Emergence of a Novel Reassortant Strain of Bluetongue Serotype 6 in Israel, 2017: Clinical Manifestations of the Disease and Molecular Characterization
Abstract
1. Introduction
2. Materials and Methods
2.2. Pan-BTV qRT-PCR
2.3. BT Virus Isolation.
2.4. BTV Serotype Identification
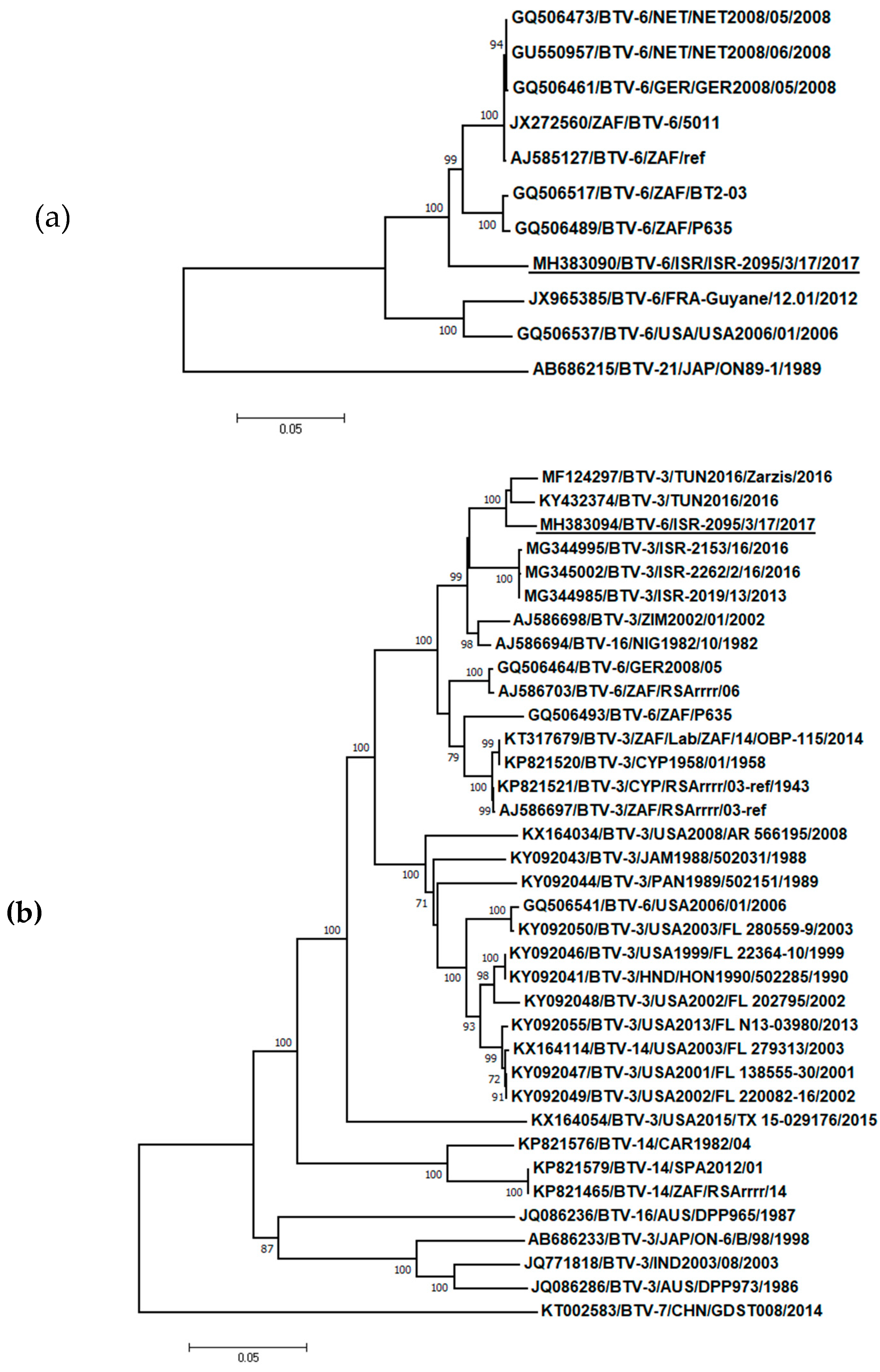
2.5. Sequencing and Phylogenetic Analysis
3. Results
3.1. Clinical Signs in Sheep and Cattle Naturally Infected with BTV-6
3.2. BTV Detection and Isolation, 2017
3.3. Phylogenetic, BLAST and Pairwise Analysis of BTV-6 Isolates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maclachlan, N.J.; Drew, C.P.; Darpel, K.E.; Worwa, G. The pathology and pathogenesis of bluetongue. J. Comparat. Pathol. 2009, 141, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, N.J.; Mayo, C.E.; Daniels, P.W.; Savini, G.; Zientara, S.; Gibbs, E.P. Bluetongue. Rev. Sci. Tech. 2015, 34, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; McArthur, C.; Selby, R.; Ward, R.; Nolan, D.V.; Luntz, A.J.; Dallas, J.F.; Tripet, F.; Mellor, P.S. Experimental infection studies of uk culicoides species midges with bluetongue virus serotypes 8 and 9. Vet. Rec. 2008, 163, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Wilson, A.; Mellor, P.S. Culicoides and the emergence of bluetongue virus in northern europe. Trends Microbiol. 2009, 17, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, N.J. Bluetongue: History, global epidemiology, and pathogenesis. Prev. Vet. Med. 2011, 102, 107–111. [Google Scholar] [CrossRef]
- Purse, B.V.; Mellor, P.S.; Rogers, D.J.; Samuel, A.R.; Mertens, P.P.; Baylis, M. Climate change and the recent emergence of bluetongue in europe. Nat. Rev. Microbiol. 2005, 3, 171–181. [Google Scholar] [CrossRef]
- Belbis, G.; Zientara, S.; Breard, E.; Sailleau, C.; Caignard, G.; Vitour, D.; Attoui, H. Bluetongue virus: From btv-1 to btv-27. Adv. Virus Res. 2017, 99, 161–197. [Google Scholar]
- Puggioni, G.; Pintus, D.; Melzi, E.; Meloni, G.; Rocchigiani, A.M.; Maestrale, C.; Manunta, D.; Savini, G.; Dattena, M.; Oggiano, A.; et al. Testicular degeneration and infertility following arbovirus infection. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Brenner, J.; Batten, C.; Yadin, H.; Bumbarov, V.; Friedgut, O.; Rotenberg, D.; Golender, N.; Oura, C.A. Clinical syndromes associated with the circulation of multiple serotypes of bluetongue virus in dairy cattle in israel. Vet. Rec. 2011, 169, 389. [Google Scholar] [CrossRef]
- Bumbarov, V.; Golender, N.; Rotenberg, D.; Brenner, J. Unusual clinical manifestations in israeli ruminant populations infected with orbiviruses. Vet. Ital. 2016, 52, 343–351. [Google Scholar]
