The Diagnosis of Feline Leukaemia Virus (FeLV) Infection in Owned and Group-Housed Rescue Cats in Australia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Determination of FeLV Exposure/Infection Status
2.2.1. FeLV PoC p27 Testing
2.2.2. FeLV qPCR Testing
2.2.3. FeLV qRT-PCR Testing
2.2.4. Laboratory-Based FeLV p27 ELISA Testing
2.2.5. FeLV NAb Testing
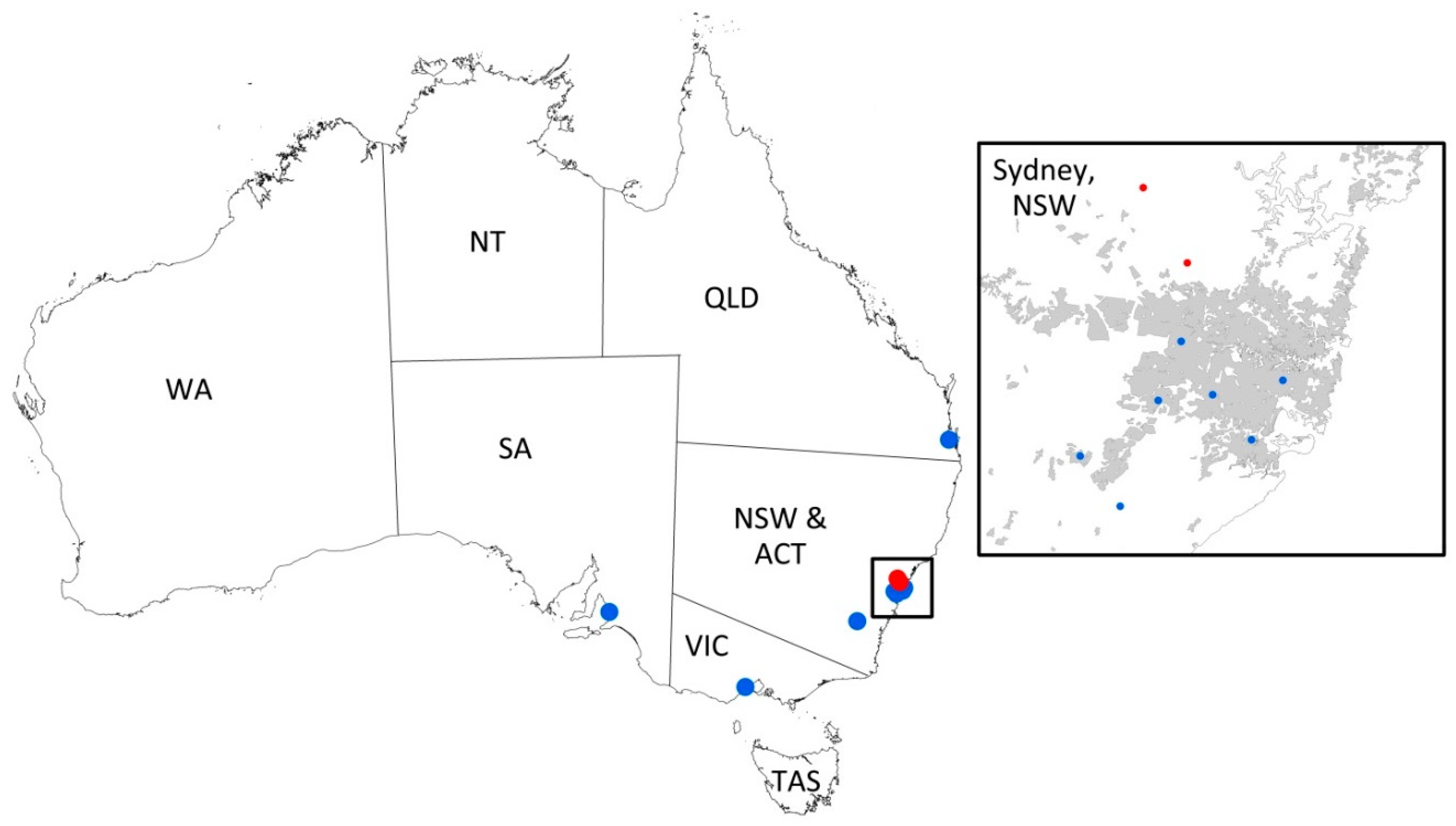
2.3. Statistical Analysis Including Mapping of Cases of FeLV Exposure and Infection
3. Results
3.1. Description of Study Population (n = 529)
3.2. Identification of FeLV-Infected Cats
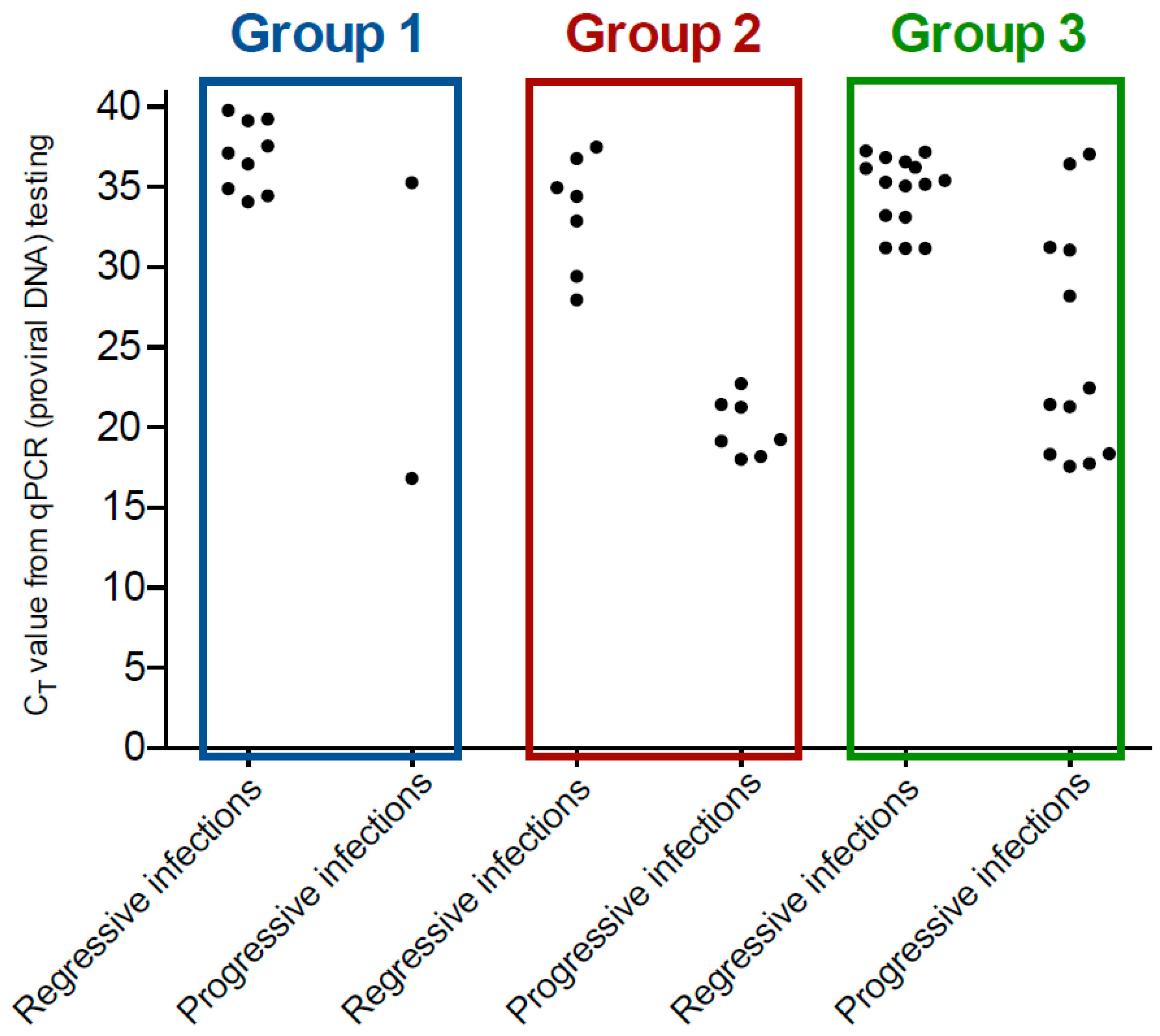
3.3. Detection of FeLV Provirus by qPCR
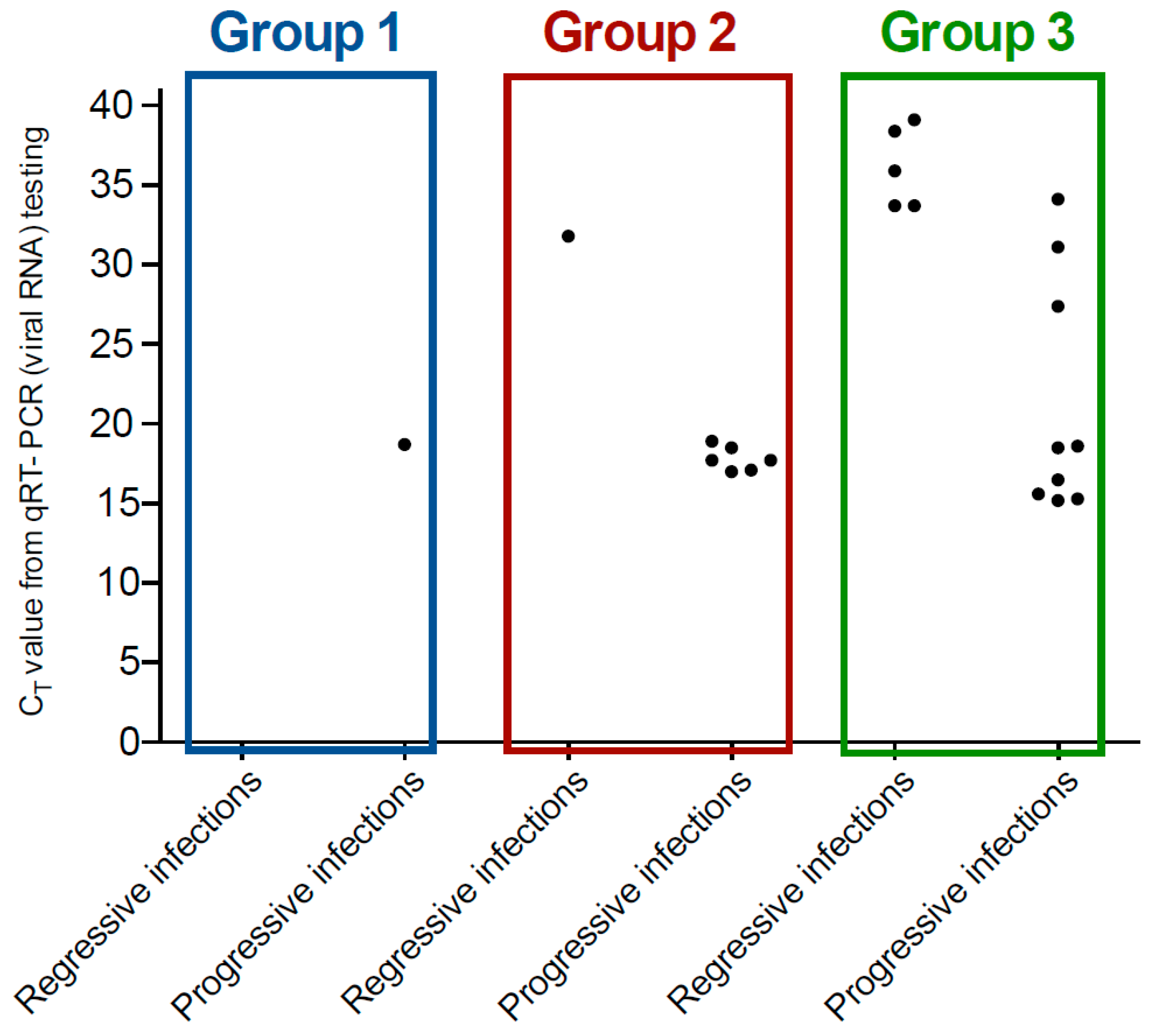
3.4. Detection of FeLV RNA by qRT-PCR
3.5. Laboratory-Based FeLV p27 ELISA
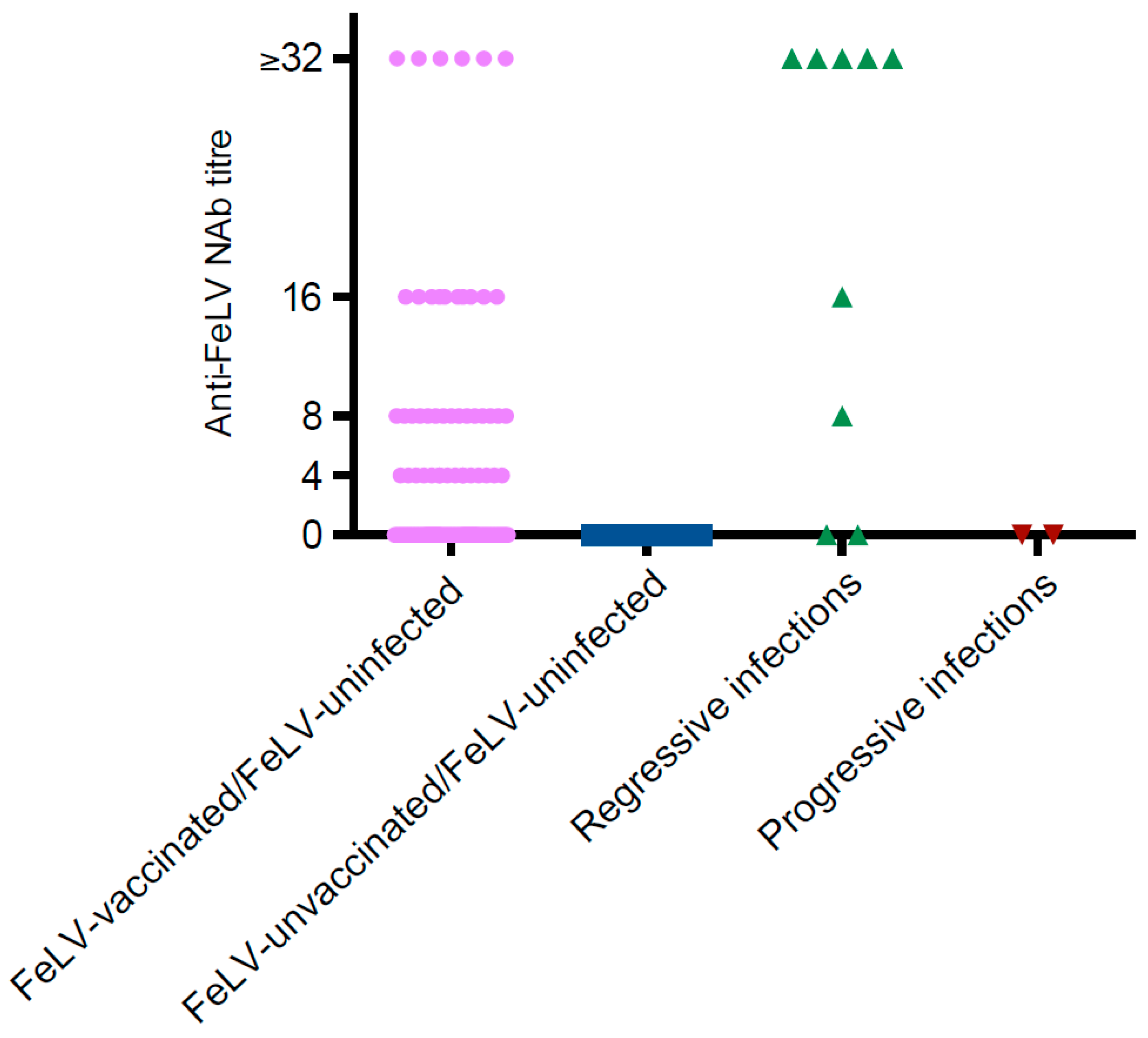
3.6. Detection of FeLV NAb
3.7. FIV Infection Status and FeLV/FIV Co-Infections
3.8. Mapping of Cases of FeLV Exposure and Infection (Group 1 Only)
3.9. Follow-up of Rescue Cats and FeLV-Infected Cats (Groups 1 and 2 Only)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levy, J.; Crawford, C.; Hartmann, K.; Hofmann-Lehmann, R.; Little, S.; Sundahl, E.; Thayer, V. American Association of Feline Practitioners’ feline retrovirus management guidelines. J. Feline Med. Surg. 2008, 10, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Horzinek, Feline Leukaemia: ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2009, 11, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Firth, C.L.; Möstl, K. A survey of feline leukaemia virus antigenaemia among cats in eastern Austria: A retrospective analysis of serum samples routinely tested between 1996 and 2011. J. Feline Med. Surg. Open Rep. 2015, 1, 2055116915598336. [Google Scholar] [CrossRef]
- Ravi, M.; Wobeser, G.A.; Taylor, S.M.; Jackson, M.L. Naturally acquired feline immunodeficiency virus (FIV) infection in cats from western Canada: Prevalence, disease associations, and survival analysis. Can. Vet. J. 2010, 51, 271–276. [Google Scholar] [PubMed]
- Westman, M.E.; Paul, A.; Malik, R.; McDonagh, P.; Ward, M.P.; Hall, E.; Norris, J.M. Seroprevalence of feline immunodeficiency virus and feline leukaemia virus in Australia: Risk factors for infection and geographical influences (2011–2013). J. Feline Med. Surg. Open Rep. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Gonczil, E.; Riond, B.; Meli, M.L.; Willi, B.; Howard, J.; Schaarschmidt, D.; Regli, W.; Gilli, U.; Boretti, F.S. Feline leukemia virus infection: Importance and current situation in Switzerland. Schweizer Archiv. Fur. Tierheilkunde 2018, 160, 95–105. [Google Scholar] [CrossRef]
- Willett, B.J.; Hosie, M.J. Feline leukaemia virus: Half a century since its discovery. Vet. J. 2013, 195, 16–23. [Google Scholar] [CrossRef]
- Möstl, K.; Egberink, H.; Addie, D.; Frymus, T.; Boucraut-Baralon, C.; Truyen, U.; Hartmann, K.; Lutz, H.; Gruffydd-Jones, T.; Radford, A.D.; et al. Prevention of infectious diseases in cat shelters: ABCD guidelines. J. Feline Med. Surg. 2013, 15, 546–554. [Google Scholar] [CrossRef]
- de Almeida, N.R.; Danelli, M.G.; da Silva, L.H.; Hagiwara, M.K.; Mazur, C. Prevalence of feline leukemia virus infection in domestic cats in Rio de Janeiro. J. Feline Med. Surg. 2012, 14, 583–586. [Google Scholar] [CrossRef]
