Van Den Hurk, J.; Berube, N.; Allan, B.; Gerdts, V.; Zakhartchouk, A. S1 Domain of the Porcine Epidemic Diarrhea Virus Spike Protein as a Vaccine Antigen. Virol. J. 2016, 13. [Google Scholar] [CrossRef]
- Khamis, Z.; Menassa, R. Porcine Epidemic Diarrhea Virus. In Prospects of Plant-Based Vaccines in Veterinary Medicine; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 255–266. ISBN 9783319901374. [Google Scholar]
- Crawford, K.; Lager, K.M.; Kulshreshtha, V.; Miller, L.C.; Faaberg, K.S. Status of Vaccines for Porcine Epidemic Diarrhea Virus in the United States and Canada. Virus Res. 2016, 226. [Google Scholar] [CrossRef]
- Kao, C.F.; Chiou, H.Y.; Chang, Y.C.; Hsueh, C.S.; Jeng, C.R.; Tsai, P.S.; Cheng, I.C.; Pang, V.F.; Chang, H.W. The Characterization of Immunoprotection Induced by a Cdna Clone Derived from the Attenuated Taiwan Porcine Epidemic Diarrhea Virus Pintung 52 Strain. Viruses 2018, 10, 543. [Google Scholar] [CrossRef]
- Chang, Y.C.; Kao, C.F.; Chang, C.Y.; Jeng, C.R.; Tsai, P.S.; Pang, V.F.; Chiou, H.Y.; Peng, J.Y.; Cheng, I.C.; Chang, H.W. Evaluation and Comparison of the Pathogenicity and Host Immune Responses Induced by a G2b Taiwan Porcine Epidemic Diarrhea Virus (Strain Pintung 52) and Its Highly Cell-Culture Passaged Strain in Conventional 5-Week-Old Pigs. Viruses 2017, 9, 121. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chang, C.Y.; Tsai, P.S.; Chiou, H.Y.; Jeng, C.R.; Pang, V.F.; Chang, H.W. Efficacy of Heat-Labile Enterotoxin B Subunit-Adjuvanted Parenteral Porcine Epidemic Diarrhea Virus Trimeric Spike Subunit Vaccine in Piglets. Appl. Microbiol. Biotechnol. 2018, 102. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsu, W.T.; Chao, Y.C.; Chang, H.W. Display of Porcine Epidemic Diarrhea Virus Spike Protein on Baculovirus to Improve Immunogenicity and Protective Efficacy. Viruses 2018, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Amanna, I.J.; Slifka, M.K. Wanted, Dead or Alive: New Viral Vaccines. Antiviral Res. 2009, 84. [Google Scholar] [CrossRef] [PubMed]
- Gerdts, V.; Zakhartchouk, A. Vaccines for Porcine Epidemic Diarrhea Virus and Other Swine Coronaviruses. Vet. Microbiol. 2017, 206. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.M.C. Recombination in Large RNA Viruses: Coronaviruses. Semin. Virol. 1996, 7. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Gomes, A.C.; Cabral-Miranda, G.; Krueger, C.C.; Leoratti, F.M.; Stein, J.V.; Bachmann, M.F. Delivering Adjuvants and Antigens in Separate Nanoparticles Eliminates the Need of Physical Linkage for Effective Vaccination. J. Control. Release 2017, 251. [Google Scholar] [CrossRef]
- Ahsan, F.; Rivas, I.P.; Khan, M.A.; Torres Suárez, A.I. Targeting to Macrophages: Role of Physicochemical Properties of Particulate Carriers—Liposomes and Microspheres—On the Phagocytosis by Macrophages. J. Control. Release 2002, 79. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic Cells and the Control of Immunity. Nature 1998, 392. [Google Scholar] [CrossRef]
- Den Haan, J.M.M.; Lehar, S.M.; Bevan, M.J. CD8+ but Not CD8- Dendritic Cells Cross-Prime Cytotoxic T Cells in Vivo. J. Exp. Med. 2000, 192. [Google Scholar] [CrossRef]
- Dudziak, D.; Trumpfheller, C.; Yamazaki, S.; Cheong, C.; Liu, K.; Lee, H. Differential Antigen Processing by Dendritic Cell Subsets in Vivo. Science 2007. [Google Scholar] [CrossRef]
