The Avocado Sunblotch Viroid: An Invisible Foe of Avocado
Abstract
1. The Origin of the Avocado and the Avocado Sunblotch Viroid
2. Symptoms
3. Taxonomy and Structure of ASBVd
4. Host range of ASBVd
5. Transmission
6. Economic Importance
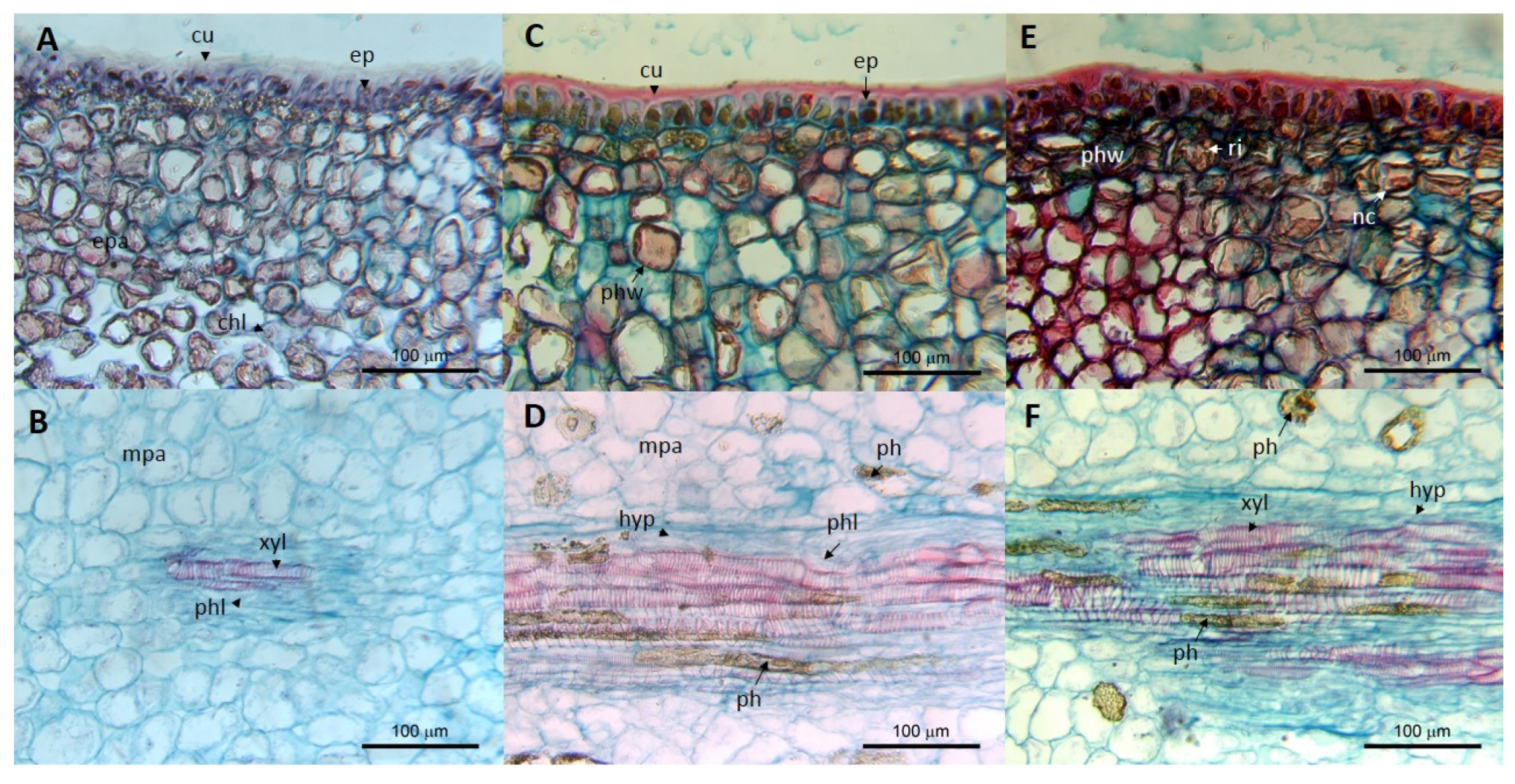
7. Postharvest and Histological Effects of ASBVd
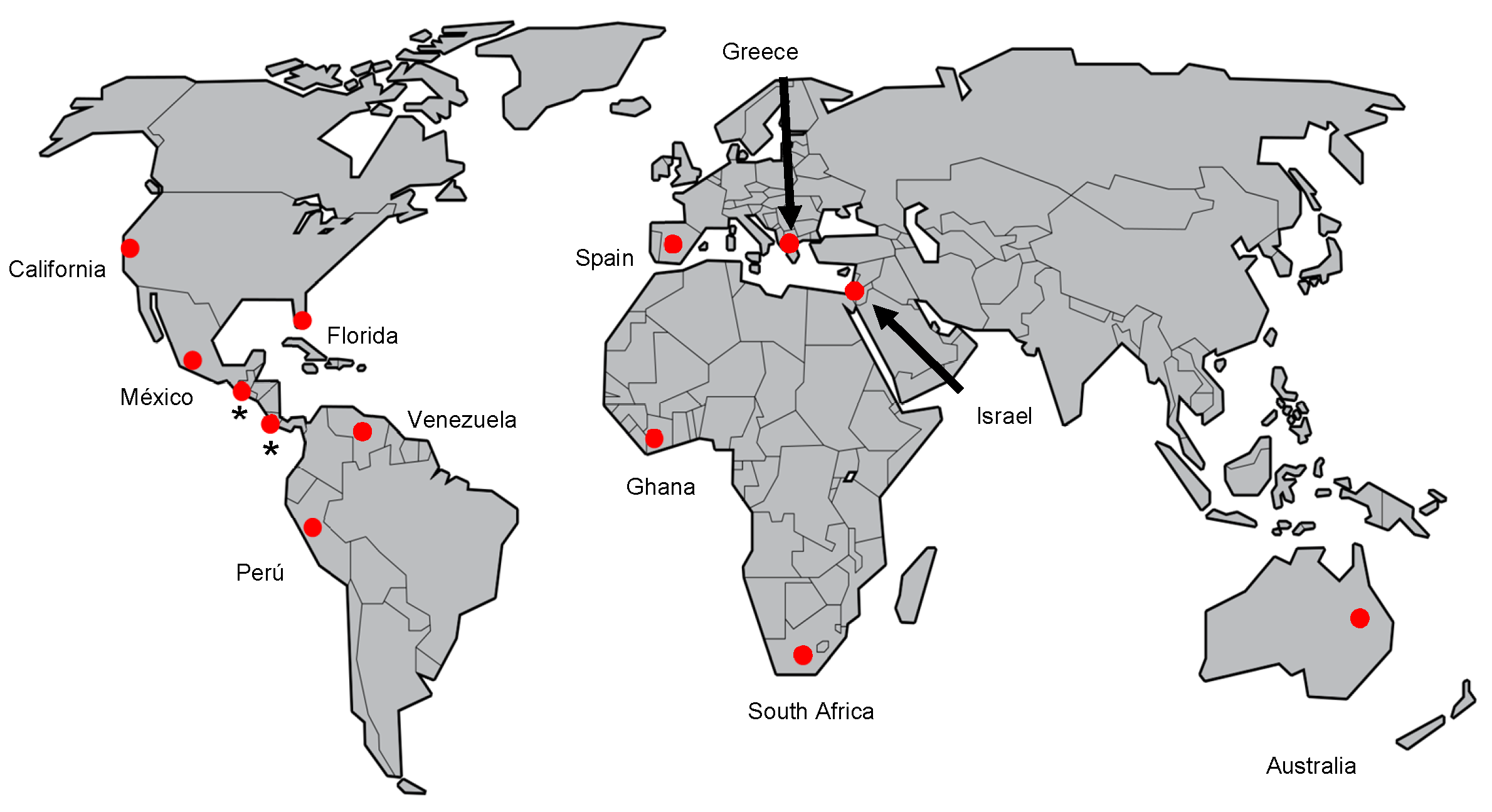
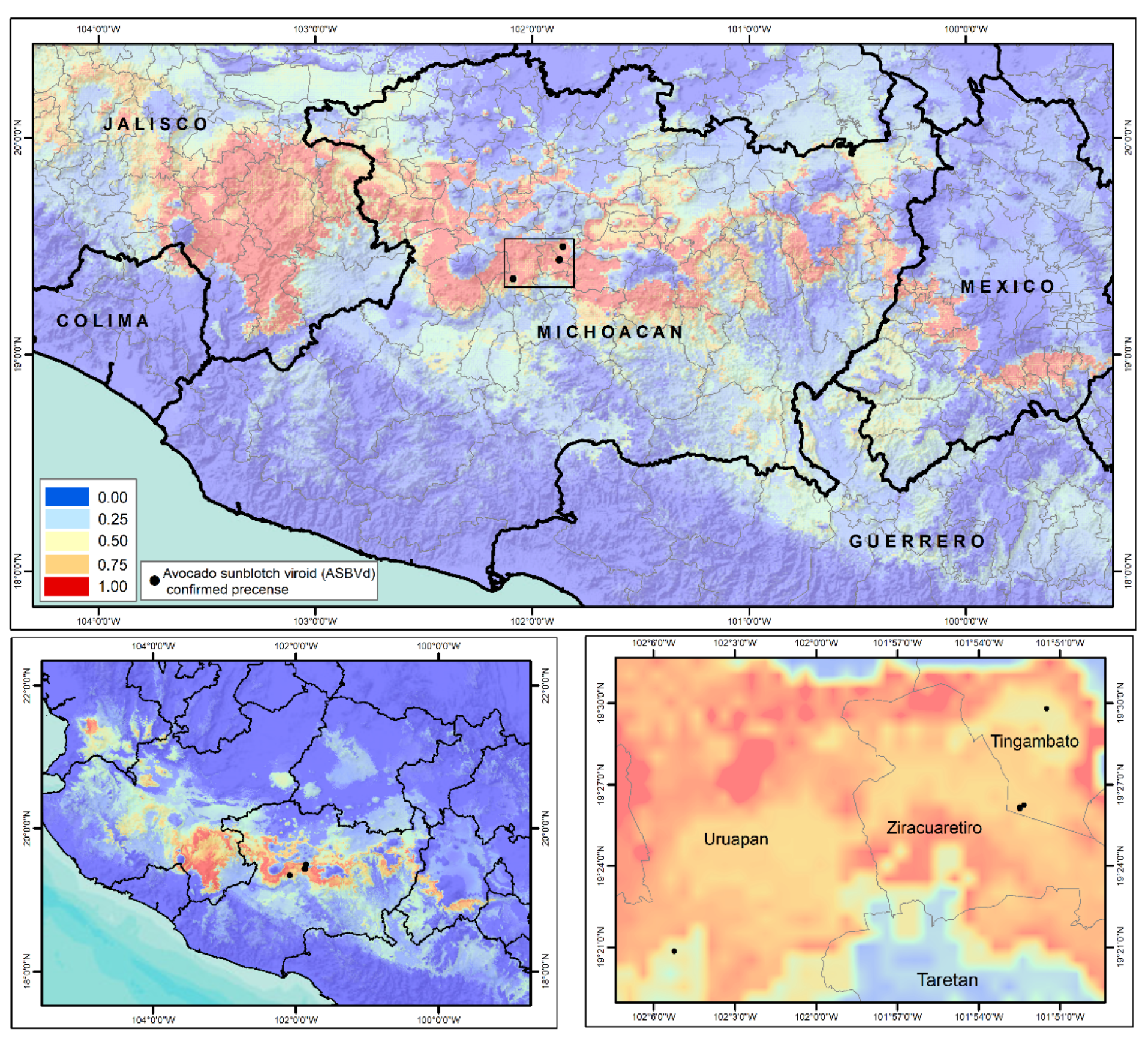
8. Geographic Distribution
9. Diagnostic Methods
10. Management of ASBVd
11. Future perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, L.O. The avocados, a synopsis of the genus Persea, subg. Persea. Econ. Bot. 1977, 31, 315–320. [Google Scholar] [CrossRef]
- Smith, C.E. Archeological evidence for selection in avocado. Econ. Bot. 1966, 20, 166–175. [Google Scholar] [CrossRef]
- Téliz, D.; Mora, G.; Morales, L. Importancia histórica y socioeconómica del aguacate. In El Aguacate y su Manejo Integrado; Téliz, D., Mora, A., Eds.; Mundi-Prensa: México, D.F, México, 2000; p. 231. ISBN 968-7462-15-9. [Google Scholar]
- California Avocado History. Available online: https://www.californiaavocado.com/avocado101/the-california-difference/avocado-history (accessed on 17 March 2019).