- Biezus, G.; Machado, G.; Ferian, P.E.; da Costa, U.M.; Pereira, L.H.H.d.S.; Withoeft, J.A.; Nunes, I.A.C.; Muller, T.R.; de Cristo, T.G.; Casagrande, R.A. Prevalence of and factors associated with feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) in cats of the state of Santa Catarina, Brazil. Comp. Immun. Microbiol. Infect. Dis. 2019, 63, 17–21. [Google Scholar] [CrossRef]
- Bande, F.; Arshad, S.S.; Hassan, L.; Zakaria, Z.; Sapian, N.A.; Rahman, N.A.; Alazawy, A. Prevalence and risk factors of feline leukaemia virus and feline immunodeficiency virus in peninsular Malaysia. BMC Vet. Res. 2012, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.T.; Beatty, J.A.; Barrs, V.R.; Morris, A.K.; Reagan, K.; Lappin, M.R. Evidence for feline immunodeficiency virus, feline leukemia virus, Bartonella spp, haemoplasmas, and Toxoplasma spp. exposure in Singaporean cats. J. Vet. Intern. Med. 2013, 27, 729. [Google Scholar]
- Sukhumavasi, W.; Bellosa, M.L.; Lucio-Forster, A.; Liotta, J.L.; Lee, A.C.Y.; Pornmingmas, P.; Chungpivat, S.; Mohammed, H.O.; Lorentzen, L.; Dubey, J.P.; Bowman, D.D. Serological survey of Toxoplasma gondii, Dirofilaria immitis, Feline Immunodeficiency Virus (FIV) and Feline Leukemia Virus (FeLV) infections in pet cats in Bangkok and vicinities, Thailand. Vet. Parasitol. 2012, 188, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Westman, M.E.; Malik, R.; Hall, E.; Sheehy, P.A.; Norris, J.M. Comparison of three feline leukaemia virus (FeLV) point-of-care antigen test kits using blood and saliva. Comp. Immun. Microbiol. Infect. Dis. 2017, 50, 88–96. [Google Scholar] [CrossRef]
- Hardy, W.D.; Hess, P.W.; MacEwen, E.G.; McClelland, A.J.; Zuckerman, E.E.; Essex, M.; Cotter, S.M.; Jarrett, O. Biology of feline leukemia virus in the natural environment. Cancer Res. 1976, 36, 582–588. [Google Scholar] [PubMed]
- Hartmann, K. Clinical aspects of feline retroviruses: A review. Viruses 2012, 4, 2684–2710. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Cattori, V.; Tandon, R.; Boretti, F.S.; Meli, M.L.; Riond, B.; Pepin, A.C.; Willi, B.; Ossent, P.; Lutz, H. Vaccination against the feline leukaemia virus: Outcome and response categories and long-term follow-up. Vaccine 2007, 25, 5531–5539. [Google Scholar] [CrossRef]
- Englert, T.; Lutz, H.; Sauter-Louis, C.; Hartmann, K. Survey of the feline leukemia virus infection status of cats in Southern Germany. J. Feline Med. Surg. 2012, 14, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Cristo, T.G.; Biezus, G.; Noronha, L.F.; Pereira, L.H.H.S.; Withoeft, J.A.; Furlan, L.V.; Costa, L.S.; Traverso, S.D.; Casagrande, R.A. Feline lymphoma and a high correlation with feline leukaemia virus infection in Brazil. J. Comp. Pathol. 2019, 166, 20–28. [Google Scholar] [CrossRef]
- Helfer-Hungerbuehler, A.K.; Widmer, S.; Kessler, Y.; Riond, B.; Boretti, F.S.; Grest, P.; Lutz, H.; Hofmann-Lehmann, R. Long-term follow up of feline leukemia virus infection and characterization of viral RNA loads using molecular methods in tissues of cats with different infection outcomes. Virus Res. 2015, 197, 137–150. [Google Scholar] [CrossRef]
- Gabor, L.J.; Jackson, M.L.; Trask, B.; Malik, R.; Canfield, P.J. Feline leukaemia virus status of Australian cats with lymphosarcoma. Aust. Vet. J. 2001, 79, 476–481. [Google Scholar] [CrossRef]