- Ohteki, B.T.; Fukao, T.; Suzue, K.; Maki, C. Interleukin 12-Dependent Interferon Gamma Production by CD8alpha+ Lymphoid Dendritic Cells. J. Exp. Med. 1999, 189. [Google Scholar] [CrossRef] [PubMed]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles Target Distinct Dendritic Cell Populations According to Their Size. Eur. J. Immunol. 2008, 38. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Teng, C.-Y.; Villaflores, O.B.; Chen, Y.-J.; Liu, M.-K.; Chan, H.-L.; Jinn, T.-R.; Wu, T.-Y. Using Internal Ribosome Entry Sites to Facilitate Engineering of Insect Cells and Used in Secretion Proteins Production. J. Taiwan Inst. Chem. Eng. 2017, 71. [Google Scholar] [CrossRef]
- Song, D.S.; Oh, J.S.; Kang, B.K.; Yang, J.S.; Moon, H.J.; Yoo, H.S.; Jang, Y.S.; Park, B.K. Oral Efficacy of Vero Cell Attenuated Porcine Epidemic Diarrhea Virus DR13 Strain. Res. Vet. Sci. 2007, 82. [Google Scholar] [CrossRef]
- Meurens, F.; Berri, M.; Whale, J.; Dybvig, T.; Strom, S.; Thompson, D.; Brownlie, R.; Townsend, H.G.G.; Salmon, H.; Gerdts, V. Expression of TECK/CCL25 and MEC/CCL28 Chemokines and Their Respective Receptors CCR9 and CCR10 in Porcine Mucosal Tissues. Vet. Immunol. Immunopathol. 2006, 113. [Google Scholar] [CrossRef]
- Wang, C.; Yan, F.; Zheng, X.; Wang, H.; Jin, H.; Wang, C.; Zhao, Y.; Feng, N.; Wang, T.; Gao, Y.; et al. Porcine Epidemic Diarrhea Virus Virus-like Particles Produced in Insect Cells Induce Specific Immune Responses in Mice. Virus Genes 2017, 53. [Google Scholar] [CrossRef]
- Chattha, K.S.; Roth, J.A.; Saif, L.J. Strategies for Design and Application of Enteric Viral Vaccines. Annu. Rev. Anim. Biosci. 2015, 3. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Johansen, F.E. Mucosal B Cells: Phenotypic Characteristics, Transcriptional Regulation, and Homing Properties. Immunol. Rev. 2005, 206. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Wang, S. Effect of Vaccine Administration Modality on Immunogenicity and Efficacy. Expert Rev. Vaccines 2015, 14. [Google Scholar] [CrossRef]
- Yuan, L.; Kang, S.-Y.; Ward, L.A.; To, T.L.; Saif, L.J. Antibody-Secreting Cell Responses and Protective Immunity Assessed in Gnotobiotic Pigs Inoculated Orally or Intramuscularly with Inactivated Human Rotavirus. J. Virol. 1998, 72. [Google Scholar] [CrossRef]
- Aldon, Y.; Kratochvil, S.; Shattock, R.J.; McKay, P.F. Chemokine-Adjuvanted Plasmid DNA Induces Homing of Antigen-Specific and Non–Antigen-Specific B and T Cells to the Intestinal and Genital Mucosae. J. Immunol. 2020, 204. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A.; Wise, M.C.; Hutnick, N.A.; Moldoveanu, Z.; Hunter, M.; Reuter, M.A.; Yuan, S.; Yan, J.; Ginsberg, A.A.; Sylvester, A.; et al. Chemokine-Adjuvanted Electroporated DNA Vaccine Induces Substantial Protection from Simian Immunodeficiency Virus Vaginal Challenge. Mucosal Immunol. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Rainone, V.; Dubois, G.; Temchura, V.; Überla, K.; Clivio, A.; Nebuloni, M.; Lauri, E.; Trabattoni, D.; Veas, F.; Clerici, M. CCL28 Induces Mucosal Homing of HIV-1-Specific IgA-Secreting Plasma Cells in Mice Immunized with HIV-1 Virus-like Particles. PLoS ONE 2011, 6, e26979. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Jaimes, M.C.; Lazarus, N.H.; Monak, D.; Zhang, C.; Butcher, E.C.; Greenberg, H.B. Redundant Role of Chemokines CCL25/TECK and CCL28/MEC in IgA + Plasmablast Recruitment to the Intestinal Lamina Propria after Rotavirus Infection. J. Immunol. 