Coxsackievirus-B4 Infection of Human Primary Pancreatic Ductal Cell Cultures Results in Impairment of Differentiation into Insulin-Producing Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Pancreatic Ductal Cells
2.2. Virus
2.3. Cell Infection
2.4. RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.5. Quantitative RT-PCR
2.6. C-Peptide Quantification
3. Results
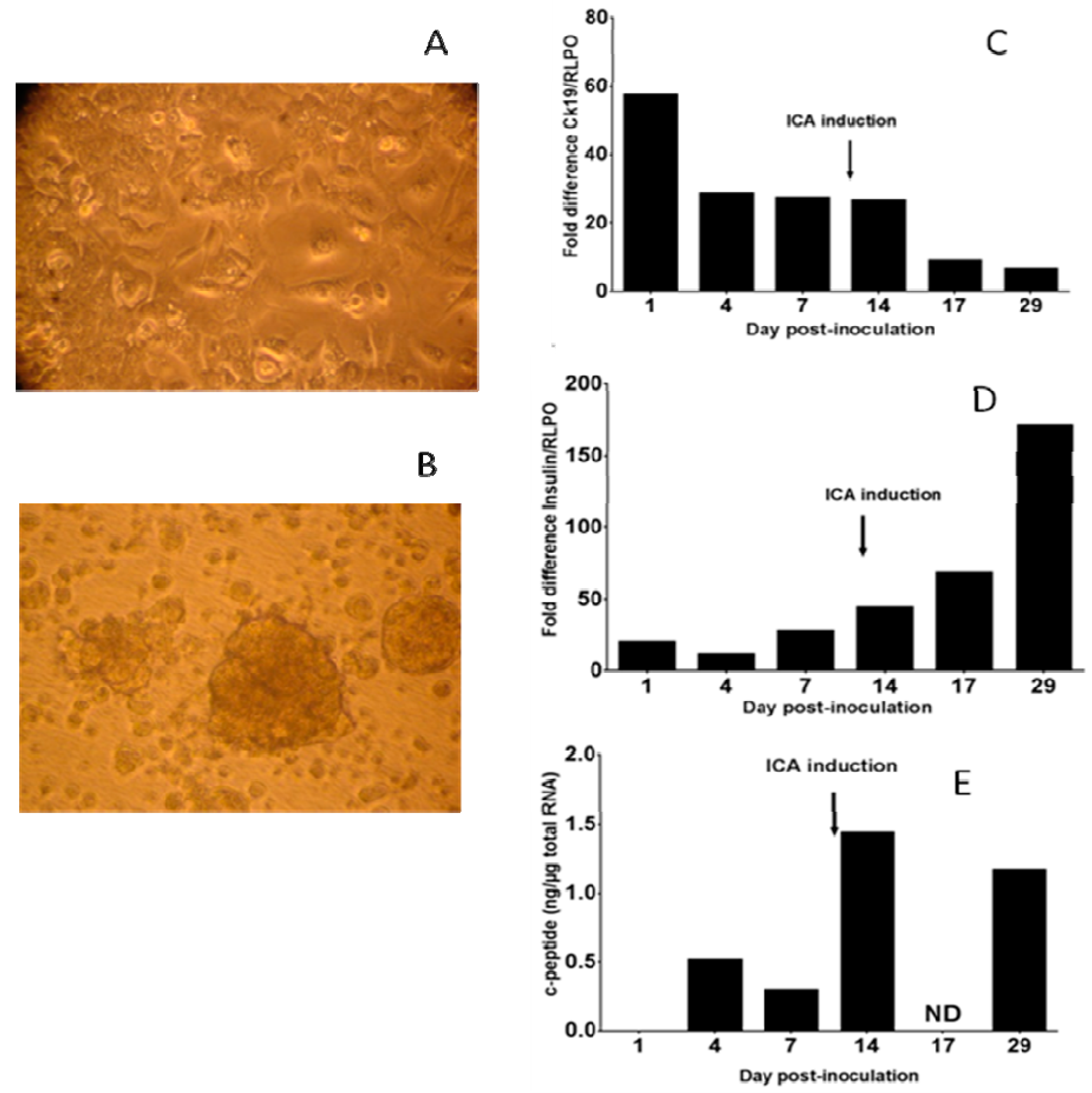
3.1. Human Primary Pancreatic Ductal Cells Can Be Differentiated into Insulin-Producing Islet-Like Cell Aggregates
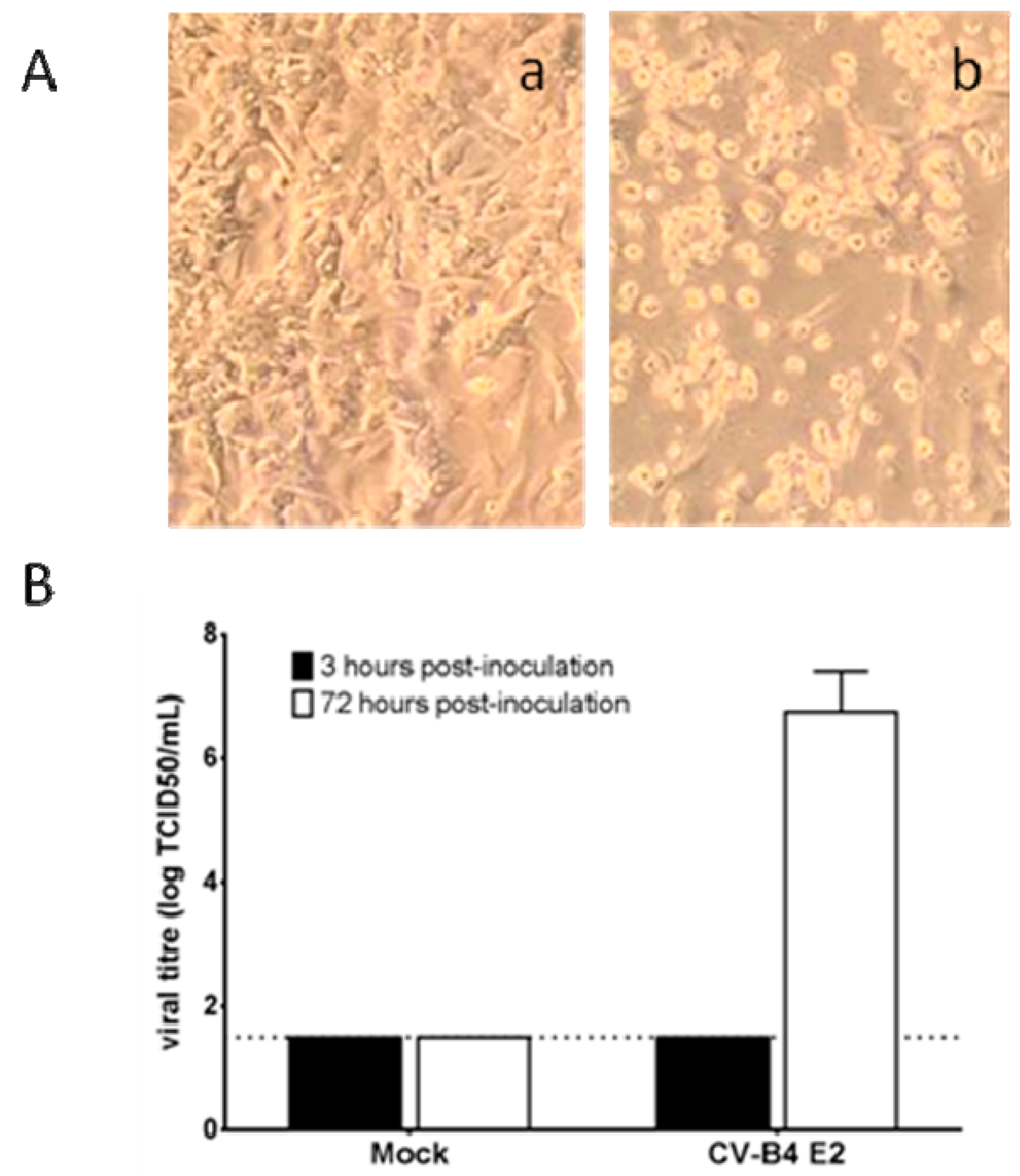
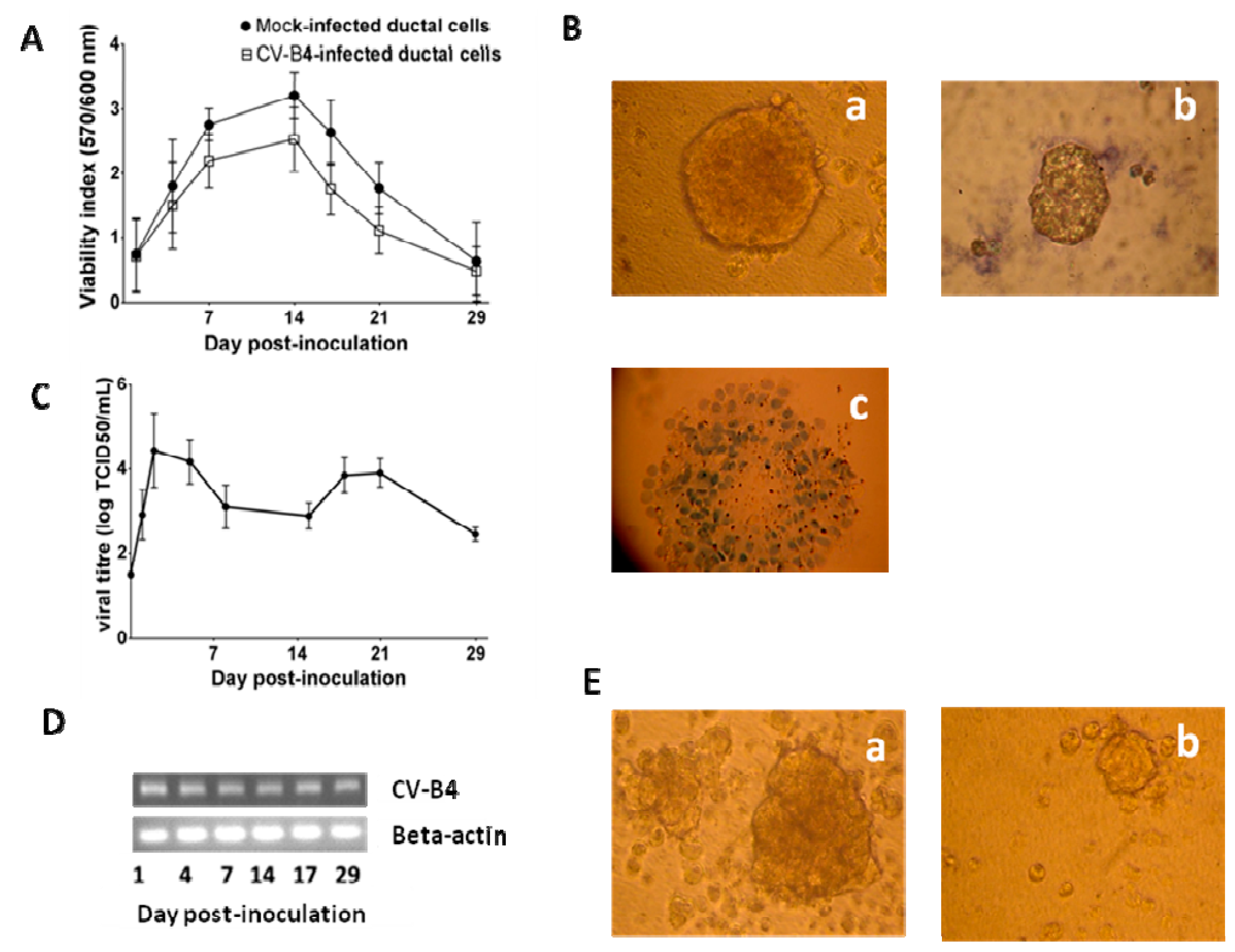
3.2. CV-B4 E2 Can Persist in Human Primary Pancreatic Ductal Cells In Vitro
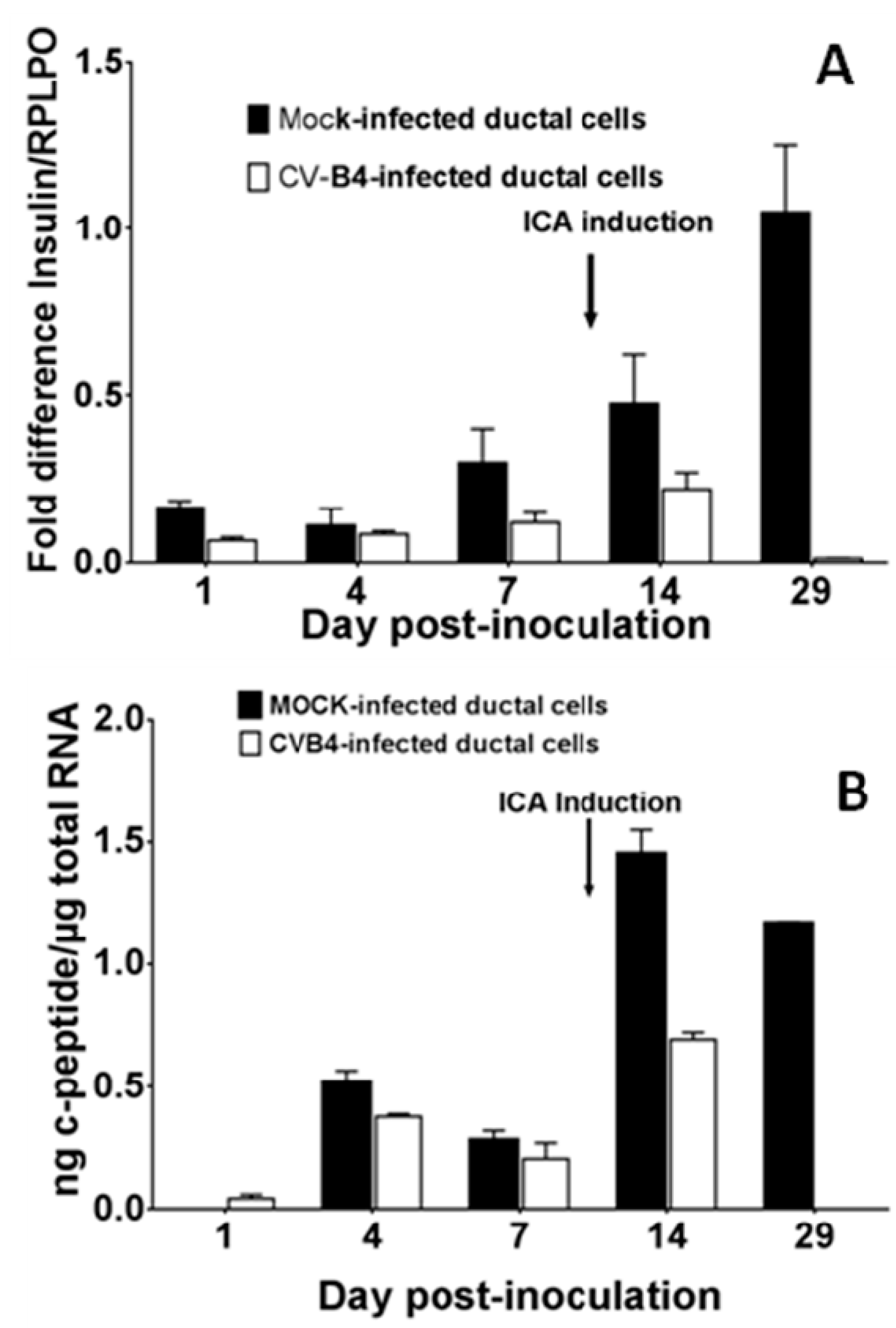
3.3. CV-B4 E2 Can Inhibit the Production of Insulin by Differentiated Human Primary Pancreatic Ductal Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Yeung, W.C.; Rawlinson, W.D.; Craig, M.E. Enterovirus infection and type 1 diabetes mellitus: Systematic review and meta-analysis of observational molecular studies. BMJ 2011, 342, d35. [Google Scholar] [CrossRef] [PubMed]
- Hyöty, H. Viruses in type 1 diabetes. Pediatr. Diabetes 2016, 17 (Suppl. 22), 56–64. [Google Scholar] [CrossRef] [PubMed]