- Golender, N.; Panshin, A.; Brenner, J.; Rotenberg, D.; Oura, C.; Khinich, E.; Bumbarov, V. Bluetongue virus serotype 24 (btv-24) in israel: Phylogenetic characterization and clinical manifestation of the disease. Vet. Ital. 2016, 52, 333–341. [Google Scholar] [PubMed]
- Stewart, M.; Hardy, A.; Barry, G.; Pinto, R.M.; Caporale, M.; Melzi, E.; Hughes, J.; Taggart, A.; Janowicz, A.; Varela, M.; et al. Characterization of a second open reading frame in genome segment 10 of bluetongue virus. J. Gen. Virol. 2015, 96, 3280–3293. [Google Scholar] [CrossRef] [PubMed]
- Bumbarov, V.; Golender, N.; Erster, O.; Khinich, Y. Detection and isolation of bluetongue virus from commercial vaccine batches. Vaccine 2016, 34, 3317–3323. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, A.; Sghaier, S.; Di Domenico, M.; Barbria, M.E.; Zaccaria, G.; Megdich, A.; Portanti, O.; Seliman, I.B.; Spedicato, M.; Pizzurro, F.; et al. Analysis of bluetongue serotype 3 spread in tunisia and discovery of a novel strain related to the bluetongue virus isolated from a commercial sheep pox vaccine. Infect. Genet. Evol. 2018, 59, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Puggioni, G.; Meloni, G.; Marcacci, M.; Di Domenico, M.; Rocchigiani, A.M.; Spedicato, M.; Oggiano, A.; Manunta, D.; Teodori, L.; et al. Novel putative bluetongue virus in healthy goats from sardinia, italy. Infect. Genet. Evol. 2017, 51, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.C.; Huang, L.P.; Xu, Q.Y.; Wang, H.X.; Xue, X.M.; Lu, P.; Li, W.J.; Liu, W.; Bu, Z.G.; Wu, D.L. Emergence of a novel bluetongue virus serotype, china 2014. Transbound. Emerg. Dis. 2016, 63, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Attoui, H.; Maan, S.; Anthony, S.J. Bluetongue virus, other orbiviruses and other reoviruses: Their relationships and taxonomy. In Bluetongue, 1st ed.; Mertens, P., Baylis, M., Mellor, P., Eds.; Elsevier/Academic Press: London, UK, 2008; pp. 23–52. [Google Scholar]
- Coetzee, P.; Stokstad, M.; Venter, E.H.; Myrmel, M.; Van Vuuren, M. Bluetongue: A historical and epidemiological perspective with the emphasis on south africa. Virol. J. 2012, 9, 198. [Google Scholar] [CrossRef]
- Schirtzinger, E.E.; Jasperson, D.C.; Ostlund, E.N.; Johnson, D.J.; Wilson, W.C. Recent us bluetongue virus serotype 3 isolates found outside of florida indicate evidence of reassortment with co-circulating endemic serotypes. J. Gen. Virol. 2018, 99, 157–168. [Google Scholar] [CrossRef]
- Shimshony, A. Bluetongue in israel—A brief historical overview. Vet. Ital. 2004, 40, 116–118. [Google Scholar]