2006, 176. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, F.C.; Chang, Y.C.; Kao, C.F.; Hsu, C.W.; Chang, H.W. Intramuscular Immunization with Chemokine-Adjuvanted Inactive Porcine Epidemic Diarrhea Virus Induces Substantial Protection in Pigs. Vaccines 2020, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-W.; Wu, T.-Y. Development of Flu Vaccine by a Novel Polycistronic Baculovirus Expression Vector; Chung Yuan Christian University: Taoyuan City, Taiwan, 2010. [Google Scholar]
- Chang, C.-Y.; Peng, J.-Y.; Cheng, Y.-H.; Chang, Y.-C.; Wu, Y.-T.; Tsai, P.-S.; Chiou, H.-Y.; Jeng, C.-R.; Chang, H.-W. Development and Comparison of Enzyme-Linked Immunosorbent Assays Based on Recombinant Trimeric Full-Length and Truncated Spike Proteins for Detecting Antibodies against Porcine Epidemic Diarrhea Virus. BMC Vet. Res. 2019, 15. [Google Scholar] [CrossRef]
- Jung, K.; Wang, Q.; Scheuer, K.A.; Lu, Z.; Zhang, Y.; Saif, L.J. Pathology of US Porcine Epidemic Diarrhea Virus Strain PC21A in Gnotobiotic Pigs. Emerg. Infect. Dis. 2014, 20. [Google Scholar] [CrossRef]
- Muth, C.; Bales, K.L.; Hinde, K.; Maninger, N.; Mendoza, S.P.; Ferrer, E. Alternative Models for Small Samples in Psychological Research: Applying Linear Mixed Effects Models and Generalized Estimating Equations to Repeated Measures Data. Educ. Psychol. Meas. 2016, 76. [Google Scholar] [CrossRef]
- Maxwell, S.E.; Delaney, H.D.; Kelley, K. Designing Experiments and Analyzing Data: A Model Comparison Perspective, 3rd ed.; Routledge: New York, NY, USA, 2018; ISBN 978-1-315-16978-1. [Google Scholar]
- Ma, Y.; Mazumdar, M.; Memtsoudis, S.G. Beyond Repeated-Measures Analysis of Variance: Advanced Statistical Methods for the Analysis of Longitudinal Data in Anesthesia Research. Reg. Anesth. Pain Med. 2012, 37. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, J.; Ahn, C. A GEE Approach to Determine Sample Size for Pre- and Post-Intervention Experiments with Dropout. Comput. Stat. Data Anal. 2014, 69. [Google Scholar] [CrossRef]
- Wang, M.; Kong, L.; Li, Z.; Zhang, L. Covariance Estimators for Generalized Estimating Equations (GEE) in Longitudinal Analysis with Small Samples. Stat. Med. 2016, 35. [Google Scholar] [CrossRef]
- Abe, T.; Takahashi, H.; Hamazaki, H.; Miyano-Kurosaki, N.; Matsuura, Y.; Takaku, H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 2003, 171, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Heinimäki, S.; Tamminen, K.; Malm, M.; Vesikari, T.; Blazevic, V. Live baculovirus acts as a strong B and T cell adjuvant for monomeric and oligomeric protein antigens. Virology 2017, 511, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Chang, M.O.; Kitajima, M.; Takaku, H. Baculovirus activates murine dendritic cells and induces non-specific NK cell and T cell immune responses. Cell. Immunol. 2010, 262, 35–43. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lo, C.F.; Huang, C.J.; Yu, H.T.; Wang, C.H. The complete genome sequence of Perina nuda picorna-like virus, an insect-infecting RNA virus with a genome organization similar to that of the mammalian picornaviruses. Virology 2002, 294, 312–323. [Google Scholar] [CrossRef]
- Altmann, F.; Staudacher, E.; Wilson, I.B.; Marz, L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj. J. 1999, 16, 109–123. [Google Scholar] [CrossRef]