- Jackson, M.L.; Haines, D.M.; Meric, S.M.; Misra, V. Feline leukemia virus detection by immunohistochemistry and polymerase chain reaction in formalin-fixed, paraffin-embedded tumor tissue from cats with lymphosarcoma. Can. J. Vet. Res. 1993, 57, 269–276. [Google Scholar]
- McLuckie, A.; Barrs, V.; Lindsay, S.; Aghazadeh, M.; Sangster, C.; Beatty, J. Molecular Diagnosis of Felis catus Gammaherpesvirus 1 (FcaGHV1) Infection in Cats of Known Retrovirus Status with and without Lymphoma. Viruses 2018, 10, 128. [Google Scholar] [CrossRef]
- Westman, M.E.; Malik, R.; Hall, E.; Harris, M.; Norris, J.M. The protective rate of the feline immunodeficiency virus vaccine: An Australian field study. Vaccine 2016, 34, 4752–4758. [Google Scholar] [CrossRef]
- Westman, M.E.; Malik, R.; Hall, E.; Sheehy, P.A.; Norris, J.M. Determining the feline immunodeficiency virus (FIV) status of FIV-vaccinated cats using point-of-care antibody kits. Comp. Immun. Microbiol. Infect. Dis. 2015, 42, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, O.; Ganiere, J.P. Comparative studies of the efficacy of a recombinant feline leukaemia virus vaccine. Vet. Rec. 1996, 138, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hofmann-Lehmann, R.; Tandon, R.; Boretti, F.S.; Meli, M.L.; Willi, B.; Cattori, V.; Gomes-Keller, M.A.; Ossent, P.; Golder, M.C.; Flynn, J.N.; Lutz, H. Reassessment of feline leukaemia virus (FeLV) vaccines with novel sensitive molecular assays. Vaccine 2006, 24, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.N.; O’Halloran, K.P.; Larson, L.J.; Schultz, R.D.; Hoover, E.A. Feline leukemia virus immunity induced by whole inactivated virus vaccination. Vet. Immunol. Immunopathol. 2010, 134, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Hofmann-Lehmann, R.; Cattori, V.; Tandon, R.; Boretti, F.S.; Meli, M.L.; Riond, B.; Lutz, H. How molecular methods change our views of FeLV infection and vaccination. Vet. Immunol. Immunopathol. 2008, 123, 119–123. [Google Scholar] [CrossRef]
- Westman, M.E.; Malik, R.; Norris, J.M. Diagnosing feline immunodeficiency virus (FIV) and feline leukaemia virus (FeLV) infection: An update for clinicians. Aust. Vet. J. 2019, 97, 47–55. [Google Scholar] [CrossRef]
- Tandon, R.; Cattori, V.; Gomes-Keller, M.A.; Meli, M.L.; Golder, M.C.; Lutz, H.; Hofmann-Lehmann, R. Quantitation of feline leukaemia virus viral and proviral loads by TaqMan (R) real-time polymerase chain reaction. J. Virol. Methods 2005, 130, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Helps, C.R.; Lait, P.; Damhuis, A.; Björnehammar, U.; Bolta, D.; Brovida, C.; Chabanne, L.; Egberink, H.; Ferrand, G.; Fontbonne, A.; et al. Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: Experience from 218 European catteries. Vet. Rec. 2005, 156, 669–673. [Google Scholar] [CrossRef]
- Pinches, M.D.; Helps, C.R.; Gruffydd-Jones, T.J.; Egan, K.; Jarrett, O.; Tasker, S. Diagnosis of feline leukaemia virus infection by semi-quantitative real-time polymerase chain reaction. J. Feline Med. Surg. 2007, 9, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H.; Pedersen, N.C.; Durbin, R.; Theilen, G.H. Monoclonal antibodies to three epitopic regions of feline leukemia virus p27 and their use in enzyme-linked immunosorbent assay of p27. J. Immunol. Methods 1983, 56, 209–220. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Huder, J.B.; Gruber, S.; Boretti, F.; Sigrist, B.; Lutz, H. Feline leukaemia provirus load during the course of experimental infection and in naturally infected cats. J. Gen. Virol. 2001, 82, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Monograph of the European Pharmacopoeia Commission. Council of Europe, and Convention on the Elaboration of a European Pharmacopoeia. Feline leukemia vaccine (inactivated). In European Pharmacopoiea, 5th ed.; Sainte-Ruffine: Maisonneuve, France, 2005; p. 761. [Google Scholar]
- Beatty, J.A.; Tasker, S.; Jarrett, O.; Lam, A.; Gibson, S.; Noe-Nordberg, A.; Phillips, A.; Fawcett, A.; Barrs, V.R. Markers of feline leukaemia virus infection or exposure in cats from a region of low seroprevalence. J. Feline Med. Surg. 2011, 13, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Keller, M.A.; Gönczi, E.; Tandon, R.; Riondato, F.; Hofmann-Lehmann, R.; Meli, M.L.; Lutz, H. Detection of feline leukemia virus RNA in saliva from naturally infected cats and correlation of PCR results with those of current diagnostic methods. J. Clin. Microbiol. 2006, 44, 916–922. [Google Scholar] [CrossRef]
- Malik, R.; Kendall, K.; Cridland, J.; Coulston, S.; Stuart, A.J.; Snow, D.; Love, D.N. Prevalences of feline leukaemia virus and feline immunodeficiency virus infections in cats in Sydney. Aust. Vet. J. 1997, 75, 323–327. [Google Scholar] [CrossRef]
- Norris, J.M.; Bell, E.T.; Hales, L.; Toribio, J.A.L.; White, J.D.; Wigney, D.I.; Baral, R.M.; Malik, R. Prevalence of feline immunodeficiency virus infection in domesticated and feral cats in eastern Australia. J. Feline Med. Surg. 2007, 9, 300–308. [Google Scholar] [CrossRef]
- Bęczkowski, P.M.; Litster, A.; Lin, T.L.; Mellor, D.J.; Willett, B.J.; Hosie, M.J. Contrasting clinical outcomes in two cohorts of cats naturally infected with feline immunodeficiency virus (FIV). Vet. Microbiol. 2015, 176, 50–60. [Google Scholar] [CrossRef]
- Wilson, S.; Greenslade, J.; Saunders, G.; Holcroft, C.; Bruce, L.; Scobey, A.; Childers, T.; Sture, G.; Thompson, J. Difficulties in demonstrating long term immunity in FeLV vaccinated cats due to increasing age-related resistance to infection. BMC Vet. Res. 2012, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Benka, V.A.; Briggs, J.R.; Maki, J.; Morris, K.N.; Myers, K.A.; Rhodes, L.; Weedon, G.R.; Levy, J.K. Hybrid model intermediate between a laboratory and field study: A humane paradigm shift in feline research. J. Feline Med. Surg. 2018, 20, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Boenzli, E.; Hadorn, M.; Hartnack, S.; Huder, J.; Hofmann-Lehmann, R.; Lutz, H. Detection of antibodies to the feline leukemia virus (FeLV) transmembrane protein p15E: an alternative approach for serological FeLV detection based on antibodies to p15E. J. Clin. Microbiol. 2014, 52, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Day, M.J.; Thiry, E.; Lloret, A.; Frymus, T.; Addie, D.; Boucraut-Baralon, C.; Egberink, H.; Gruffydd-Jones, T.; Horzinek, M.C.; Hosie, M.J. Feline injection site sarcoma: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2015, 17, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, A.H. Feline leukaemia virus and vaccination. J. Feline Med. Surg. 2003, 5, 97–100. [Google Scholar] [CrossRef]