- Viarouge, C.; Lancelot, R.; Rives, G.; Breard, E.; Miller, M.; Baudrimont, X.; Doceul, V.; Vitour, D.; Zientara, S.; Sailleau, C. Identification of bluetongue virus and epizootic hemorrhagic disease virus serotypes in french guiana in 2011 and 2012. Vet. Microbiol. 2014, 174, 78–85. [Google Scholar] [CrossRef]
- Zientara, S.; Sanchez-Vizcaino, J.M. Control of bluetongue in europe. Vet. Microbiol. 2013, 165, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Maan, S.; Maan, N.S.; van Rijn, P.A.; van Gennip, R.G.; Sanders, A.; Wright, I.M.; Batten, C.; Hoffmann, B.; Eschbaumer, M.; Oura, C.A.; et al. Full genome characterisation of bluetongue virus serotype 6 from the netherlands 2008 and comparison to other field and vaccine strains. PLoS ONE 2010, 5, e10323. [Google Scholar] [CrossRef] [PubMed]
- Simon-Loriere, E.; Holmes, E.C. Why do rna viruses recombine? Nat. Rev. Microbiol. 2011, 9, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Janowicz, A.; Caporale, M.; Shaw, A.; Gulletta, S.; Di Gialleonardo, L.; Ratinier, M.; Palmarini, M. Multiple genome segments determine virulence of bluetongue virus serotype 8. J. Virol. 2015, 89, 5238–5249. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, P.; Van Vuuren, M.; Stokstad, M.; Myrmel, M.; van Gennip, R.G.; van Rijn, P.A.; Venter, E.H. Viral replication kinetics and in vitro cytopathogenicity of parental and reassortant strains of bluetongue virus serotype 1, 6 and 8. Vet. Microbiol. 2014, 171, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.E.; Ratinier, M.; Nunes, S.F.; Nomikou, K.; Caporale, M.; Golder, M.; Allan, K.; Hamers, C.; Hudelet, P.; Zientara, S.; et al. Reassortment between two serologically unrelated bluetongue virus strains is flexible and can involve any genome segment. J. Virol. 2013, 87, 543–557. [Google Scholar] [CrossRef]
- Nomikou, K.; Hughes, J.; Wash, R.; Kellam, P.; Breard, E.; Zientara, S.; Palmarini, M.; Biek, R.; Mertens, P. Widespread reassortment shapes the evolution and epidemiology of bluetongue virus following european invasion. PLoS Pathog. 2015, 11, e1005056. [Google Scholar] [CrossRef]
- Erster, O.; Stram, R.; Menasherow, S.; Rubistein-Giuni, M.; Sharir, B.; Kchinich, E.; Stram, Y. High-resolution melting (hrm) for genotyping bovine ephemeral fever virus (befv). Virus Res. 2017, 229, 1–8. [Google Scholar] [CrossRef]
- Golender, N.; Khinich, Y.; Gorohov, A.; Abramovitz, I.; Bumbarov, V. Epizootic hemorrhagic disease virus serotype 6 outbreak in israeli cattle in 2015. J. Vet. Diagn. Investg. 2017, 29, 885–888. [Google Scholar] [CrossRef]