- Jarvis, D.L. Developing baculovirus-insect cell expression systems for humanized recombinant glycoprotein production. Virology 2003, 310, 1–7. [Google Scholar] [CrossRef]
- Gimenez-Lirola, L.G.; Zhang, J.; Carrillo-Avila, J.A.; Chen, Q.; Magtoto, R.; Poonsuk, K.; Baum, D.H.; Pineyro, P.; Zimmerman, J. Reactivity of Porcine Epidemic Diarrhea Virus Structural Proteins to Antibodies against Porcine Enteric Coronaviruses: Diagnostic Implications. J. Clin. Microbiol. 2017, 55, 1426–1436. [Google Scholar] [CrossRef]
- Slifka, M.K.; Amanna, I. How Advances in Immunology Provide Insight into Improving Vaccine Efficacy. Vaccine 2014, 32. [Google Scholar] [CrossRef]
- Langel, S.N.; Paim, F.C.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Lactogenic Immunity and Vaccines for Porcine Epidemic Diarrhea Virus (PEDV): Historical and Current Concepts. Virus Res. 2016, 226. [Google Scholar] [CrossRef]
- Id, V.D.K.; Kim, Y.; Yang, M.; Vannucci, F.; Molitor, T.; Cheeran, M.C. Immune Responses to Porcine Epidemic Diarrhea Virus (PEDV) in Swine and Protection against Subsequent Infection. PLoS ONE 2020, 15, e0231723. [Google Scholar] [CrossRef]
- Chang, C.Y.; Cheng, I.C.; Chang, Y.C.; Tsai, P.S.; Lai, S.Y.; Huang, Y.L.; Jeng, C.R.; Pang, V.F.; Chang, H.W. Identification of Neutralizing Monoclonal Antibodies Targeting Novel Conformational Epitopes of the Porcine Epidemic Diarrhoea Virus Spike Protein. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Hsu, W.T.; Tsai, P.S.; Chen, C.M.; Cheng, I.C.; Chao, Y.C.; Chang, H.W. Oral Administration of Porcine Epidemic Diarrhea Virus Spike Protein Expressing in Silkworm Pupae Failed to Elicit Immune Responses in Pigs. AMB Express 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, K.W.; Choi, H.W.; Lee, C. Immunogenicity and Protective Efficacy of Recombinant S1 Domain of the Porcine Epidemic Diarrhea Virus Spike Protein. Arch. Virol. 2014, 159. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Yugo, D.M.; Heffron, C.L.; Rogers, A.J.; Sooryanarain, H.; LeRoith, T.; Overend, C.; Cao, D.; Meng, X.J. Vaccination of Sows with a Dendritic Cell-Targeted Porcine Epidemic Diarrhea Virus S1 Protein-Based Candidate Vaccine Reduced Viral Shedding but Exacerbated Gross Pathological Lesions in Suckling Neonatal Piglets. J. Gen. Virol. 2018, 99. [Google Scholar] [CrossRef] [PubMed]
- Zeltins, A. Construction and Characterization of Virus-like Particles: A Review. Mol. Biotechnol. 2013, 53. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like Particles as a Highly Efficient Vaccine Platform: Diversity of Targets and Production Systems and Advances in Clinical Development. Vaccine 2012, 31. [Google Scholar] [CrossRef]
- Buonaguro, L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M. Developments in Virus-like Particle-Based Vaccines for Infectious Diseases and Cancer. Expert Rev. Vaccines 2011, 10. [Google Scholar] [CrossRef]
- Hieshima, K.; Kawasaki, Y.; Hanamoto, H.; Nakayama, T.; Nagakubo, D.; Kanamaru, A.; Yoshie, O. CC Chemokine Ligands 25 and 28 Play Essential Roles in Intestinal Extravasation of IgA Antibody-Secreting Cells. J. Immunol. 2004, 173. [Google Scholar] [CrossRef]
- Kathuria, N.; Kraynyak, K.A.; Carnathan, D.; Betts, M.; Weiner, D.B.; Kutzler, M.A. Generation of Antigen-Specific Immunity Following Systemic Immunization with DNA Vaccine Encoding CCL25 Chemokine Immunoadjuvant. Hum. Vaccines Immunother. 2012, 8. [Google Scholar] [CrossRef]