- Torres, A.N.; Mathiason, C.K.; Hoover, E.A. Re-examination of feline leukemia virus: Host relationships using real-time PCR. Virology 2005, 332, 272–283. [Google Scholar] [CrossRef]
- Patel, M.; Carritt, K.; Lane, J.; Jayappa, H.; Stahl, M.; Bourgeois, M. Comparative Efficacy of Feline Leukemia Virus (FeLV) Inactivated Whole-Virus Vaccine and Canarypox Virus-Vectored Vaccine during Virulent FeLV Challenge and Immunosuppression. Clin. Vaccine Immunol. 2015, 22, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Stuke, K.; King, V.; Southwick, K.; Stoeva, M.I.; Thomas, A.; Winkler, M.T.C. Efficacy of an inactivated FeLV vaccine compared to a recombinant FeLV vaccine in minimum age cats following virulent FeLV challenge. Vaccine 2014, 32, 2599–2603. [Google Scholar] [CrossRef]
- Rojko, J.L.; Hoover, E.A.; Quackenbush, S.L.; Olsen, R.G. Reactivation of latent feline leukaemia virus infection. Nature 1982, 298, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Nesina, S.; Helfer-Hungerbuehler, A.K.; Riond, B.; Boretti, F.S.; Willi, B.; Meli, M.L.; Grest, P.; Hofmann-Lehmann, R. Retroviral DNA - the silent winner: blood transfusion containing latent feline leukemia provirus causes infection and disease in naive recipient cats. Retrovirology 2015, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Hartmann, K.; Addie, D.D.; Lutz, H.; Gruffydd-Jones, T.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Horzinek, M.C.; Hosie, M.J.; et al. Blood transfusion in cats: ABCD guidelines for minimising risks of infectious iatrogenic complications. J. Feline Med. Surg. 2015, 17, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, O.; Pacitti, A.M.; Hosie, M.J.; Reid, G. Comparison of diagnostic methods for feline leukemia virus and feline immunodeficiency virus. J. Am. Vet. Med. Assoc. 1991, 199, 1362–1364. [Google Scholar] [PubMed]
- Miyazawa, T.; Jarrett, O. Feline leukaemia virus proviral DNA detected by polymerase chain reaction in antigenaemic but non-viraemic (‘discordant’) cats. Arch. Virol. 1997, 142, 323–332. [Google Scholar] [CrossRef] [PubMed]
| FeLV Infection Status | Category | Results from FeLV Tests Performed | ||
|---|---|---|---|---|
| PoC Testing (No. of Positive Kits) | qPCR | Nab 1 | ||
| FeLV-unexposed | 0/3 | − | − | |
| FeLV-uninfected | Definitively abortive infection (FeLV-unvaccinated cats) | 0/3 | − | + |
| Presumptively abortive infection (FeLV-vaccinated cats) | 0/3 | − | + | |
| FeLV-infected | Presumptively regressive infection | 0/3 | + | −/+ |
| Presumptively progressive infection | 1/3, 2/3 or 3/3 | + | −/+ | |
| Group | Description | FeLV-Uninfected 1 | FeLV-Infected | ||
|---|---|---|---|---|---|
| Presumptively Regressive Infections 2 | Presumptively Progressive Infections 3 | Ratio Regressive:Progressive Infections | |||
| Group 1 (n = 440) | Healthy, client-owned cats recruited predominantly from eastern Australia as part of a study into the effectiveness of a commercially available FIV vaccine | 429/440 (98%), including 47/440 (11%) presumptively abortive infections | 9/440 (2%), including 0/8 with detectable circulating vRNA | 2/440 (0.5%) | 4.5 |
| Group 2 (n = 38) | Mixture of healthy and sick cats being group-housed at a rescue facility with recent cases of FeLV infection | 24/38 (63%), including 4/38 (11%) abortive infections | 7/38 (18%), including 1/6 (17%) with detectable circulating vRNA | 7/38 (18%) | 1.0 |
