Identification of an Immunosuppressive Cell Population during Classical Swine Fever Virus Infection and Its Role in Viral Persistence in the Host
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Experimental Infection
2.3. CSFV RNA and Antibody Detection
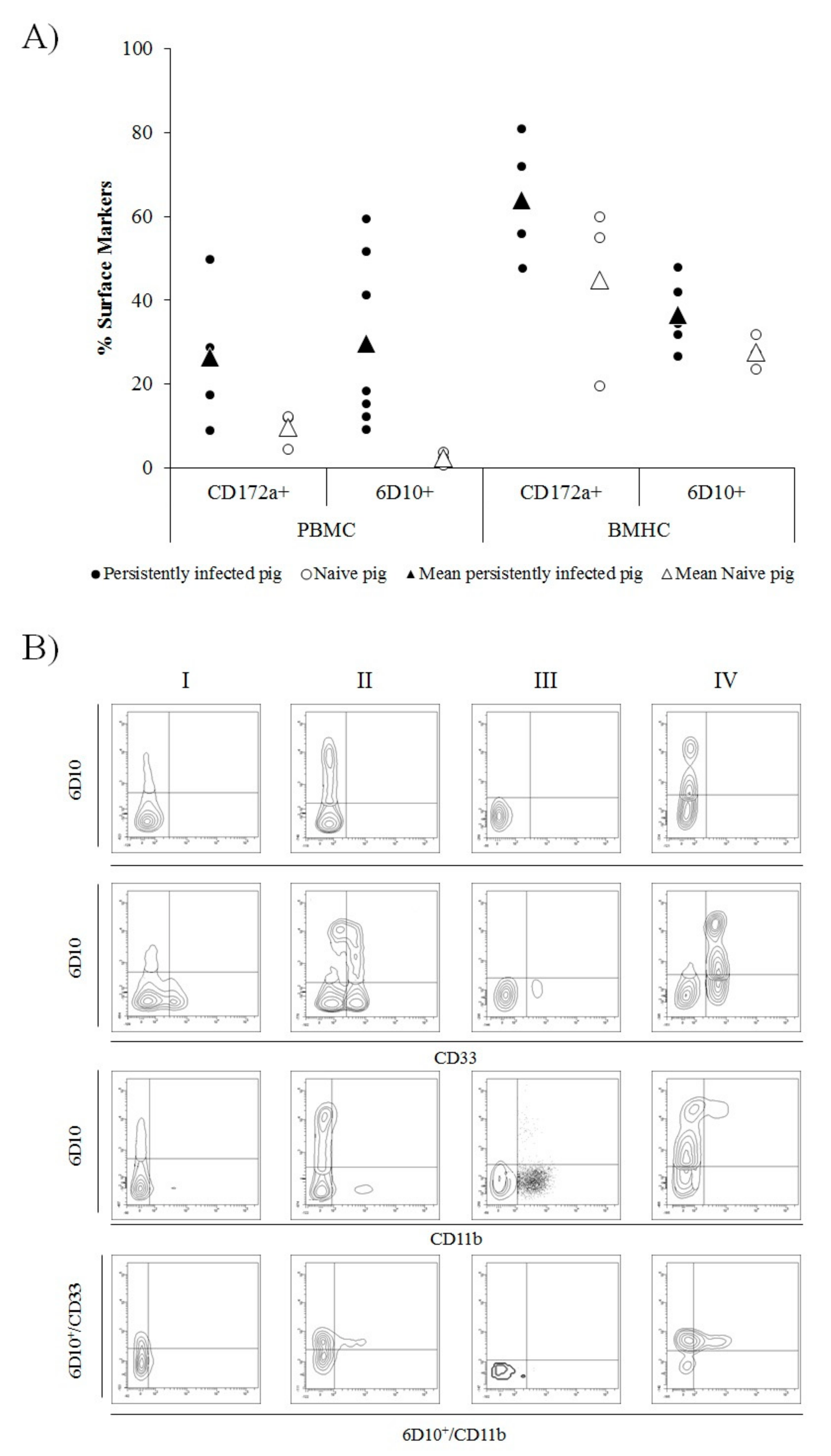
2.4. PBMC and BMHC Collection and Phenotypical Analysis
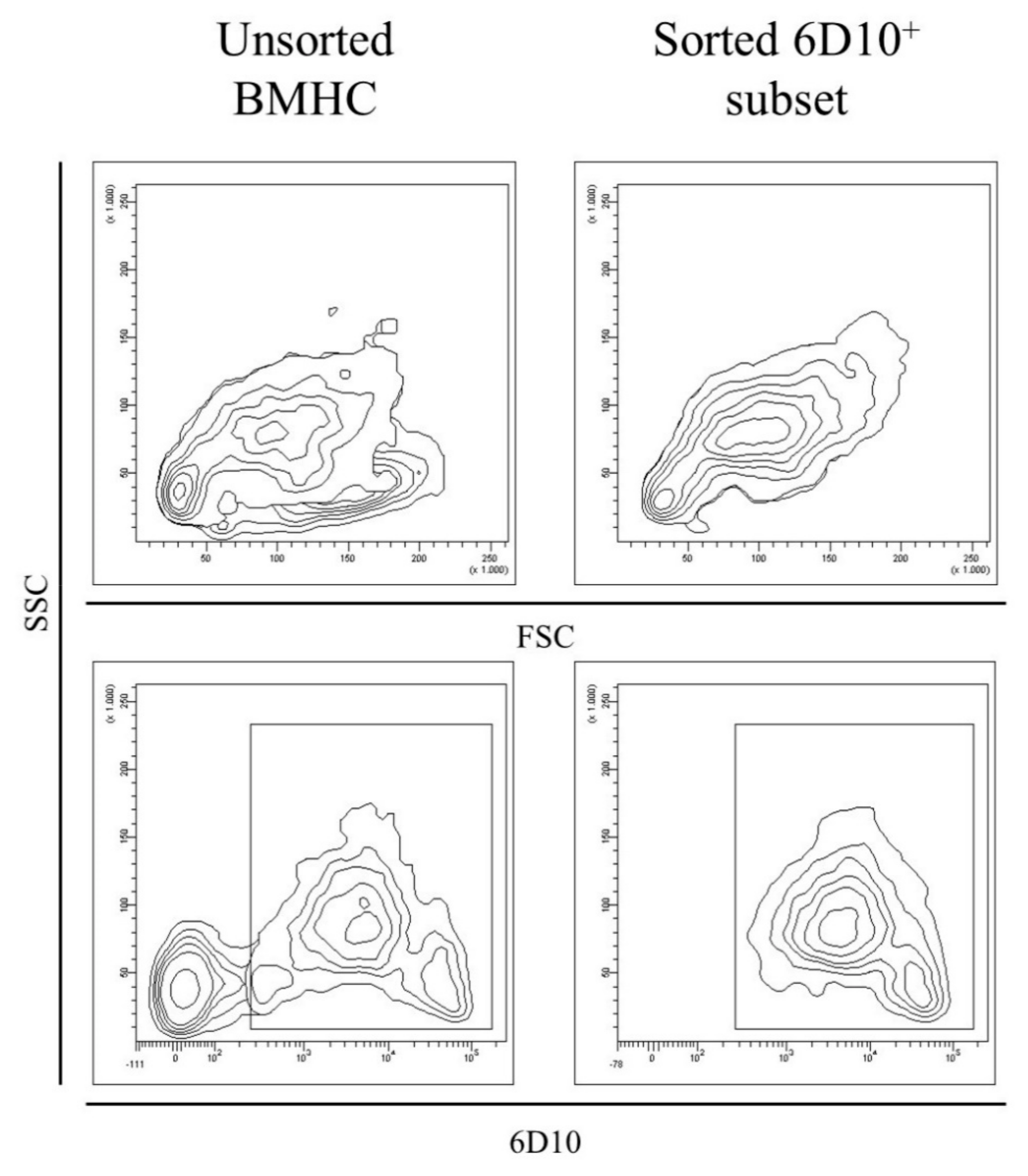
2.5. Sorting of 6D10+ Cells
2.6. Co-Culture Experiments and Determination of IFN-γ Production by ELISPOT
3. Results
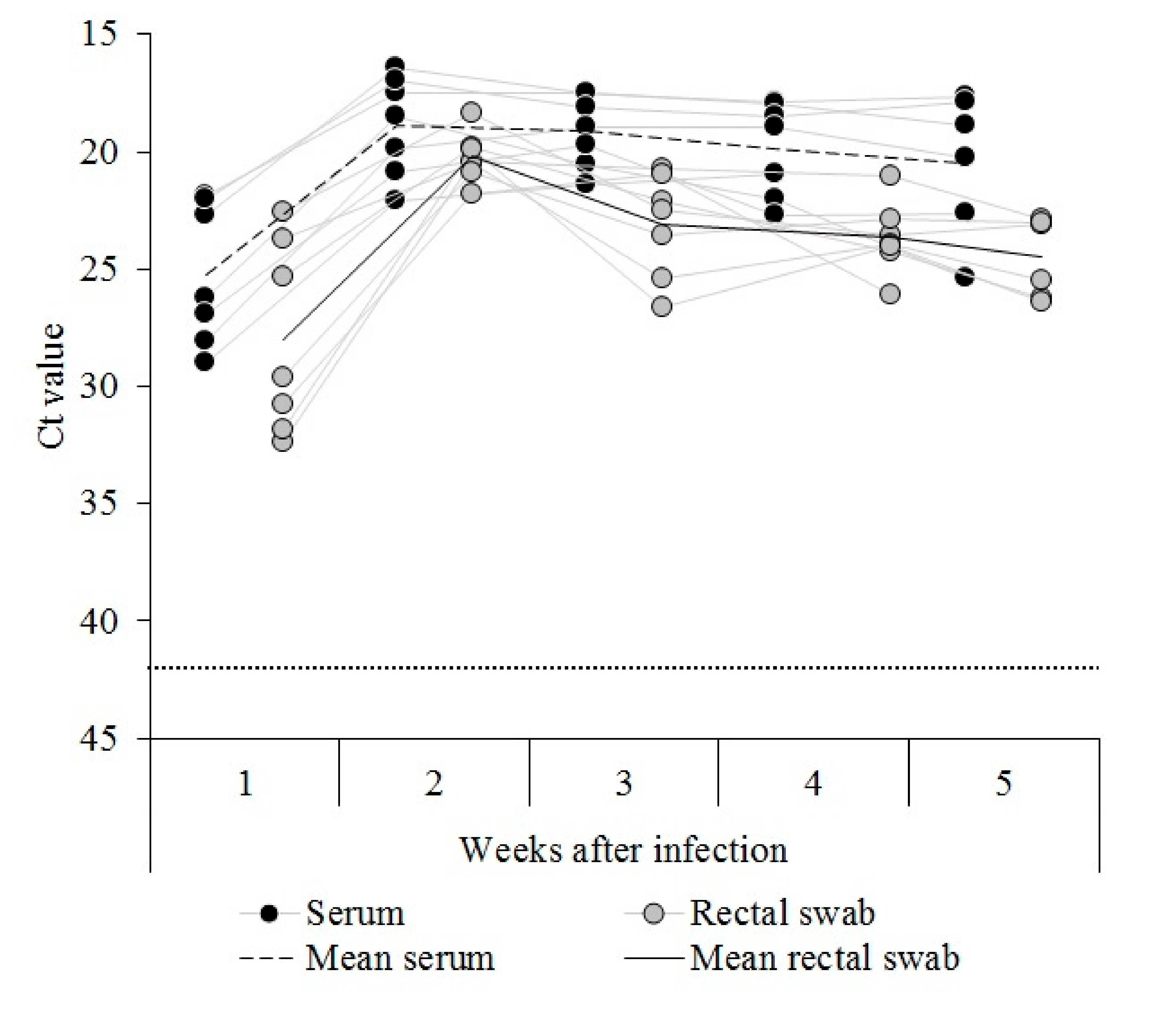
3.1. Establishment of CSFV Postnatal Persistent Infection
3.2. Granulocyte Precursor Cells Are Increased in CSFV Persistently Infected Animals and Show a Similar Phenotype to MDSC Populations
3.3. Granulocyte Precursor Cells Are a Target for CSFV
3.4. Granulocyte Precursor Cells from CSFV Persistently Infected Animals Are Able to Induce Immunosuppression
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical swine fever—An updated review. Viruses 2017, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Nishi, T.; Kameyama, K.; Meyer, D.; Suckstorff, O.; Fukai, K.; Becher, P. Reemergence of Classical Swine Fever, Japan, 2018. Emerg. Infect. Dis. 2019, 25, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Becher, P.; Collett, M.; Gould, E.A.; Heinz, F.X.; Meyers, G.; Monath, T.; Pletney, A.; Rice, C.M.; Stiasny, K.; et al. Family Flaviviridae. In Ninth Report of the International Commitee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2012; pp. 1004–1020. [Google Scholar]
- Tacke, R.S.; Lee, H.-C.; Goh, C.; Courtney, J.; Polyak, S.J.; Rosen, H.R.; Hahn, Y.S. Myeloid Suppressor Cells Induced by Hepatitis C Virus Suppress T-Cell Responses Through the Production of Reactive Oxygen Species. Hepatology 2012, 55, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Pan, T.; Wu, K.; Yang, Q.; Liu, Y.; Yan, D.; Zhang, H.; Zhou, J.; Cai, W.; Hu, F.; et al. