Current Understanding of Human Enterovirus D68
Abstract
1. Introduction
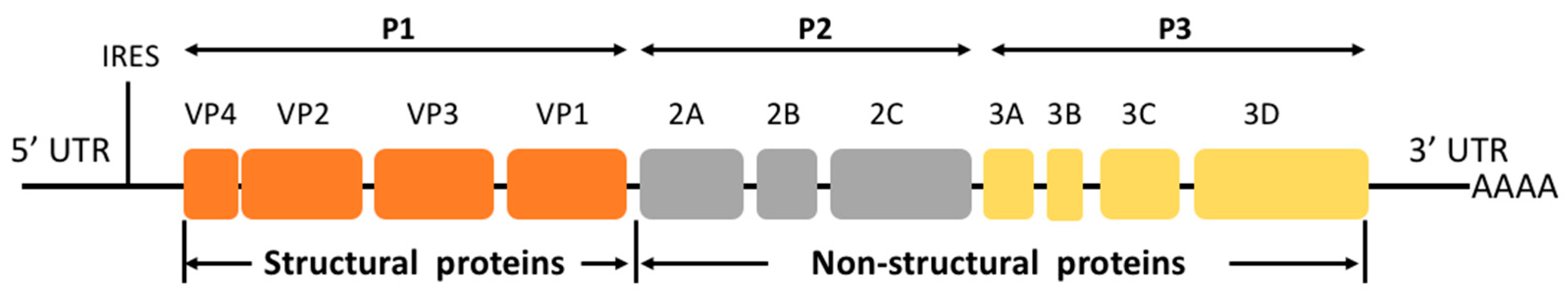
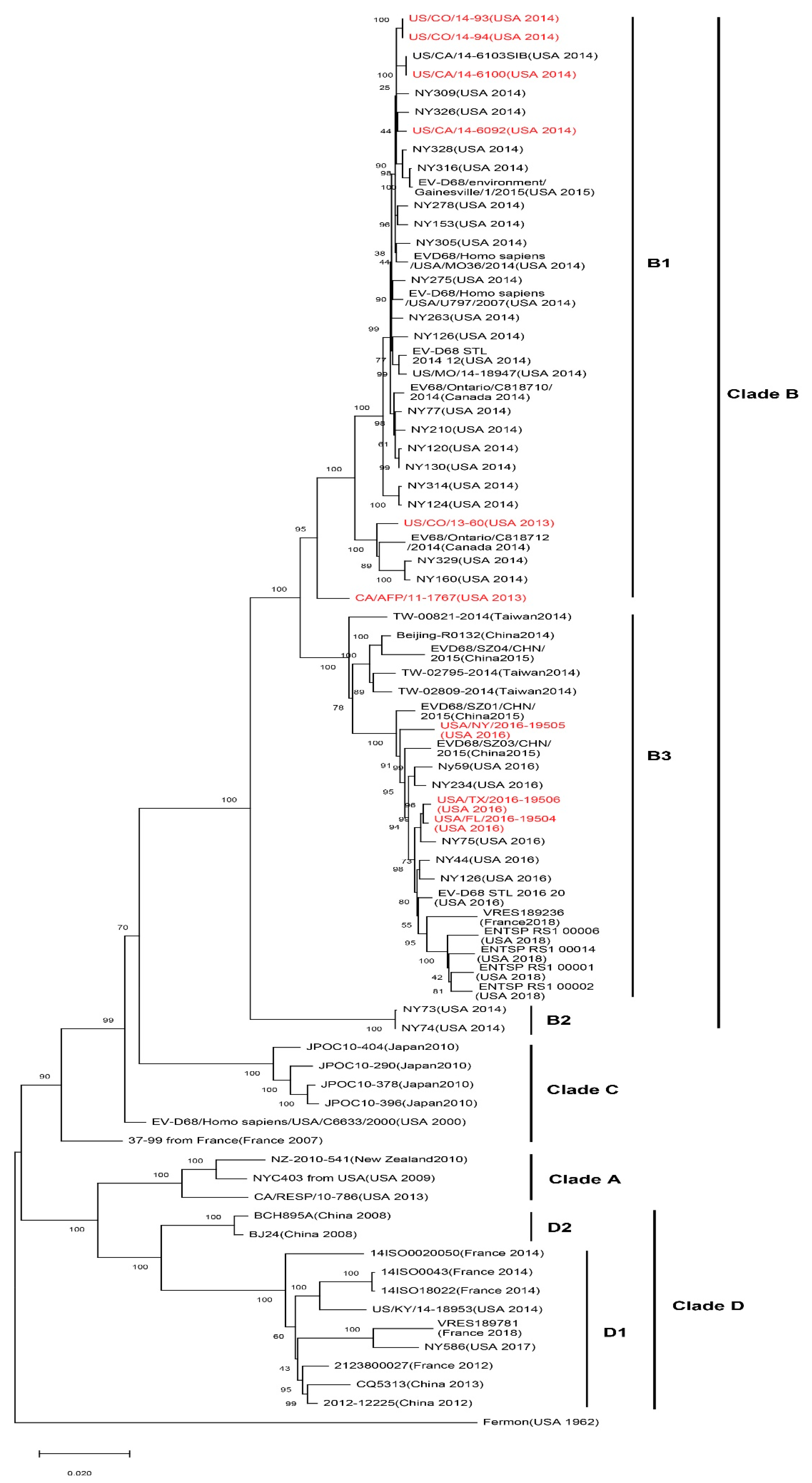
2. Genetic Characteristics of EV-D68
3. Infection Mechanisms of EV-D68
4. Virus-Host Interactions of EV-D68
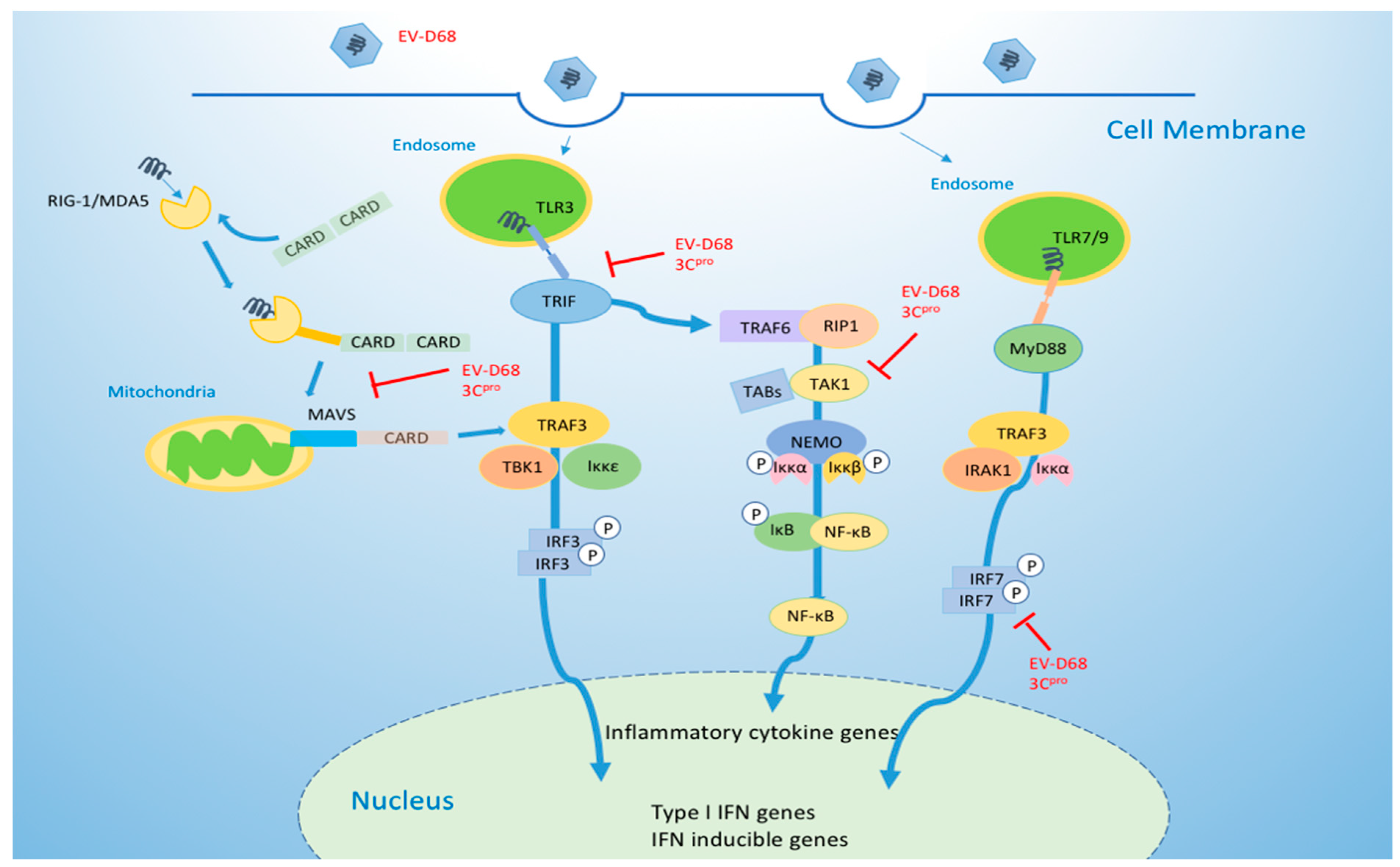
4.1. EV-D68 Suppresses the Innate Immune Response
4.2. EV-D68 Manipulates Cell Cycle Progression
4.3. Development of Antiviral Strategies
5. EV-D68 and Acute Flaccid Myelitis (AFM)
5.1. Epidemic Evidence of the Association between EV-D68 and AFM
5.2. Neuropathogenicity of EV-D68 In Vitro and In Vivo
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schieble, J.H.; Fox, V.L.; Lennette, E.H. A probable new human picornavirus associated with respiratory disease. Am. J. Epidemiol. 1967, 85, 297–310. [Google Scholar] [CrossRef]
- Midgley, C.M.; Watson, J.T.; Nix, W.A.; Curns, A.T.; Rogers, S.L.; Brown, B.A.; Conover, C.; Dominguez, S.R.; Feikin, D.R.; Gray, S.; et al. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): A descriptive epidemiological investigation. Lancet. Res. Med. 2015, 3, 879–887. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. MMWR 2011, 60, 1301–1304. [Google Scholar]
- Poelman, R.; Schuffenecker, I.; Van Leer-Buter, C.; Josset, L.; Niesters, H.G.M.; Lina, B. European surveillance for enterovirus D68 during the emerging North-American outbreak in 2014. J. Clin. Virol. 2015, 71, 1–9. [Google Scholar] [CrossRef]
- Oberste, M.S.; Maher, K.; Schnurr, D.; Flemister, M.R.; Lovchik, J.C.; Peters, H.; Sessions, W.; Kirk, C.; Chatterjee, N.; Fuller, S.; et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J. Gen. Virol. 2004, 85, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Messacar, K.; Asturias, E.J.; Hixon, A.M.; Van Leer-Buter, C.; Niesters, H.G.M.; Tyler, K.L.; Abzug, M.J.; Dominguez, S.R. Enterovirus D68 and acute flaccid myelitis—evaluating the evidence for causality. Lancet Infect. Dis. 2018, 18, e239–e247. [Google Scholar] [CrossRef]