- Luttig, M.; Manicom, B.Q. Application of highly sensitive indexing method. South Afr. Avocado Grow. Assoc. Yearb. 1999, 22, 55–60. [Google Scholar]
- Suárez, I.E.; Litz, R.E.; Schnell, R.J.; Kuhn, D.N. El viroide de la mancha de sol (ASBVd) es persistente en cultivos nucelares de aguacate (Persea americana Mill.). Rev. Colomb. Biotecnol. 2005, 7, 10–18. [Google Scholar]
- Geering, A.D. A review of the status of Avocado sunblotch viroid in Australia. Australas. Plant Pathol. 2018, 47, 555–559. [Google Scholar] [CrossRef]
- Whitsell, R. Sunblotch disease of avocados. Calif. Avocado Soc. Yearb. 1952, 37, 215–240. [Google Scholar]
- Coit, J.E. Sunblotch of the avocado. Calif. Avocado Soc. Yearb. 1928, 20, 27–32. [Google Scholar]
- Horne, W.T. Progress in the study of certain disease of avocado. Phytopathology 1929, 19, 1144. [Google Scholar]
- Horne, W.T.; Parker, E.R.; Rounds, M.B. The nature of Sun-blotch and its practical control. Calif. Avocado Soc. Yearb. 1941, 26, 35–38. [Google Scholar]
- Wallace, J.M. The sun-blotch disease of avocados. Calif. Avocado Soc. Yearb. 1958, 42, 86–89. [Google Scholar]
- Dale, J.L.; Allen, R.N. Avocado affected by sunblotch disease contains low molecular weight ribonucleic acid. Australas. Plant Pathol. 1979, 8, 3–4. [Google Scholar] [CrossRef]
- Desjardins, P.R.; Drake, R.J.; Swiecki, S.A. Infectivity studies of avocado sunblotch disease causal agent, possibly a viroid rather than a virus. Plant Dis. 1980, 64, 313–315. [Google Scholar] [CrossRef]
- Thomas, W.; Mohamed, N.A. Avocado sunblotch-a viroid disease? Australas. Plant Pathol. 1979, 8, 1–3. [Google Scholar] [CrossRef]
- Allen, R.; Palukaitis, P.; Symons, R. Purified Avocado sunblotch viroid causes disease in avocado seedlings. Australas. Plant Pathol. 1981, 10, 31–32. [Google Scholar] [CrossRef]
- Lopez-Rivera, L.A.; Ramírez-Ramírez, I.; Gonzalez-Hernandez, V.A.; Cruz-Huerta, N.; Teliz Ortiz, D. Differential gene expression of avocado defense genes in response to avocado sunblotch viroid infection. Revista Mexicana de Fitopatología 2017, 36, 151–161. [Google Scholar]
- MacKenzie, D.J.; McLean, M.A.; Mukerji, S.; Green, M. Improved RNA extraction from woody plants for the detection of viral pathogens by reverse transcription-polymerase chain reaction. Plant Dis. 1997, 81, 222–226. [Google Scholar] [CrossRef]
- Trask, E.E. Observations on the avocado industry in México. Calif. Avocado Soc. Yearb. 1948, 33, 50–53. [Google Scholar]
- Dale, J.L.; Symons, R.H.; Allen, R.N.; Avocado sunblotch viroid. CMI/AAB Descriptions of Plant Viruses. 1982, p. 254. Available online: http://www.dpvweb.net/dpv/showdpv.php?dpvno=254 (accessed on 11 February 2019).