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J. Virol. 2013, 87, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Cui, M.; Lv, Z.; Lu, J.; Zhang, X.; Zhao, Z.; Wang, Y.; Gao, L.; Tsuji, N.; Yan, H. Expression and significance of peripheral myeloid-derived suppressor cells in chronic hepatitis B patients. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Carossino, M.; Dini, P.; Kalbfleisch, T.S.; Loynachan, A.T.; Canisso, I.F.; Cook, R.F.; Timoney, P.J.; Balasuriya, U.B.R. Equine arteritis virus long-term persistence is orchestrated by CD8+ T lymphocyte transcription factors, inhibitory receptors, and the CXCL16/CXCR6 axis. PLOS Pathog. 2019, 15, e1007950. [Google Scholar] [CrossRef]
- Lutz, H.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline Leukaemia: ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2009, 11, 565–574. [Google Scholar] [CrossRef]
- Taniwaki, S.A.; Figueiredo, A.S.; Araujo Jr., J.P. Virus–host interaction in feline immunodeficiency virus (FIV) infection. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 549–557. [Google Scholar] [CrossRef]
- Gomez-Lucia, E.; Barquero, N.; Domenech, A. Maedi-Visna virus: current perspectives. Vet. Med. Res. Rep. 2018, 9, 11. [Google Scholar] [CrossRef]
- Van Oirschot, J.T. Experimental production of congenital persistent swine fever infections. I. Clinical, pathological and virological observations. Vet. Microbiol. 1979, 4, 117–132. [Google Scholar] [CrossRef]
- Van Oirschot, J.T. Experimental production of congenital persistent swine fever infections: II. Effect on functions of the immune system. Vet. Microbiol. 1979, 4, 133–147. [Google Scholar] [CrossRef]
- Johnson, D.W.; Muscoplat, C.C. Immunologic abnormalities in calves with chronic bovine viral diarrhea. Am. J. Vet. Res. 1973, 34, 1139–1141. [Google Scholar] [PubMed]
- Terpstra, C. Border disease: Virus persistence, antibody response and transmission studies. Res. Vet. Sci. 1981, 30, 185–191. [Google Scholar] [CrossRef]