- Komarov, A.; Goldsmith, L. A disease, similar to bt in cattle and sheep in israel. Isr. J. Vet. Med. 1951, 8, 96–100. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Cappai, S.; Rolesu, S.; Loi, F.; Liciardi, M.; Leone, A.; Marcacci, M.; Teodori, L.; Mangone, I.; Sghaier, S.; Portanti, O.; et al. Western bluetongue virus serotype 3 in sardinia, diagnosis and characterization. Transbound. Emerg. Dis. 2019, 66, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.S.; Savini, G.; Spedicato, M.; Monaco, F.; Carmine, I.; Lorusso, A.; Francesco, T.; Mazzei, M.; Forzan, M.; Eldaghayes, I.; et al. Exploiting serological data to understand the epidemiology of bluetongue virus serotypes circulating in libya. Vet. Med. Sci. 2019, 5, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Sghaier, S.; Lorusso, A.; Portanti, O.; Marcacci, M.; Orsini, M.; Barbria, M.E.; Mahmoud, A.S.; Hammami, S.; Petrini, A.; Savini, G. A novel bluetongue virus serotype 3 strain in tunisia, november 2016. Transbound. Emerg. Dis. 2017, 64, 709–715. [Google Scholar] [CrossRef] [PubMed]

| Type of Animals | ||||||
|---|---|---|---|---|---|---|
| RT-qPCR | Cattle | Wild/Zoo Animals | Positive/Total Num of Samples | |||
| w. blood | spleen | a. fetus | spleen | a. fetus | ||
| BEFV | 7/212 | 0/1 | 0/1 | NT | NT | 7/214 |
| EHDV | 24/217 | 0/6 | 0/4 | 0/8 | 0/1 | 24/236 |
| Cattle | Sheep | Goat | Wild Animals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| w. b. | s. | a. f. | w. b. | s. | a. f. | w. b. | s. | w. b. | s. | a. f. | Total | total VI | |
| Num of pos. samples | 93 | 6 | 0 | 51 | 9 | 0 | 0 | 6 | 1 | 2 | 0 | 166 | |
| Num of tested samples | 402 | 35 | 7 | 173 | 41 | 10 | 9 | 24 | 17 | 23 | 3 | 693 | |
| Num isolated BTV-2 | 1 | 1 | |||||||||||
| Num isolated BTV-3 | 4 | 4 | |||||||||||
| Num isolated BTV-4 | 1 | 12 | 13 | ||||||||||
| Num isolated BTV-6 | 6 | 10 | 16 | ||||||||||
| Num isolated BTV-15 | 6 | 6 | |||||||||||
| Total Num VI | 14 | 26 | 40 | ||||||||||
| Locality/Geographic Zone/Distinct | Farm | Species/ Num Animals in the Farm | Breed | Affected Group | Num of Dead Animals/ Num of Ill Animals/ Total Num of Animals in Affected Group | Morbidity/Case Mortality (%) |
|---|---|---|---|---|---|---|
| Yonatan/Golan Height/Northern distinct | 1 | sheep/500 | Marino | recently bought 17-20 month-old pregnant ewes | 27/78/ no data | no data/34.6 |
| Sde David/Negev desert/Sothern distinct | 2 | sheep/450 | Asaf x Merino | lambs of different ages | 10/50/150 | 33.3/20 |
| Rosh HaNikra/Golan Height/Northern distinct | 3 | cattle/no data | Holstein-Friesian | no data | 0/no data/no data | no data |
| Moshav Nahalal/Jezreel Valley/Northern Distinct | 4 | cattle/no data | mixed breed | male fattening calves 3–7 month old | 2/no data | no data/no data |
| Moshav Lachish/Negev Desert/Southern Distinct | 5 | sheep/1350 | Merino× Romanov × Asaf × Puld-Dorset | lambs 4–15 month and primipara ewes | 20–30/100/ no data | no data/20-30 |