- Greninger, A.L.; Naccache, S.N.; Messacar, K.; Clayton, A.; Yu, G.; Somasekar, S.; Federman, S.; Stryke, D.; Anderson, C.; Yagi, S.; et al. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14): A retrospective cohort study. Lancet Infect. Dis. 2015, 15, 671–682. [Google Scholar] [CrossRef]
- Opanda, S.M.; Wamunyokoli, F.; Khamadi, S.; Coldren, R.; Bulimo, W.D. Genetic diversity of human enterovirus 68 strains isolated in Kenya using the hypervariable 3’-end of VP1 gene. PLoS ONE 2014, 9, e102866. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, R.; Firth, C.; Madhi, S.A.; Howie, S.R.C.; Wu, W.; Sall, A.A.; Haq, S.; Briese, T.; Lipkin, W.I. Worldwide emergence of multiple clades of enterovirus 68. J. Gen. Virol. 2012, 93, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Ulferts, R.; van der Linden, L.; Thibaut, H.J.; Lanke, K.H.W.; Leyssen, P.; Coutard, B.; De Palma, A.M.; Canard, B.; Neyts, J.; van Kuppeveld, F.J.M. Selective serotonin reuptake inhibitor fluoxetine inhibits replication of human enteroviruses B and D by targeting viral protein 2C. Antimicrob. Agents Chemother. 2013, 57, 1952–1956. [Google Scholar] [CrossRef]
- Messacar, K.; Sillau, S.; Hopkins, S.E.; Otten, C.; Wilson-Murphy, M.; Wong, B.; Santoro, J.D.; Treister, A.; Bains, H.K.; Torres, A.; et al. Safety, tolerability, and efficacy of fluoxetine as an antiviral for acute flaccid myelitis. Neurology 2018. [CrossRef]
- Musharrafieh, R.; Ma, C.; Zhang, J.; Hu, Y.; Diesing, J.M.; Marty, M.T.; Wang, J. Validating Enterovirus D68-2Apro as an Antiviral Drug Target and the Discovery of Telaprevir as a Potent D68-2Apro Inhibitor. J. Virol. 2019, 93, e02221-18. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, L.; Lei, X.; Zhou, H.; Zhou, Z.; He, B.; Wang, J. Enterovirus 68 3C Protease Cleaves TRIF To Attenuate Antiviral Responses Mediated by Toll-Like Receptor 3. J. Virol. 2014, 88, 6650. [Google Scholar] [CrossRef]