- Muñoz-González, S.; Ruggli, N.; Rosell, R.; Pérez, L.J.; Frías-Leuporeau, M.T.; Fraile, L.; Montoya, M.; Cordoba, L.; Domingo, M.; Ehrensperger, F.; et al. Postnatal Persistent Infection with Classical Swine Fever Virus and Its Immunological Implications. PLoS ONE 2015, 10, e0125692. [Google Scholar] [CrossRef] [PubMed]
- Bohórquez, J.A.; Wang, M.; Pérez-Simó, M.; Vidal, E.; Rosell, R.; Ganges, L. Low CD4/CD8 ratio in classical swine fever postnatal persistent infection generated at 3 weeks after birth. Transbound. Emerg. Dis. 2019, 66, 752–762. [Google Scholar] [CrossRef]
- Muñoz-González, S.; Pérez-Simó, M.; Colom-Cadena, A.; Cabezón, O.; Bohórquez, J.A.; Rosell, R.; Pérez, L.J.; Marco, I.; Lavín, S.; Domingo, M.; et al. Classical swine fever virus vs. Classical swine fever virus: The superinfection exclusion phenomenon in experimentally infected wild boar. PLoS ONE 2016, 11, e0149469. [Google Scholar] [CrossRef]
- Rojas, J.M.; Avia, M.; Martín, V.; Sevilla, N. IL-10: A Multifunctional Cytokine in Viral Infections. J. Immunol. Res. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Cabezón, O.; Muñoz-González, S.; Colom-Cadena, A.; Pérez-Simó, M.; Rosell, R.; Lavín, S.; Marco, I.; Fraile, L.; De La Riva, P.M.; Rodríguez, F.; et al. African swine fever virus infection in Classical swine fever subclinically infected wild boars. BMC Vet. Res. 2017, 13, 227. [Google Scholar] [CrossRef]
- Pérez, C.; Revilla, C.; Alvarez, B.; Chamorro, S.; Correa, C.; Domenech, N.; Alonso, F.; Ezquerra, A.; Domínguez, J. Phenotypic and functional characterization of porcine granulocyte developmental stages using two new markers. Dev. Comp. Immunol. 2007, 31, 296–306. [Google Scholar] [CrossRef]
- Goh, C.; Narayanan, S.; Hahn, Y.S. Myeloid derived suppressor cells: The Dark Knight or The Joker in viral infections? Immunol. Rev. 2013, 255, 210–221. [Google Scholar] [CrossRef]