- Desjardins, P.R.; Saski, P.J.; Drake, R.J. Chemical inactivation of avocado sunblotch viroid on pruning and propagation tools. Calif. Avocado Soc. Yearb. 1987, 71, 259–262. [Google Scholar]
- Schnell, R.J.; Kuhn, D.N.; Olano, C.T.; Quintanilla, W.E. Sequence diversity among avocado sunblotch viroids isolated from single avocado trees. Phytoparasitica 2001, 29, 451–460. [Google Scholar] [CrossRef]
- Semancik, J.S. Avocado viroids: Avocado Sunblotch viroid. In The Viroids; Hadidi, A., Flores, R., Randles, J.W., Semancik, J.S., Eds.; CSIRO Publishing: Melbourne, Australia, 2003; pp. 171–177. [Google Scholar]
- Semancik, J.S.; Szychowski, J.A. Avocado sunblotch disease a persistent viroid infection in which variants are associated with differential symptoms. J. Gen. Virol. 1994, 75, 1543–1549. [Google Scholar]
- Kuhn, D.N.; Geering, D.W.; Dixon, J. Avocado sunblotch viroid. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J., Palukaitis, P., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 299–300. ISBN 9780128017029. [Google Scholar]
- Saucedo-Carabez, J.R.; Téliz-Ortiz, D.; Ochoa-Ascensio, S.; Ochoa-Martinez, D.; Vallejo-Pérez, M.R.; Beltrán-Pena, H. Effect of Avocado sunblotch viroid (ASBVd) on avocado yield in Michoacan, México. Eur. J. Plant Pathol. 2014, 138, 799–805. [Google Scholar] [CrossRef]
- Vallejo-Pérez, M.R.; Téliz-Ortiz, D.; De La Torre-Almaraz, R.; Valdovinos-Ponce, G.; Colinas-León, M.T.; Nieto-Ángel, D.; Ochoa-Martínez, D.L. Histopathology of avocado fruit infected by avocado sunblotch viroid. J. Agric. Sci. 2014, 6, 158–165. [Google Scholar] [CrossRef]
- Vallejo-Pérez, M.R.; Téliz-Ortiz, D.; Colinas-León, M.T.; De La Torre-Almaraz, R.; Valdovinos-Ponce, G.; Nieto-Ángel, D.; Ochoa-Martinez, D.L. Alterations induced by Avocado sunblotch viroid in the postharvest physiology and quality of avocado Hass fruit. Phytoparasitica 2015, 43, 355–364. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Zentmyer, G.A.; Nishijima, R.T.; Rohrbach, K.G.; Ohr, D. Compendium of Tropical Fruit Diseases; APS Press: St Paul, MN, USA, 1998; p. 88. [Google Scholar]
- Ploetz, R.C.; Dann, E.; Pegg, K.; Eskalen, A.; Ochoa, S.; Campbell, A. Pathogen exclusion: Options and implementation. In Proceedings of the 7th World Avocado Congress, Cairns, Queensland, Australia, 5–9 September 2011. [Google Scholar]
- Flores, R.; Gago-Zachert, S.; Serra, P.; Sanjuán, R.; Elena, S.F. Viroids: Survivors from the RNA world? Annu. Rev. Microbiol. 2014, 68, 395–414. [Google Scholar] [CrossRef]
- Diener, O.T. Circular RNAs: Relics of precellular evolution? Proc. Natl. Acad. Sci. USA 1989, 86, 9370–9374. [Google Scholar] [CrossRef]
- Flores, R. A naked plant-specific RNA ten-fold smaller than the smallest known viral RNA: The viroid. C. R. Acad. Sci. Paris Sci. de la vie/Life Sci. 2001, 324, 943–952. [Google Scholar] [CrossRef]
- Navarro, J.A.; Vera, A.; Flores, R. A chloroplastic RNA polymerase resistant to tagetitoxin is involved in replication of Avocado sunblotch viroid. Virology 2000, 268, 218–225. [Google Scholar] [CrossRef]