- Xiang, Z.; Liu, L.; Lei, X.; Zhou, Z.; He, B.; Wang, J. 3C Protease of Enterovirus D68 Inhibits Cellular Defense Mediated by Interferon Regulatory Factor 7. J. Virol. 2016, 90, 1613. [Google Scholar] [CrossRef]
- Rui, Y.; Su, J.; Wang, H.; Chang, J.; Wang, S.; Zheng, W.; Cai, Y.; Wei, W.; Gordy, J.T.; Markham, R.; et al. Disruption of MDA5-Mediated Innate Immune Responses by the 3C Proteins of Coxsackievirus A16, Coxsackievirus A6, and Enterovirus D68. J. Virol. 2017, 91, e00546-17. [Google Scholar] [CrossRef]
- Eshaghi, A.; Duvvuri, V.R.; Isabel, S.; Banh, P.; Li, A.; Peci, A.; Patel, S.N.; Gubbay, J.B. Global Distribution and Evolutionary History of Enterovirus D68, with Emphasis on the 2014 Outbreak in Ontario, Canada. Front. Microbiol. 2017, 8, 257. [Google Scholar] [CrossRef]
- Pellegrinelli, L.; Giardina, F.; Lunghi, G.; Uceda Renteria, S.C.; Greco, L.; Fratini, A.; Galli, C.; Piralla, A.; Binda, S.; Pariani, E.; et al. Emergence of divergent enterovirus (EV) D68 sub-clade D1 strains, northern Italy, September to October 2018. Euro Surv. 2019, 24, 1900090. [Google Scholar] [CrossRef]
- Bal, A.; Sabatier, M.; Wirth, T.; Coste-Burel, M.; Lazrek, M.; Stefic, K.; Brengel-Pesce, K.; Morfin, F.; Lina, B.; Schuffenecker, I.; et al. Emergence of enterovirus D68 clade D1, France, August to November 2018. Euro Surv. 2019, 24, 1800699. [Google Scholar] [CrossRef]
- Xiang, Z.; Xie, Z.; Liu, L.; Ren, L.; Xiao, Y.; Paranhos-Baccalà, G.; Wang, J. Genetic divergence of enterovirus D68 in China and the United States. Sci. Rep. 2016, 6, 27800. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, L.; Ren, L.; Guo, L.; Xie, Z.; Liu, C.; Li, T.; Luo, M.; Paranhos-Baccalà, G.; Xu, W.; et al. Seroepidemiology of enterovirus D68 infection in China. Emerg. Microbes Infect. 2017, 6, e32. [Google Scholar] [CrossRef]
- Kyriakopoulou, Z.; Pliaka, V.; Amoutzias, G.D.; Markoulatos, P. Recombination among human non-polio enteroviruses: Implications for epidemiology and evolution. Virus Genes 2015, 50, 177–188. [Google Scholar] [CrossRef]
