Evaluation of Real-Time RT-PCR for Diagnostic Use in Detection of Puumala Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Samples
2.2. Construction of Synthetic Standard PUUV RNA
2.3. Primers and Probes
2.4. PUUV-qRT-PCR
2.5. RT-Nested-PCR
2.6. Detection of Immunoglobulins
3. Results
3.1. PUUV-qRT-PCR Is Both Sensitive and Specific
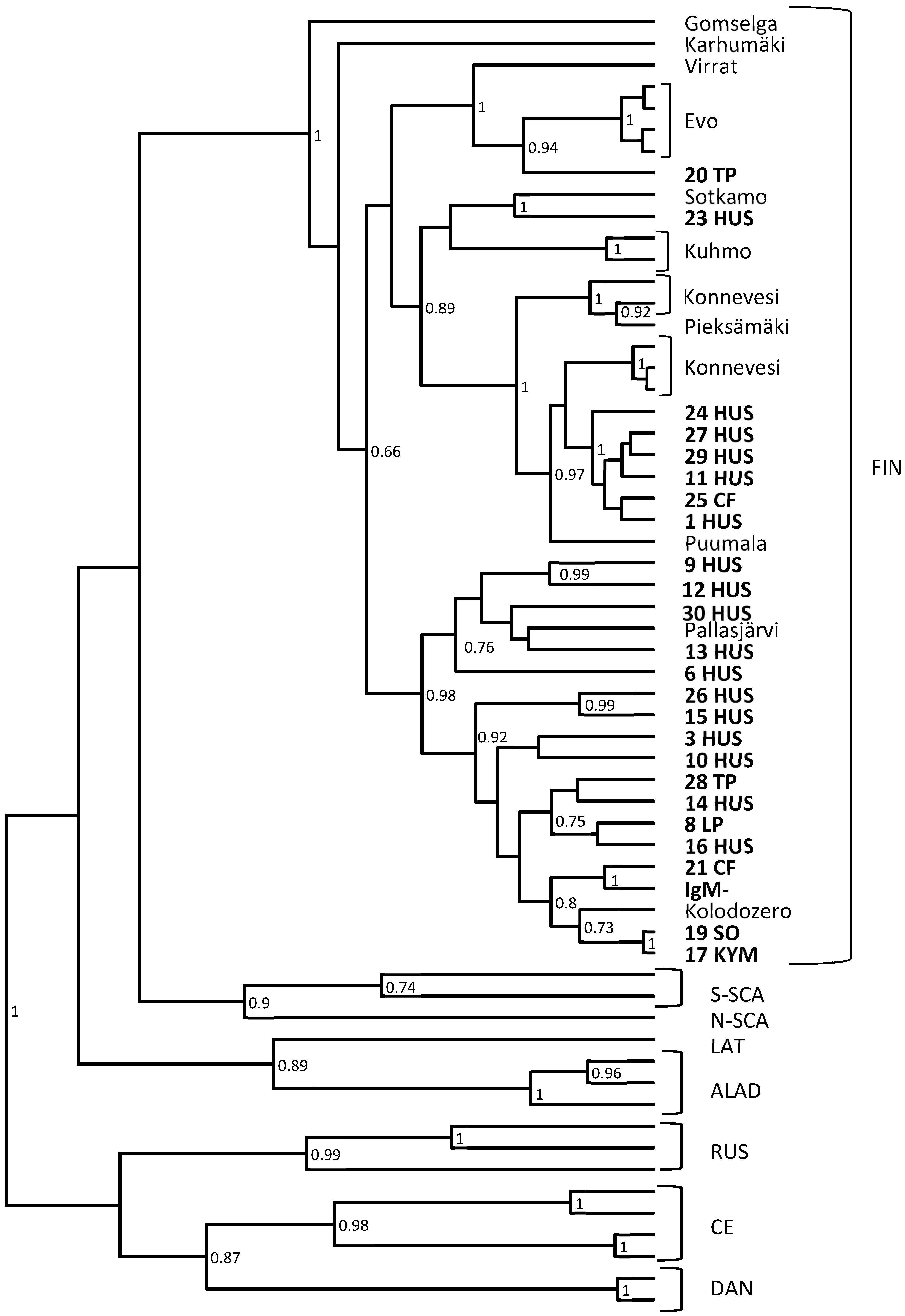
3.2. RT-Nested-PCR and Subsequent Sequencing Shows the High Variability of PUUV Strains Detected
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krüger, D.; Figueiredo, L.T.M.; Song, J.W.; Klempa, B. Hantaviruses—Globally emerging pathogens. J. Clin. Virol. 2015, 64, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Makary, P.; Kanerva, M.; Ollgren, J.; Virtanen, M.J.; Vapalahti, O.; Lyytikäinen, O. Disease burden of Puumala virus infections, 1995–2008. Epidemiol. Infect. 2010, 138, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Sironen, T.; Voutilainen, L.; Hepojoki, S.; Niemimaa, J.; Isoviita, V.M. Hantaviruses in Finnish soricomorphs: Evidence for two distinct hantaviruses carried by Sorex araneus suggesting ancient host–switch. Infect. Genet. Evol. 2014, 27, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Vaheri, A.; Hepojoki, S.; Levanov, L.; Jääskeläinen, A.; Henttonen, H. Serological survey of Seewis virus antibodies in patients suspected for hantavirus infection in Finland; a cross-reaction between Puumala virus antiserum with Seewis virus N protein. J. Gen. Virol. 2015, 96, 1664–1675. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Arai, S.; Hope, A.G.; Song, J.W.; Cook, J.A.; Yanagihara, R. Genetic diversity and phylogeography of Seewis virus in the Eurasian common shrew in Finland and Hungary. Virol. J. 2009, 24, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Lähdevirta, J. Nephropathia epidemica in Finland. A clinical histological and epidemiological study. Ann. Clin. Res. 1971, 3, 1–54. [Google Scholar] [PubMed]
- Mustonen, J.; Brummer-Korvenkontio, M.; Hedman, K.; Pasternack, A.; Pietila, K.; Vaheri, A. Nephropathia epidemica in Finland: A retrospective study of 126 cases. Scand. J. Infect. Dis. 1994, 26, 7–13. [Google Scholar] [CrossRef]
- Vapalahti, O.; Mustonen, J.; Lundkvist, A.; Henttonen, H.; Plyusnin, A.; Vaheri, A. Hantavirus infections in Europe. Lancet. Infect. Dis. 2003, 3, 653–661. [Google Scholar] [CrossRef]