| Mishav Nehalim/Central distinct | 6 | sheep/no data | Marino x Safolk | 6 month old | 10/12/70 | 17.1/83.3 |
| Kefar Blum/Galilee/Northern distinct | 7 | cattle/350 | Holstein-Friesian | heifers | 0/ no data/ 70 | no data/0 |
| Kibutz Gonen/Galilee/Northern distinct | 8 | cattle/350 | Holstein-Friesian | heifers | 0/no data/70 | no data/0 |
| Havat Shaharim/Samaria/Central distinct | 9 | sheep/100 | mixed breed | lambs 4–6 month old | 2/no data | no data/no data |
| Moshav Moledet/Galilee/Northern distinct | 10 | sheep/3500 | Asaf | lambs 4–6 month old and adult animals | 24/ 1200/3500/ | 34.3/2 |
| Kibutz Ma’ale HaHamesha/Judean hills/Central distinct | 11 | cattle/500 | Holstein-Friesian | 5 month old female calf | 0/1/50 | 2/0 |
| Farm | Date of Beginning BT Disease in the Farm | Clinical Signs/Duration | Sample Date | RT-qPCR Positive/Total | VI | Serotype |
|---|---|---|---|---|---|---|
| 1 | no data | Lameness, rejection of moving, bloody nasal discharge/no data | 29-Aug-2017 | 3/3 | 2 | BTV-6 |
| 2 | beg-Sep-2017 | Pyrexia and skin hyperemia/no data | 6-Sep-2017 | 3/3 | 2 | BTV-6 BTV-3 |
| 3 | no data | No data/no data | 12-Sep-2017 | 3/3 | 1 | BTV-6 |
| 4 | end-Aug-2017 | Inappetence, cachexia, dyspnea and cough/two months | 12-Sep-2017 | 2/2 | 2 | BTV-6 |
| 5 | mid-Sep-2017 | Pyrexia, followed by lameness and stiffness in legs and back muscles, conjunctivitis, nasal discharge, ulceration of oral and nasal mucosa, recumbency, fatigue, mild respiratory distress and a few abortions | 14-Sep-2017;28-Nov-2017 | 3/3; 2/2 | 2; 1 | BTV-6 BTV-3 BTV-3 |
| 6 | no data | No data/no data | 19-Sep-2017 | 2/2 | 2 | BTV-6 |
| 7 | no data | Symmetrical bilateral edema for all length of the hind limbs; skin of udders in some infected animals was hyperemic, dry and scaly/no data | 24-Sep-2017 | 1/1 | 1 | BTV-6 |
| 8 | no data | Same as in farm 7; cow fever and icterus in some/no data | 28-Sep-2017 | 2/2 | 1 | BTV-6 |
| 9 | end-Sep-2017 | Heavy hyperemia of udder and face/3 weeks | 6-Sep-2017 | 1/1 | 1 | BTV-6 |
| 10 | beg-Sep-2017 | Pyrexia and skin hyperemia/light perinasal and perioral edema, milk reduction/several days | 3-Oct-2017 | 3/3 | 3 | BTV-6 |
| 11 | mid-Oct-2017 | Symmetrical bilateral edema for all length of the hind limbs/two weeks | 18-Oct-2017 | 1/1 | 1 | BTV-6 |
| Num of RT-qPCR positive /VI | RT-qPCR Positive/Total | BTV Serotype (VI) | ||||
|---|---|---|---|---|---|---|
| Ct Value | <25.0 | 25.1–30.0 | 30.1–35.0 | >35.1 | ||
| Month | ||||||
| Jan | 0 | 6/0 | 10/0 | 3/0 | 19/89 | - |
| Feb | 0 | 0 | 4/0 | 3/0 | 7/47 | - |
| Mar | 0 | 2/0 | 0 | 1/0 | 3/49 | - |