- Alidjinou, E.K.; Sané, F.; Engelmann, I.; Geenen, V.; Hober, D. Enterovirus persistence as a mechanism in the pathogenesis of type 1 diabetes. Discov. Med. 2014, 18, 273–282. [Google Scholar] [PubMed]
- Hober, D.; Sauter, P. Pathogenesis of type 1 diabetes mellitus: Interplay between enterovirus and host. Nat. Rev. Endocrinol. 2010, 6, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Jaïdane, H.; Sauter, P.; Sane, F.; Goffard, A.; Gharbi, J.; Hober, D. Enteroviruses and type 1 diabetes: Towards a better understanding of the relationship. Rev. Med. Virol. 2010, 20, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Jaïdane, H.; Sané, F.; Gharbi, J.; Aouni, M.; Romond, M.B.; Hober, D. Coxsackievirus B4 and type 1 diabetes pathogenesis: Contribution of animal models. Diabetes Metab. Res. Rev. 2009, 25, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Ylipaasto, P.; Klingel, K.; Lindberg, A.M.; Otonkoski, T.; Kandolf, R.; Hovi, T.; Roivainen, M. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 2004, 47, 225–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.W.; Austin, M.; Onodera, T.; Notkins, A.L. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N. Engl. J. Med. 1979, 300, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, X.; Yu, L.; Zou, X.; Zhao, H. Characterization of insulin-immunoreactive cells and endocrine cells within the duct system of the adult human pancreas. Pancreas 2016, 45, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Martin-Pagola, A.; Sisino, G.; Allende, G.; Dominguez-Bendala, J.; Gianani, R.; Reijonen, H.; Nepom, G.T.; Ricordi, C.; Ruiz, P.; Sageshima, J.; et al. Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia 2008, 51, 1803–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pin, C.L.; Ryan, J.F.; Mehmood, R. Acinar cell reprogramming: A clinically important target in pancreatic disease. Epigenomics 2015, 7, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Hardikar, A.A.; Marcus-Samuels, B.; Geras-Raaka, E.; Raaka, B.M.; Gershengorn, M.C. Human pancreatic precursor cells secrete FGF2 to stimulate clustering into hormone-expressing islet-like cell aggregates. Proc. Natl. Acad. Sci. USA 2003, 100, 7117–7122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Li, J.; Saleem, S.; Yee, S.P.; Hardikar, A.A.; Wang, R. c-Kit and stem cell factor regulate PANC-1 cell differentiation into insulin- and glucagon-producing cells. Lab. Investig. 