- Kallio-Kokko, H.; Leveelahti, R.; Brummer-Korvenkontio, M.; Lundkvist, A.; Vaheri, A.; Vapalahti, O. Human immune response to Puumala virus glycoproteins and nucleocapsid protein expressed in mammalian cells. J. Med. Virol. 2001, 65, 605–613. [Google Scholar] [CrossRef]
- Mäkelä, S.; Ala-Houhala, I.; Mustonen, J.; Koivisto, A.M.; Kouri, T.; Turjanmaa, V. Renal function and blood pressure five years after Puumala virus-induced nephropathy. Kidney Int. 2000, 58, 1711–1718. [Google Scholar] [CrossRef]
- Miettinen, M.H.; Makela, S.M.; Ala-Houhala, I.O.; Huhtala, H.S.; Koobi, T.; Vaheri, A.I. Ten-year prognosis of Puumala hantavirus-induced acute interstitial nephritis. Kidney Int. 2006, 69, 2043–2048. [Google Scholar] [CrossRef]
- Mäkelä, S.; Jaatinen, P.; Miettinen, M.; Salmi, J.; Ala-Houhala, I.; Huhtala, H.; Hurme, M.; Pörsti, I.; Vaheri, A.; Mustonen, J. Hormonal deficiencies during and after Puumala hantavirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 705–713. [Google Scholar] [CrossRef]
- Rasmuson, J.; Andersson, C.; Norrman, E.; Haney, M.; Evander, M.; Ahlm, C. Time to revise the paradigm of hantavirus syndromes? Hantavirus pulmonary syndrome caused by European hantavirus. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 685–690. [Google Scholar] [CrossRef]
- Sironen, T.; Sane, J.; Lokki, M.L.; Meri, S.; Andersson, L.C.; Hautala, T.; Kauma, H.; Vuorinen, S.; Rasmuson, J.; Evander, M.; et al. Fatal Puumala hantavirus disease: Involvement of complement activation and vascular leakage in the pathobiology. Open Forum Infect. Dis. 2017, 4, 229. [Google Scholar] [CrossRef]
- Pettersson, L.; Thunberg, T.; Rocklov, J.; Klingström, J.; Evander, M.; Ahlm, C. Viral load and humoral immune response in association with disease severity in Puumala hantavirus-infected patients-implications for treatment. Clin. Microbiol. Infect. 2014, 20, 235–241. [Google Scholar] [CrossRef]
- Kallio-Kokko, H.; Vapalahti, O.; Lundkvist, Å.; Vaheri, A. Evaluation of Puumala virus IgG and IgM enzyme immunoassays based on recombinant baculovirus-expressed nucleocapsid protein for early nephropathia epidemica diagnosis. Clin. Diagn. Virol. 1998, 10, 83–90. [Google Scholar] [CrossRef]