| Group 3 (n = 51) | Mixture of healthy and sick cats being group-housed at a rescue facility with recent cases of FeLV infection | 24/51 (47%), including 4/51 (8%) abortive infections | 15/51 (29%) including 5/14 (36%) with detectable circulating vRNA | 12/51 (24%) | 1.25 |
| Group | Negative NAb Result (<4) | Positive NAb Result | Positive Laboratory-Based p27 ELISA | Positive qRT-PCR | |||||
|---|---|---|---|---|---|---|---|---|---|
| 4 (weak) | 8 | 16 | ≥32 (strong) | Total NAb Positive | |||||
| 1 | FeLV-uninfected (n = 429) | 370 | 16 | 15 | 10 | 6 | 47/417 1 | NP | NP |
| 1 | Presumptively regressive infections (n = 9) | 2 | 0 | 1 | 1 | 5 | 7/9 | 0/6 | 0/8 |
| 1 | Presumptively progressive infections (n = 2) | 2 | 0 | 0 | 0 | 0 | 0/2 | 1/1 | 1/1 |
| 2 | FeLV-uninfected (n = 24) | 15 | 0 | 0 | 2 | 2 | 4/19 | 0/18 | 0/19 |
| 2 | Presumptively regressive infections (n = 7) | 0 | 0 | 1 | 0 | 5 | 6/6 | 0/5 | 1/6 |
| 2 | Presumptively progressive infections (n = 7) | 1 | 0 | 0 | 0 | 0 | 0/1 | 6/6 | 6/6 |
| 3 | FeLV-uninfected (n = 24) | 16 | 0 | 1 | 1 | 2 | 4/20 | 0/20 | 0/20 |
| 3 | Presumptively regressive infections (n = 15) | 0 | 0 | 0 | 1 | 13 | 14/14 | 0/13 | 5/14 |
| 3 | Presumptively progressive infections (n = 12) | 9 | 0 | 0 | 0 | 3 | 3/12 | 8/10 | 9/11 |
| Cat ID | Presumptive Category of Infection | FeLV Vaccination Status | Test Performed | ||||
|---|---|---|---|---|---|---|---|
| PoC Testing (No. of Positive Kits) | Laboratory-Based p27 ELISA | qPCR CT | qRT-PCR CT | NAb Assay | |||
| Group 1 - #41 | Regressive | Unvaccinated | 0/3 | - | 39.8 | - | - |
| Group 1 - #386 | Regressive | Unvaccinated | 0/3 | NP | 39.2 | NP | + (≥ 1/32) |
| Group 1 - #141 | Regressive | On-time | 0/3 | - | 39.1 | - | + (1/8) |
| Group 1 - #251 | Regressive | Unvaccinated | 0/3 | NP | 37.6 | - | + (≥ 1/32) |
| Group 2 - #15 | Regressive | Unvaccinated | 0/3 | - | 37.5 | - | + (1/8) |
| Group 3 - #19 | Regressive | Unvaccinated | 0/3 | - | 37.3 | - | + (≥ 1/32) |
| Group 3 - #5 | Regressive | Unvaccinated | 0/3 | - | 37.2 | - | + (≥ 1/32) |
| Group 1 - #71 | Regressive | On-time | 0/3 | NP | 37.1 | - | + (≥ 1/32) |
| Group 3 - #13 | Regressive | Unvaccinated | 0/3 | - | 36.9 | - | + (1/16) |
| Group 2 - #3 | Regressive | Unvaccinated | 0/3 | - | 36.8 | - | + (≥ 1/32) |
| Group 3 - #7 | Regressive | Unvaccinated | 0/3 | NP | 36.6 | - | + (≥ 1/32) |
| Group 1 - #48 | Regressive | Overdue (last given 11.2 years prior) | 0/3 | - | 36.4 | - | + (1/16) |
| Group 3 - #38 | Regressive | Unvaccinated | 0/3 | - | 36.2 | 39.1 | + (≥ 1/32) |
| Group 3 - #6 | Regressive | Unvaccinated | 0/3 | - | 36.2 | - | + (≥ 1/32) |
| Group 3 - #36 | Regressive | Unvaccinated | 0/3 | - | 35.4 | - | + (≥ 1/32) |
| Group 3 - #18 | Regressive | Unvaccinated | 0/3 | - | 35.3 | - | + (≥ 1/32) |
| Group 3 - #34 | Regressive | Unvaccinated | 0/3 | - | 35.2 | - | + (≥ 1/32) |
| Group 3 - #17 | Regressive | Unvaccinated | 0/3 | NP | 35.1 | NP | NP |
| Group 2 - #22 | Regressive | Unvaccinated | 0/3 | - | 35.0 | - | + (≥ 1/32) |
| Group 1 - #287 | Regressive | Unvaccinated | 0/3 | - | 34.9 | - | + (≥ 1/32) |
| Group 1 - #252 | Regressive | Unvaccinated | 0/3 | - | 34.5 | - | + (≥ 1/32) |