- Flores, R.; Hernandez, C.; Martinez de Alba, A.E.; Daros, J.A.; di Serio, F. Viroids and viroid-host interactions. Annu. Rev. Phytopathol. 2005, 43, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Giguère, T.; Adkar-Purushothama, C.R.; Bolduc, F.; Perreault, J.P. Elucidation of the structures of all the members of the Avsunviroidae family. Mol. Plant Pathol. 2014, 15, 767–779. [Google Scholar] [CrossRef]
- Daros, J.A.; Marcos, J.F.; Marcos, J.F.; Hernández, C.; Flores, R. Replication of avocado sunblotch viroid: Evidence for a symmetric pathway into two rolling circles and hammerhead ribozyme processing. Proc. Natl. Acad. Sci. USA 1994, 91, 12813–12817. [Google Scholar] [CrossRef]
- Hutchins, C.J.; Rathjen, P.D.; Foster, A.C.; Symons, R.H. Hammerhead ribozymes structure and function in plant RNA replication. Nucleic Acids Res. 1986, 14, 3627–3640. [Google Scholar] [CrossRef]
- López, C.A.; Flores, R. The predominant circular form of avocado sunblotch viroid accumulates in planta as a free RNA adopting a rod-shaped secondary structure unprotected by tightly bound host proteins. J. Gen. Virol. 2017, 98, 1913–1922. [Google Scholar]
- Da Graca, J.V. Avocado sunblotch research in South Africa. S. Afr. Avo. Grow. Avocado Res. Rep. 1978, 2, 53. [Google Scholar]
- Da Graca, J.V.; Van Vuuren, S.P. Transmission of avocado sunblotch disease to cinnamon. Plant Dis. 1980, 64, 475. [Google Scholar] [CrossRef]
- Da Graca, J.V.; Van Vuuren, S.P. Host range studies on avocado sunblotch. S. Afr. Avocado Grow. Assoc. Yearb. 1981, 4, 81–82. [Google Scholar]
- Delan-Forino, C.; Maurel, M.C.; Torchet, C. Replication of Avocado sunblotch viroid in yeast Saccharomyces cerevisae. J. Virol. 2011, 85, 3229–3238. [Google Scholar] [CrossRef]
- Latifi, A.; Bernard, C.; da Silva, L.; Andéol, Y.; Elleuch, A.; Riouls, V.; Vergne, J.; Maurel, M.C. Replication of Avocado sunblotch viroid in the cyanobacterium Nostoc sp. PCC7120. J. Plant Pathol. Microbiol. 2016, 7, 4. [Google Scholar] [CrossRef]
- Horne, W.T.; Parker, E.R. The avocado disease called sun-blotch. Phytopathology 1931, 21, 235–238. [Google Scholar]
- Wallace, J.M.; Drake, R.J. The high rate of seed transmission of avocado sun-blotch virus from symptomless trees and the origin of such trees. Phytopathology 1962, 52, 237–241. [Google Scholar]
- Suarez, I.E.; Schnell, R.A.; Kuhn, D.N.; Litz, R.E. Micrografting of ASBVd-infected avocado (Persea americana) plants. Plant Cell Tissue Organ Cult. 2005, 80, 179–185. [Google Scholar] [CrossRef]
- Barret, C.; Rounds, M.B.; Coit, J.E.; Shepard, S.; Hazzard, A.G.; Adams, W.; Biery, E.; Griswold, H.B.; Thille, J.N.; Trask, E.E.; et al. Report of the variety committee on avocados California Avocado Society-1945. Calif. Avocado Soc. Yearb. 1945, 30, 12–18. [Google Scholar]
- Desjardins, P.R.; Drake, R.J.; Atkins, E.L.; Bergh, B.O. Pollen transmission of avocado sunblotch virus experimentally demonstrated. Calif. Agri. 1979, 33, 14–15. [Google Scholar]
- Pegg, K.G.; Coates, L.M.; Korsten, L.; Harding, R.M. Foliar, fruit and soilborne disease. In The Avocado: Botany, Production and Uses; Whiley, A.W., Schaffer, B., Wolstenholme, B.N., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 299–358. [Google Scholar]