- Young, M.R.; Newby, M.; Wepsic, H.T. Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res. 1987, 47, 100–105. [Google Scholar] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S.; Fenselau, C. Myeloid-derived suppressor cells: Immune suppressive cells that impair antitumor immunity and are sculpted by their environment. J. Immunol. 2018, 200, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Stegelmeier, A.A.; van Vloten, J.P.; Mould, R.C.; Klafuric, E.M.; Minott, J.A.; Wootton, S.K.; Bridle, B.W.; Karimi, K.; Stegelmeier, A.A.; van Vloten, J.P.; et al. Myeloid Cells during Viral Infections and Inflammation. Viruses 2019, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Tarradas, J.; De La Torre, M.E.; Rosell, R.; Pérez, L.J.; Pujols, J.; Muñoz, M.; Muñoz, I.; Muñoz, S.; Abad, X.; Domingo, M.; et al. The impact of CSFV on the immune response to control infection. Virus Res. 2014, 185, 82–91. [Google Scholar] [CrossRef]
- Wensvoort, G.; Terpstra, C.; Boonstra, J.; Bloemraad, M.; Van Zaane, D. Production of monoclonal antibodies against swine fever virus and their use in laboratory diagnosis. Vet. Microbiol. 1986, 12, 101–108. [Google Scholar] [CrossRef]
- Reed, L.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Muñoz-González, S.; Pérez-Simó, M.; Muñoz, M.; Bohórquez, J.A.; Rosell, R.; Summerfield, A.; Domingo, M.; Ruggli, N.; Ganges, L. Efficacy of a live attenuated vaccine in classical swine fever virus postnatally persistently infected pigs. Vet. Res. 2015, 46, 78. [Google Scholar] [CrossRef]
- Blome, S.; Moß, C.; Reimann, I.; König, P.; Beer, M. Classical swine fever vaccines—State-of-the-art. Vet. Microbiol. 2017, 206, 10–20. [Google Scholar] [CrossRef]
- Aragon, V.; Cerdà-Cuéllar, M.; Fraile, L.; Mombarg, M.; Nofrarias, M.; Olvera, A.; Sibila, M.; Solanes, D.; Segalés, J. Correlation between clinico-pathological outcome and typing of Haemophilus parasuis field strains. Vet. Microbiol. 2010, 142, 387–393. [Google Scholar] [CrossRef]