| Group 2 - #38 | Regressive | Unvaccinated | 0/3 | NP | 34.4 | - | + (≥ 1/32) |
| Group 1 - #258 | Regressive | Unvaccinated | 0/3 | - | 34.1 | - | - |
| Group 3 - #27 | Regressive | Unvaccinated | 0/3 | - | 33.2 | - | + (≥ 1/32) |
| Group 3 - #15 | Regressive | Unvaccinated | 0/3 | - | 33.1 | 38.4 | + (≥ 1/32) |
| Group 2 - #13 | Regressive | Unvaccinated | 0/3 | - | 32.9 | 31.8 | + (≥ 1/32) |
| Group 3 - #33 | Regressive | Unvaccinated | 0/3 | - | 31.2 | 33.7 | + (≥ 1/32) |
| Group 3 - #52 | Regressive | Unvaccinated | 0/3 | - | 31.2 | 35.9 | + (≥ 1/32) |
| Group 3 - #29 | Regressive | Unvaccinated | 0/3 | - | 31.2 | 33.7 | + (≥ 1/32) |
| Group 2 - #36 | Regressive | Unvaccinated | 0/3 | NP | 29.4 | NP | NP |
| Group 2 - #4 | Regressive | Unvaccinated | 0/3 | - | 28.0 | - | + (≥ 1/32) |
| TOTAL POSITIVE (presumptively regressive) | 3/31 vaccinated | 31 × 0/3 | 0/24 | 31/31 | 6/28 | 27/29 | |
| Group 3 - #3 | Progressive | Unvaccinated | 1/3 1 | + (43.5%) | 37.1 | - | + (≥ 1/32) |
| Group 3 - #12 | Progressive | Unvaccinated | 1/3 1 | - | 36.4 | - | + (≥ 1/32) |
| Group 1 - #330 | Progressive | Unvaccinated | 1/3 1 | NP | 35.3 | NP | - |
| Group 3 - #40 | Progressive | Unvaccinated | 3/3 | + (13.4%) | 31.3 | 31.1 | - |
| Group 3 - #9 | Progressive | Unvaccinated | 3/3 | + (6.4%) | 31.1 | 34.1 | - |
| Group 3 - #50 | Progressive | Unvaccinated | 3/3 | - (1.5%) | 28.2 | 27.4 | + (≥ 1/32) |
| Group 2 - #21 | Progressive | Unvaccinated | 3/3 | + (24.9%) | 22.7 | 18.9 | NP |
| Group 3 - #51 | Progressive | Unvaccinated | 3/3 | + (61.2%) | 22.5 | 18.6 | - |
| Group 3 - #10 | Progressive | Unvaccinated | 3/3 | + (80.0%) | 21.5 | 18.5 | - |
| Group 2 - #30 | Progressive | Unvaccinated | 3/3 | + (35.1%) | 21.4 | 17.7 | NP |
| Group 3 - #16 | Progressive | Unvaccinated | 3/3 | NP | 21.3 | NP | - |
| Group 2 - #31 | Progressive | Unvaccinated | 3/3 | + (36.6%) | 21.3 | 17.0 | NP |
| Group 2 - #5 | Progressive | Unvaccinated | 3/3 | + (59.2%) | 19.2 | 17.7 | NP |
| Group 2 - #20 | Progressive | Unvaccinated | 3/3 | + (27.2%) | 19.2 | 17.1 | NP |
| Group 3 - #26 | Progressive | Unvaccinated | 3/3 | NP | 18.4 | 16.5 | - |
| Group 3 - #31 | Progressive | Unvaccinated | 3/3 | + (105.2%) | 18.3 | 15.2 | - |
| Group 2 - #7 | Progressive | Unvaccinated | 3/3 | + (26.7%) | 18.2 | 18.5 | NP |
| Group 2 - #14 | Progressive | Unvaccinated | 3/3 | NP | 18.0 | NP | - |
| Group 3 - #39 | Progressive | Unvaccinated | 3/3 | + (49.3%) | 17.8 | 15.6 | - |
| Group 3 - #42 | Progressive | Unvaccinated | 3/3 | + (65.2%) | 17.6 | 15.3 | - |
| Group 1 - #62 | Progressive | On-time | 3/3 | + (55.9%) | 16.8 | 18.7 | - |
| TOTAL POSITIVE (presumptively progressive) | 1/21 vaccinated | 3 × 1/3, 18 × 3/3 | 15/17 | 21/21 | 16/18 | 3/15 | |
| Cat ID | Age at Time of Diagnosis (Years) | Sex | Outcome |
|---|---|---|---|
| PRESUMPTIVELY REGRESSIVE INFECTIONS | |||
| Group 1 - #41 | 15 | MN | Found dead 25 months after diagnosis, suspected due to progression of CKD |
| Group 1 - #48 | 12 | MN | Lost to follow up |
| Group 1 - #71 | 6 | FS | Alive and doing well at the time of writing |
| Group 1 - #141 | 15.8 | MN | Euthanased 15 months after diagnosis due to progression of CKD |
| Group 1 - #251 | 2.3 | MN | Lost to follow up |
| Group 1 - #252 | 4.9 | MN | Lost to follow up |
| Group 1 - #258 | 10.2 | FS | Lost to follow up |