| Apr | 0 | 0 | 3/0 | 1/0 | 4/42 | - |
| May | 2/0 | 0 | 3/0 | 2/0 | 7/72 | - |
| Jun | 0 | 0 | 0 | 1/0 | 1/44 | - |
| Jul | 4/2 | 2/1 | 0 | 0 | 6/48 | BTV-4 |
| Aug | 5/3 | 4/0 | 1/0 | 3/0 | 13/45 | BTV-2, -6 |
| Sep | 16/10 | 12/9 | 10/2 | 4/0 | 42/83 | BTV-3, -4, -6 |
| Oct | 5/4 | 11/4 | 7/0 | 1/0 | 24/60 | BTV-3, -6, -15 |
| Nov | 2/1 | 3/0 | 15/1 | 7/1 | 27/73 | BTV-3, -4, -15 |
| Dec | 0 | 2/0 | 9/2 | 8/0 | 19/41 | BTV-15 |
| Genome Segment/acc. Num | Closest Sequence-nt Identity (%) |
|---|---|
| Seg-1/MH383089 | KY432369/BTV-3/TUN2016/2016-99.47 |
| Seg-2/MH383090 | JX272560/BTV-6/ZAF/5011-93.88 |
| Seg-3/MH383091 | DQ186825/BTV-4/SUD1983/01/1983-97.00 |
| Seg-4/MH383092 | KY432372/BTV-3/TUN2016/2016-99.33 |
| Seg-5/MH383093 | MK348541/BTV-3/ITL/SAR2018/2018-99.41 |
| Seg-6/MH383094 | MF124297/BTV-3/TUN2016/Zarzis/2016-97.62 |
| Seg-7/MH383095 | MG344986/BTV-3/ISR-2019/13/2013-97.78 |
| Seg-8/MH383096 | KP821811/BTV-9/LIB2008/03/2008-97.81 |
| Seg-9/MH383097 | MK348545/BTV-3/ITL/SAR2018/2018-99.13 |
| Seg-10/MH383098 | KP196612/ZAF/BT 57/08/2008- 97.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golender, N.; Eldar, A.; Ehrlich, M.; Khinich, Y.; Kenigswald, G.; Varsano, J.S.; Ertracht, S.; Abramovitz, I.; Assis, I.; Shlamovitz, I.; et al. Emergence of a Novel Reassortant Strain of Bluetongue Serotype 6 in Israel, 2017: Clinical Manifestations of the Disease and Molecular Characterization. Viruses 2019, 11, 633. https://doi.org/10.3390/v11070633
Golender N, Eldar A, Ehrlich M, Khinich Y, Kenigswald G, Varsano JS, Ertracht S, Abramovitz I, Assis I, Shlamovitz I, et al. Emergence of a Novel Reassortant Strain of Bluetongue Serotype 6 in Israel, 2017: Clinical Manifestations of the Disease and Molecular Characterization. Viruses. 2019; 11(7):633. https://doi.org/10.3390/v11070633
Chicago/Turabian StyleGolender, Natalia, Avi Eldar, Marcelo Ehrlich, Yevgeny Khinich, Gabriel Kenigswald, Joseph Seffi Varsano, Shachar Ertracht, Itzik Abramovitz, Itay Assis, Ily Shlamovitz, and et al. 2019. "Emergence of a Novel Reassortant Strain of Bluetongue Serotype 6 in Israel, 2017: Clinical Manifestations of the Disease and Molecular Characterization" Viruses 11, no. 7: 633. https://doi.org/10.3390/v11070633
APA StyleGolender, N., Eldar, A., Ehrlich, M., Khinich, Y., Kenigswald, G., Varsano, J. S., Ertracht, S., Abramovitz, I., Assis, I., Shlamovitz, I., Tiomkin, E., Yonay, E., Sharir, B., & Bumbarov, V. Y. (2019). Emergence of a Novel Reassortant Strain of Bluetongue Serotype 6 in Israel, 2017: Clinical Manifestations of the Disease and Molecular Characterization. Viruses, 11(7), 633. https://doi.org/10.3390/v11070633