- Lundkvist, Å.; Hukic, M.; Horling, J.; Gilljam, M.; Nichol, S.; Niklasson, B. Puumala and Dobrava viruses cause hemorrhagic fever with renal syndrome in Bosnia-Herzegovina: Evidence of highly cross-neutralizing antibody responses in early patient sera. J. Med. Virol. 1997, 53, 51–59. [Google Scholar] [CrossRef]
- Plyusnin, A.; Hörling, J.; Kanerva, M.; Mustonen, J.; Cheng, Y.; Partanen, J. Puumala hantavirus genome in patients with nephropathia epidemica: Correlation of PCR positivity with HLA haplotype and link to viral sequences in local rodents. J. Clin. Microbiol. 1997, 35, 1090–1096. [Google Scholar]
- Vapalahti, O.; Lundkvist, A.; Kallio-Kokko, H.; Paukku, K.; Julkunen, I.; Lankinen, H. Antigenic properties and diagnostic potential of puumala virus nucleocapsid protein expressed in insect cells. J. Clin. Microbiol. 1996, 34, 119–125. [Google Scholar]
- Evander, M.; Eriksson, I.; Pettersson, L.; Juto, P.; Ahlm, C.; Olsson, G.E. Puumala hantavirus viremia diagnosed by real-time reverse transcriptase PCR using samples from patients with hemorrhagic fever and renal syndrome. J. Clin. Microbiol. 2007, 45, 2491–2497. [Google Scholar] [CrossRef]
- Lagerqvist, N.; Hagstrom, A.; Lundahl, M.; Nilsson, E.; Juremalm, M.; Larsson, I. Molecular diagnosis of Puumala virus-caused hemorrhagic fever with renal syndrome. J. Clin. Microbiol. 2016, 54, 1335–1339. [Google Scholar] [CrossRef]
- Voutilainen, L.; Sironen, T.; Tonteri, E.; Back, A.T.; Razzauti, M.; Karlsson, M. Life-long shedding of Puumala hantavirus in wild bank voles (Myodes glareolus). J. Gen. Virol. 2015, 96, 1238–1247. [Google Scholar] [CrossRef]
- Saksida, A.; Duh, D.; Korva, M.; Avsic-Zupanc, T. Dobrava virus RNA load in patients who have hemorrhagic fever with renal syndrome. J. Infect. Dis. 2008, 197, 681–685. [Google Scholar] [CrossRef]
- Korva, M.; Saksida, A.; Kejzar, N.; Schmaljohn, C.; Avsic-Zupanc, T. Viral load and immune response dynamics in patients with haemorrhagic fever with renal syndrome. Clin. Microbiol. Infect. 2013, 19, 358–366. [Google Scholar] [CrossRef]
- Bunz, H.; Weyrich, P.; Peter, A.; Baumann, D.; Tschritter, O.; Guthoff, M.; Beck., R.; Jahn., G.; Artunc., F.; Häring, H.U.; et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) and proteinuria predict severity of acute kidney injury in Puumala virus infection. BMC Infect. Dis. 2015, 15, 464. [Google Scholar] [CrossRef]