- Cabezon, O.; Colom-Cadena, A.; Muñoz-González, S.; Perez-Simo, M.; Bohorquez, J.A.; Rosell, R.; Marco, I.; Domingo, M.; Lavín, S.; Ganges, L.; et al. Post-Natal Persistent Infection With Classical Swine Fever Virus in Wild Boar: A Strategy for Viral Maintenance? Transbound. Emerg. Dis. 2015, 64, 651–655. [Google Scholar] [CrossRef]
- Hoffmann, B.; Beer, M.; Schelp, C.; Schirrmeier, H.; Depner, K. Validation of a real-time RT-PCR assay for sensitive and specific detection of classical swine fever. J. Virol. Methods 2005, 130, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Tarradas, J.; Álvarez, B.; Fraile, L.; Rosell, R.; Muñoz, M.; Galindo-Cardiel, I.; Domingo, M.; Domínguez, J.; Ezquerra, Ángel; Sobrino, F.; et al. Immunomodulatory effect of swine CCL20 chemokine in DNA vaccination against CSFV. Vet. Immunol. Immunopathol. 2011, 142, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, C.; Bloemraad, M.; Gielkens, A. The neutralizing peroxidase-linked assay for detection of antibody against swine fever virus. Vet. Microbiol. 1984, 9, 113–120. [Google Scholar] [CrossRef]
- Carrasco, C.P.; Rigden, R.C.; Vincent, I.E.; Balmelli, C.; Ceppi, M.; Bauhofer, O.; Tâche, V.; Hjertner, B.; McNeilly, F.; Van Gennip, H.G.; et al. Interaction of classical swine fever virus with dendritic cells. J. Gen. Virol. 2004, 85, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Mussa, T.; Rodríguez-Cariño, C.; Pujol, M.; Córdoba, L.; Busquets, N.; Crisci, E.; Domínguez, J.; Fraile, L.; Montoya, M. Interaction of porcine conventional dendritic cells with swine influenza virus. Virology 2011, 420, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ganges, L.; Barrera, M.; Núñez, J.I.; Blanco, I.; Frías, M.; Rodríguez, F.; Sobrino, F. A DNA vaccine expressing the E2 protein of classical swine fever virus elicits T cell responses that can prime for rapid antibody production and confer total protection upon viral challenge. Vaccine 2005, 23, 3741–3752. [Google Scholar] [CrossRef]