2010, 90, 1373–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sane, F.; Caloone, D.; Gmyr, V.; Engelmann, I.; Belaich, S.; Kerr-Conte, J.; Pattou, F.; Desailloud, R.; Hober, D. Coxsackievirus B4 can infect human pancreas ductal cells and persist in ductal-like cell cultures which results in inhibition of Pdx1 expression and disturbed formation of islet-like cell aggregates. Cell Mol. Life Sci. 2013, 70, 4169–4180. [Google Scholar] [CrossRef] [PubMed]
- Ricordi, C.; Lacy, P.E.; Scharp, D.W. Automated islet isolation from human pancreas. Diabetes 1989, 38 (Suppl. 1), 140–142. [Google Scholar] [CrossRef] [PubMed]
- Gmyr, V.; Kerr-Conte, J.; Belaich, S.; Vandewalle, B.; Leteurtre, E.; Vantyghem, M.C.; Lecomte-Houcke, M.; Proye, C.; Lefebvre, J.; Pattou, F. Adult human cytokeratin 19-positive cells reexpress insulin promoter factor 1 in vitro: Further evidence for pluripotent pancreatic stem cells in humans. Diabetes 2000, 49, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.; Álvarez-Cubela, S.; Lanzoni, G.; Vargas, N.; Prabakar, K.R.; Boulina, M.; Ricordi, C.; Inverardi, L.; Pastori, R.L.; Domínguez-Bendala, J. BMP-7 induces adult human pancreatic exocrine-to-endocrine conversion. Diabetes 2015, 64, 4123–4134. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sugiyama, T.; Liu, Y.; Wang, J.; Gu, X.; Lei, J.; Markmann, J.F.; Miyazaki, S.; Miyazaki, J.; Szot, G.L.; et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife 2013, 2, e00940. [Google Scholar] [CrossRef] [PubMed]
- Hodik, M.; Anagandula, M.; Fuxe, J.; Krogvold, L.; Dahl-Jørgensen, K.; Hyöty, H.; Sarmiento, L.; Frisk, G. POD-V Consortium. Coxsackie-adenovirus receptor expression is enhanced in pancreas from patients with type 1 diabetes. BMJ Open Diabetes Res. Care 2016, 4, e000219. [Google Scholar] [CrossRef] [PubMed]
| Target | Sequence | ||
|---|---|---|---|
| RT-PCR | Beta-actin | forward | 5’-GGCACTCTTCCAGCCTTCCT-3’ |
| reverse | 5’-GCAATGCCAGGGTACATGGT-3’ | ||
| CV-B4 E2 | forward | 5’-CAAGCACTTCTGTTTCCCCGG-3’ | |
| reverse | 5’-ATTGTCACCATAAGCAGCCA-3’ | ||
| RT-QPCR | RLPO | forward | 5’-ACCTCCTTTTTCCAGGCTTT-3’ |
| reverse | 5’-CCCACTTTGTCTCCAGTCTTG-3’ | ||
| Insulin | forward | 5’-TGCATCAGAAGAGGCCATCA-3’ | |
| reverse | 5’-CGTTCCCCGCACACTAGGTA-3’ | ||
| CK19 | forward | 5’-CCAGCCGGACTGAAGAATTG-3’ | |