| Patient No | Sex | Age | Hospital District | IgM | PUUV-qRT-PCR | RT-Nested-PCR |
|---|---|---|---|---|---|---|
| 1 | F | 39 | HUS | pos | pos | pos |
| 2 | M | 25 | HUS | pos | pos | NEG |
| 3 | F | 38 | HUS | pos | pos | pos |
| 4 | M | 55 | HUS | pos | NEG | NEG |
| 5 | F | 45 | HUS | pos | NEG | NEG |
| 6 | M | 65 | HUS | pos | pos | pos |
| 7 | F | 30 | HUS | pos | pos | NEG |
| 8 | F | 61 | LP | pos | pos | pos |
| 9 | F | 26 | HUS | pos | pos | pos |
| 10 | M | 57 | HUS | pos | pos | pos |
| 11 | F | 47 | HUS | pos | pos | pos |
| 12 | F | 64 | HUS | pos | pos | pos |
| 13 | M | 29 | KYM | pos | pos | pos |
| 14 | F | 61 | HUS | pos | pos | pos |
| 15 | F | 32 | HUS | pos | pos | pos |
| 16 | M | 46 | HUS | pos | pos | pos |
| 17 | F | 65 | KYM | pos | pos | pos |
| 18 | M | 66 | HUS | pos | pos | NEG |
| 19 | M | 71 | SO | pos | pos | pos |
| 20 | M | 77 | TP | pos | pos | pos |
| 21 | M | 48 | CF | pos | pos | pos |
| 22 | F | 28 | HUS | pos | pos | pos |
| 23 | M | 50 | HUS | pos | pos | pos |
| 24 | M | 39 | HUS | pos | pos | pos |
| 25 | M | 51 | CF | pos | pos | pos |
| 26 | F | 66 | HUS | pos | pos | pos |
| 27 | F | 51 | HUS | pos | pos | pos |
| 28 | M | 43 | TP | pos | pos | pos |
| 29 | M | 54 | HUS | pos | pos | pos |
| 30 | M | 57 | HUS | pos | pos | pos |
| No of positives: | 30 | 28 | 25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niskanen, S.; Jääskeläinen, A.; Vapalahti, O.; Sironen, T. Evaluation of Real-Time RT-PCR for Diagnostic Use in Detection of Puumala Virus. Viruses 2019, 11, 661. https://doi.org/10.3390/v11070661
Niskanen S, Jääskeläinen A, Vapalahti O, Sironen T. Evaluation of Real-Time RT-PCR for Diagnostic Use in Detection of Puumala Virus. Viruses. 2019; 11(7):661. https://doi.org/10.3390/v11070661
Chicago/Turabian StyleNiskanen, Silja, Anne Jääskeläinen, Olli Vapalahti, and Tarja Sironen. 2019. "Evaluation of Real-Time RT-PCR for Diagnostic Use in Detection of Puumala Virus" Viruses 11, no. 7: 661. https://doi.org/10.3390/v11070661
APA StyleNiskanen, S., Jääskeläinen, A., Vapalahti, O., & Sironen, T. (2019). Evaluation of Real-Time RT-PCR for Diagnostic Use in Detection of Puumala Virus. Viruses, 11(7), 661. https://doi.org/10.3390/v11070661