- Mohan, T.; Deng, L.; Wang, B. CCL28 Chemokine: An Anchoring Point Bridging Innate and Adaptive Immunity. Int. Immunopharmacol. 2017, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Z.; Xu, Z.; Wu, X.; Zhang, D.; Zhang, Z.; Wei, J. The Role of Chemokine Receptor 9/Chemokine Ligand 25 Signaling: From Immune Cells to Cancer Cells (Review). Oncol. Lett. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, H.; Xu, S.; Shi, L.; Dong, J.; Gao, D.; Chen, Y.; Feng, H. Membrane-Anchored CCL20 Augments HIV Env-Specific Mucosal Immune Responses. Virol. J. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Berman, Z.; Luo, Y.; Wang, C.; Wang, S.; Compans, R.W.; Wang, B.Z. Chimeric Virus-like Particles Containing Influenza HA Antigen and GPI-CCL28 Induce Long-Lasting Mucosal Immunity against H3N2 Viruses. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef]
- Ejemel, M.; Li, Q.; Hou, S.; Schiller, Z.A.; Tree, J.A.; Wallace, A.; Amcheslavsky, A.; Kurt Yilmaz, N.; Buttigieg, K.R.; Elmore, M.J.; et al. A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction. Nat. Commun. 2020, 11, 4198. [Google Scholar] [CrossRef]
- Mateus, J.; Grifoni, A.; Tarke, A.; Sidney, J.; Ramirez, S.I.; Dan, J.M.; Burger, Z.C.; Rawlings, S.A.; Smith, D.M.; Phillips, E.; et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020, 370. [Google Scholar] [CrossRef]
- Choudhury, B.; Dastjerdi, A.; Doyle, N.; Frossard, J.P.; Steinbach, F. From the field to the lab—An European view on the global spread of PEDV. Virus Res. 2016, 226, 40–49. [Google Scholar] [CrossRef]
- Lin, C.-M.; Gao, X.; Oka, T.; Vlasova, A.N.; Esseili, M.A.; Wang, Q.; Saif, L.J. Antigenic relationships among porcine epidemic diarrhea virus and transmissible gastroenteritis virus strains. J. Virol. 2015, 89, 3332–3342. [Google Scholar] [CrossRef]


| Group | Immunogen | Adjuvant | |
|---|---|---|---|
| CC Chemokine | Freund’s Adjuvant * | ||
| Control | None | None | Yes |
| VLP | 1.8 mg of VLP (0.2 µg S protein) | None | Yes |
| VLP + CCL25/28 | 1.8 mg of VLP (0.2 µg S protein) | 30 µg CCL25 and 30 µg CCL28 | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-W.; Chang, M.-H.; Chang, H.-W.; Wu, T.-Y.; Chang, Y.-C. Parenterally Administered Porcine Epidemic Diarrhea Virus-Like Particle-Based Vaccine Formulated with CCL25/28 Chemokines Induces Systemic and Mucosal Immune Protectivity in Pigs. Viruses 2020, 12, 1122. https://doi.org/10.3390/v12101122
Hsu C-W, Chang M-H, Chang H-W, Wu T-Y, Chang Y-C. Parenterally Administered Porcine Epidemic Diarrhea Virus-Like Particle-Based Vaccine Formulated with CCL25/28 Chemokines Induces Systemic and Mucosal Immune Protectivity in Pigs. Viruses. 2020; 12(10):1122. https://doi.org/10.3390/v12101122
Chicago/Turabian StyleHsu, Chin-Wei, Ming-Hao Chang, Hui-Wen Chang, Tzong-Yuan Wu, and Yen-Chen Chang. 2020. "Parenterally Administered Porcine Epidemic Diarrhea Virus-Like Particle-Based Vaccine Formulated with CCL25/28 Chemokines Induces Systemic and Mucosal Immune Protectivity in Pigs" Viruses 12, no. 10: 1122. https://doi.org/10.3390/v12101122
APA StyleHsu, C.-W., Chang, M.-H., Chang, H.-W., Wu, T.-Y., & Chang, Y.-C. (2020). Parenterally Administered Porcine Epidemic Diarrhea Virus-Like Particle-Based Vaccine Formulated with CCL25/28 Chemokines Induces Systemic and Mucosal Immune Protectivity in Pigs. Viruses, 12(10), 1122. https://doi.org/10.3390/v12101122