- GIIIA (Grupo Interdisciplinario e Interinstitucional de Investigación en Aguacate. La Mancha de Sol del Aguacate. In El Aguacate en Michoacán: Plagas y Enfermedades; APEAM AC-SENASICA, México; López Impresores: Morelia, Michoacán, México, 2013; pp. 40–42. ISBN 978-607-715-103-6. [Google Scholar]
- Da Graca, J.V.; Moon, T.E. Detection of avocado sunblotch viroid in flower buds by polyacrylamide gel electrophoresis. Phytopathology. Z. 1983, 108, 262–266. [Google Scholar] [CrossRef]
- Da Graca, J.V. Sunblotch associated reduction in fruit yield in both symptomatic and symptomless carrier trees. South Afr. Avocado Grow. Assoc. Yearb. 1985, 8, 59. [Google Scholar]
- Saucedo-Carabez, J.R.; Téliz-Ortiz, D.; Ochoa-Ascencio, S.; Ochoa-Martinez, D.; Vallejo-Pérez, M.R.; Beltrán-Peña, H. Effect of Avocado sunblotch viroid (ASBVd) on the postharvest quality of avocado fruits from México. J. Agric. Sci. 2015, 7, 85–92. [Google Scholar] [CrossRef][Green Version]
- European Plant Protection Organization (EPPO). Geographical Distribution of Avocado sunblotch viroid (ASBVd). 2016. Last Updated: 16 December 2016. Available online: https://gd.eppo.int/taxon/ASBVD0/distribution (accessed on 14 October 2018).
- Coit, J.E. Sunblotch of the avocado, a serious physiological disease. Calif. Avocado Soc. Yearb. 1928, 12, 26–29. [Google Scholar]
- Stevens, H.E.; Piper, R.B. Sunblotch in Avocado Disease in Florida; USDA: Washington, DC, USA, 1941; pp. 40–46. [Google Scholar]
- Trochoulias., T.; Allen, R.N. Sunblotch disease of avocado in New South Wales. Agric. Gaz. N. S. Wales 1970, 81, 67. [Google Scholar]
- Rondón, A.; Figueroa, M. Mancha de sol (sunblotch) de los aguacates (Persea americana) en Venezuela. Agron. Trop. 1976, 26, 463–466. [Google Scholar]
- Spiegel, S.; Alper, M.; Allen, R.N. Evaluation of biochemical methods for the diagnosis of the avocado sunblotch viroid in Israel. Phytoparasitica 1984, 12, 37–43. [Google Scholar] [CrossRef]
- López-Herrera, C.; Pliego, F.; Flores, R. Detection of avocado sunblotch viroid in Spain by double polyacrylamide gel electrophoresis. J. Phytopathol. 1987, 119, 184–189. [Google Scholar] [CrossRef]
- Vargas, C.O.; Querci, M.; Salazar, L.F. Identificación y estado de diseminación del viroide del manchado solar del palto (Persea americana L.) en el Perú y la existencia de otros viroides en palto. Fitopatología 1991, 26, 23–27. [Google Scholar]
- Acheampong, A.K.; Akromah, R.; Ofori, F.A.; Takrama, J.F.; Zeidan, M. Is there Avocado sunblotch viroid in Ghana? Afr. J. Biotechnol. 2008, 7, 3540–3545. [Google Scholar]
- De La Torre-Almaraz, R.; Téliz, O.D.; Pallás, V.; Sánchez, N.J.A. First Report of Avocado sunblotch viroid in avocado from Michoacán, México. Plant Dis. 2009, 93, 202. [Google Scholar] [CrossRef] [PubMed]
- Lotos, L.; Kavroulakis, N.; Navarro, B.; Di Serio, F.; Olmos, A.; Ruiz, G.A.; Katis, N.I.; Maliogka, V.I. First report of Avocado sunblotch viroid (ASBVd) naturally infecting Avocado (Persea americana) in Greece. Plant Dis. 2018, 102, 1470. [Google Scholar] [CrossRef]
- International Plant Protection Convention (IPPC). Determination of Pest Status in an Area. Last Updated: 29 May 2017. Available online: https://www.ippc.int/en/publications/612/ (accessed on 13 November 2018).