| Group 1 - #287 | 7.6 | MN | Lost to follow up |
| Group 1 - #386 | 11.4 | MN | Lost to follow up |
| Group 2 - #3 | 10 | FS | Alive and doing well at the time of writing. Stable diabetic (in remission at the time of writing) |
| Group 2 - #4 | 14 | FS | Euthanased due to suspected cardiomyopathy |
| Group 2 - #13 | 10 | MN | Euthanased due to geriatric-related disease |
| Group 2 - #15 | 5 | FS | Alive and doing well at the time of writing |
| Group 2 - #22 | 4 | MN | Found dead, suspected due to brown snake bite |
| Group 2 - #36 | 13 | FS | Euthanased due to progression of CKD |
| Group 2 - #38 | 3 | FS | Lost to follow up (ran away) |
| PRESUMPTIVELY PROGRESSIVE INFECTIONS | |||
| Group 1 - #62 | 3.4 | MN | Euthanased 8 months after diagnosis since not doing well (inappetent and constipated) |
| Group 1 - #330 | 6.4 | FS | Alive and doing well at the time of writing |
| Group 2 - #5 | 11 | FS | Euthanased 24 months after diagnosis due to mass in abdomen, post-mortem not performed |
| Group 2 - #7 | 11 | FS | Euthanased due to progression of CKD |
| Group 2 - #14 | 4 | MN | Euthanased due to multiple diffuse skin masses, one month after FeLV diagnosis. Feline sarcoma virus (FeSV) infection was diagnosed post-mortem (histopathology and gp70 staining of masses) |
| Group 2 - #20 | 8 | MN | Euthanased 38 months after diagnosis due to nasal cavity tumour |
| Group 2 - #21 | 9 | MN | Euthanased 13 months after diagnosis due to breathing difficulties, cardiac disease suspected due to severe heart murmur, post-mortem not performed, co-infected with FIV |
| Group 2 - #30 | 2 | MN | Euthanased 22 months after diagnosis due to breathing difficulties, post-mortem revealed mediastinal lymphoma |
| Group 2 - #31 | 2 | MN | Euthanased 6 months after diagnosis, severe non-regenerative anaemia and icterus, post-mortem revealed hepatomegaly, splenomegaly, pericardial effusion and pleural effusion |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westman, M.; Norris, J.; Malik, R.; Hofmann-Lehmann, R.; Harvey, A.; McLuckie, A.; Perkins, M.; Schofield, D.; Marcus, A.; McDonald, M.; et al. The Diagnosis of Feline Leukaemia Virus (FeLV) Infection in Owned and Group-Housed Rescue Cats in Australia. Viruses 2019, 11, 503. https://doi.org/10.3390/v11060503
Westman M, Norris J, Malik R, Hofmann-Lehmann R, Harvey A, McLuckie A, Perkins M, Schofield D, Marcus A, McDonald M, et al. The Diagnosis of Feline Leukaemia Virus (FeLV) Infection in Owned and Group-Housed Rescue Cats in Australia. Viruses. 2019; 11(6):503. https://doi.org/10.3390/v11060503
Chicago/Turabian StyleWestman, Mark, Jacqueline Norris, Richard Malik, Regina Hofmann-Lehmann, Andrea Harvey, Alicia McLuckie, Martine Perkins, Donna Schofield, Alan Marcus, Mike McDonald, and et al. 2019. "The Diagnosis of Feline Leukaemia Virus (FeLV) Infection in Owned and Group-Housed Rescue Cats in Australia" Viruses 11, no. 6: 503. https://doi.org/10.3390/v11060503
APA StyleWestman, M., Norris, J., Malik, R., Hofmann-Lehmann, R., Harvey, A., McLuckie, A., Perkins, M., Schofield, D., Marcus, A., McDonald, M., Ward, M., Hall, E., Sheehy, P., & Hosie, M. (2019). The Diagnosis of Feline Leukaemia Virus (FeLV) Infection in Owned and Group-Housed Rescue Cats in Australia. Viruses, 11(6), 503. https://doi.org/10.3390/v11060503