- Alvarez, B.; Sanchez, C.; Bullido, R.; Marina, A.; Lunney, J.; Alonso, F.; Ezquerra, A.; Dominguez, J. A porcine cell surface receptor identified by monoclonal antibodies to SWC3 is a member of the signal regulatory protein family and associates with protein-tyrosine phosphatase SHP-1. Tissue Antigens 2000, 55, 342–351. [Google Scholar] [CrossRef]
- Alvarez, B.; Escalona, Z.; Uenishi, H.; Toki, D.; Revilla, C.; Yuste, M.; Del Moral, M.G.; Alonso, F.; Ezquerra, A.; Dominguez, J. Molecular and functional characterization of porcine Siglec-3/CD33 and analysis of its expression in blood and tissues. Dev. Comp. Immunol. 2015, 51, 238–250. [Google Scholar] [CrossRef]
- Rieber, N.; Gille, C.; Köstlin, N.; Schäfer, I.; Spring, B.; Öst, M.; Spieles, H.; Kugel, H.A.; Pfeiffer, M.; Heininger, V.; et al. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin. Exp. Immunol. 2013, 174, 45–52. [Google Scholar] [CrossRef]
- Cassetta, L.; Baekkevold, E.S.; Brandau, S.; Bujko, A.; Cassatella, M.A.; Dorhoi, A.; Krieg, C.; Lin, A.; Loré, K.; Marini, O.; et al. Deciphering myeloid-derived suppressor cells: Isolation and markers in humans, mice and non-human primates. Cancer Immunol. Immunother. 2019, 68, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Mandruzzato, S.; Brandau, S.; Britten, C.M.; Bronte, V.; Damuzzo, V.; Gouttefangeas, C.; Maurer, D.; Ottensmeier, C.; Van Der Burg, S.H.; Welters, M.J.P.; et al. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: Results from an interim study. Cancer Immunol. Immunother. 2016, 65, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.A.; Moses, K.; Trellakis, S.; Lang, S.; Brandau, S. Neutrophils and granulocytic myeloid-derived suppressor cells: Immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol. Immunother. 2012, 61, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Marigo, I.; Bosio, E.; Solito, S.; Mesa, C.; Fernandez, A.; Dolcetti, L.; Ugel, S.; Sonda, N.; Bicciato, S.; Falisi, E.; et al. Tumor-Induced Tolerance and Immune Suppression Depend on the C/EBPβ Transcription Factor. Immunity 2010, 32, 790–802. [Google Scholar] [CrossRef]