- Vallejo-Pérez, M.R.; Téliz-Ortiz, D.; De La Torre Almaraz, R.; López-Martínez, J.O.; Nieto-Ángel, D. Avocado sunblotch viroid: Pest risk and potential impact in México. Crop Prot. 2017, 99, 118–127. [Google Scholar] [CrossRef]
- Beltrán-Peña, H.; Soria-Ruiz, J.; Téliz-Ortiz, D.; Ochoa-Martínez, D.L.; Nava-Díaz, C.; Ochoa Ascencio, S. Molecular and satellite spectral imaging detection of Avocado sunblotch viroid (ASBVd). Rev. Fitotec. Mex. 2014, 37, 21–29. [Google Scholar]
- Moll, J.N.; Hussey, K.M.; Van Vuuren, S.P. Sunblotch indexing for plant impromevent scheme. South Afr. Avocado Grow. Assoc. Yearb. 1984, 7, 24. [Google Scholar]
- Palukaitis, P.; Hatta, T.; Alexander, D.M.; Symons, R.H. Characterization of a viroid associated with avocado sunblotch disease. Virology 1979, 99, 145–151. [Google Scholar] [CrossRef]
- Morey-León, G.; Ortega-Ramírez, E.; Julca-Chunga, C.; Santos-Chanta, C.; Graterol-Caldera, L.; Mialhe, E. The detection of Avocado sunblotch viroid in avocado using a real-time reverse transcriptase polymerase chain reaction. BioTechnologia 2018, 99, 99–107. [Google Scholar] [CrossRef]
- Palukaitis, P.; Rakowski, A.G.; Alexander, M.; Symons, R.H. Rapid indexing of the sunblotch disease of avocados using a complementary DNA probe to avocado sunblotch viroid. Ann. Appl. Biol. 1981, 98, 439–449. [Google Scholar] [CrossRef]
- Bonfiglioli, R.G.; McFadden, G.I.; Symons, R.H. In situ hybridization localizes avocado sunblotch viroid on chloroplast thylakoid membranes and coconut cadang cadang viroid in the nucleus. Plant J. 1994, 6, 99–103. [Google Scholar] [CrossRef]
- Lima, M.I.; Fonseca, M.E.N.; Flores, R.; Kitajima, E.W. Detection of avocado sunblotch viroid in chloroplasts of avocado leaves by in situ hybridization. Arch. Virol. 1994, 138, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, N.; Aparicio, F.; Rowhani, A.; Pallas, V. Comparative analysis of ELISA, nonradioactive molecular hybridization and PCR for the detection of prunus necrotic ringspot virus in herbaceous and Prunus hosts. Plant Pathol. 1998, 47, 780–786. [Google Scholar] [CrossRef]
- Schnell, R.J.; Kuhn, D.N.; Ronning, C.M.; Harkins, D. Application of RT-PCR for indexing avocado sunblotch viroid. Plant Dis. 1997, 81, 1023–1026. [Google Scholar] [CrossRef] [PubMed][Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saucedo Carabez, J.R.; Téliz Ortiz, D.; Vallejo Pérez, M.R.; Beltrán Peña, H. The Avocado Sunblotch Viroid: An Invisible Foe of Avocado. Viruses 2019, 11, 491. https://doi.org/10.3390/v11060491
Saucedo Carabez JR, Téliz Ortiz D, Vallejo Pérez MR, Beltrán Peña H. The Avocado Sunblotch Viroid: An Invisible Foe of Avocado. Viruses. 2019; 11(6):491. https://doi.org/10.3390/v11060491
Chicago/Turabian StyleSaucedo Carabez, José Ramón, Daniel Téliz Ortiz, Moisés Roberto Vallejo Pérez, and Hugo Beltrán Peña. 2019. "The Avocado Sunblotch Viroid: An Invisible Foe of Avocado" Viruses 11, no. 6: 491. https://doi.org/10.3390/v11060491
APA StyleSaucedo Carabez, J. R., Téliz Ortiz, D., Vallejo Pérez, M. R., & Beltrán Peña, H. (2019). The Avocado Sunblotch Viroid: An Invisible Foe of Avocado. Viruses, 11(6), 491. https://doi.org/10.3390/v11060491