| reverse | 5’-TGGGCTTCAATACCGCTGAT-3’ | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertin, A.; Sane, F.; Gmyr, V.; Lobert, D.; Dechaumes, A.; Kerr-Conte, J.; Pattou, F.; Hober, D. Coxsackievirus-B4 Infection of Human Primary Pancreatic Ductal Cell Cultures Results in Impairment of Differentiation into Insulin-Producing Cells. Viruses 2019, 11, 597. https://doi.org/10.3390/v11070597
Bertin A, Sane F, Gmyr V, Lobert D, Dechaumes A, Kerr-Conte J, Pattou F, Hober D. Coxsackievirus-B4 Infection of Human Primary Pancreatic Ductal Cell Cultures Results in Impairment of Differentiation into Insulin-Producing Cells. Viruses. 2019; 11(7):597. https://doi.org/10.3390/v11070597
Chicago/Turabian StyleBertin, Antoine, Famara Sane, Valery Gmyr, Delphine Lobert, Arthur Dechaumes, Julie Kerr-Conte, François Pattou, and Didier Hober. 2019. "Coxsackievirus-B4 Infection of Human Primary Pancreatic Ductal Cell Cultures Results in Impairment of Differentiation into Insulin-Producing Cells" Viruses 11, no. 7: 597. https://doi.org/10.3390/v11070597
APA StyleBertin, A., Sane, F., Gmyr, V., Lobert, D., Dechaumes, A., Kerr-Conte, J., Pattou, F., & Hober, D. (2019). Coxsackievirus-B4 Infection of Human Primary Pancreatic Ductal Cell Cultures Results in Impairment of Differentiation into Insulin-Producing Cells. Viruses, 11(7), 597. https://doi.org/10.3390/v11070597