- Koehn, B.H.; Apostolova, P.; Haverkamp, J.M.; Miller, J.S.; McCullar, V.; Tolar, J.; Munn, D.H.; Murphy, W.J.; Brickey, W.J.; Serody, J.S.; et al. GVHD-associated, inflammasome-mediated loss of function in adoptively transferred myeloid-derived suppressor cells. Blood 2015, 126, 1621–1628. [Google Scholar] [CrossRef]
- Youn, J.-I.; Collazo, M.; Shalova, I.N.; Biswas, S.K.; Gabrilovich, D.I. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J. Leukoc. Biol. 2012, 91, 167–181. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohorquez, J.A.; Muñoz-González, S.; Pérez-Simó, M.; Revilla, C.; Domínguez, J.; Ganges, L. Identification of an Immunosuppressive Cell Population during Classical Swine Fever Virus Infection and Its Role in Viral Persistence in the Host. Viruses 2019, 11, 822. https://doi.org/10.3390/v11090822
Bohorquez JA, Muñoz-González S, Pérez-Simó M, Revilla C, Domínguez J, Ganges L. Identification of an Immunosuppressive Cell Population during Classical Swine Fever Virus Infection and Its Role in Viral Persistence in the Host. Viruses. 2019; 11(9):822. https://doi.org/10.3390/v11090822
Chicago/Turabian StyleBohorquez, Jose Alejandro, Sara Muñoz-González, Marta Pérez-Simó, Concepción Revilla, Javier Domínguez, and Llilianne Ganges. 2019. "Identification of an Immunosuppressive Cell Population during Classical Swine Fever Virus Infection and Its Role in Viral Persistence in the Host" Viruses 11, no. 9: 822. https://doi.org/10.3390/v11090822
APA StyleBohorquez, J. A., Muñoz-González, S., Pérez-Simó, M., Revilla, C., Domínguez, J., & Ganges, L. (2019). Identification of an Immunosuppressive Cell Population during Classical Swine Fever Virus Infection and Its Role in Viral Persistence in the Host. Viruses, 11(9), 822. https://doi.org/10.3390/v11090822