- Huang, W.; Wang, G.; Zhuge, J.; Nolan, S.M.; Dimitrova, N.; Fallon, J.T. Whole-Genome Sequence Analysis Reveals the Enterovirus D68 Isolates during the United States 2014 Outbreak Mainly Belong to a Novel Clade. Sci. Rep. 2015, 5, 15223. [Google Scholar] [CrossRef]
- Lee, A.J.; Zhang, Y.; Scheuermann, R.H.; Cao, J.; Zhang, S.; Larsen, C.N.; Sun, G.; Klem, E.B.; Zhao, H.; He, S.; et al. Genetic changes found in a distinct clade of Enterovirus D68 associated with paralysis during the 2014 outbreak. Virus Evolut. 2016, 2, 1. [Google Scholar]
- Esposito, S.; Chidini, G.; Cinnante, C.; Napolitano, L.; Giannini, A.; Terranova, L.; Niesters, H.; Principi, N.; Calderini, E. Acute flaccid myelitis associated with enterovirus-D68 infection in an otherwise healthy child. Virol. J. 2017, 14, 4. [Google Scholar] [CrossRef]
- Uncapher, C.R.; Dewitt, C.M.; Colonno, R.J. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 1991, 180, 814–817. [Google Scholar] [CrossRef]
- Blomqvist, S.; Savolainen, C.; Råman, L.; Roivainen, M.; Hovi, T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J. Clin. Microbiol. 2002, 40, 4218–4223. [Google Scholar] [CrossRef]
- Krautkrämer, E.; Zeier, M. Hantavirus causing hemorrhagic fever with renal syndrome enters from the apical surface and requires decay-accelerating factor (DAF/CD55). J. Virol. 2008, 82, 4257–4264. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, J.; Baggen, J.; Meng, G.; Xiao, C.; Thibaut, H.J.; van Kuppeveld, F.J.M.; Rossmann, M.G. Sialic acid-dependent cell entry of human enterovirus D68. Nat. Commun. 2015, 6, 8865. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheng, J.; Fokine, A.; Meng, G.; Shin, W.-H.; Long, F.; Kuhn, R.J.; Kihara, D.; Rossmann, M.G. Structure and inhibition of EV-D68, a virus that causes respiratory illness in children. Science 2015, 347, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Okamoto, M.; Nakakita, S.-i.; Suzuki, A.; Saito, M.; Tamaki, R.; Lupisan, S.; Roy, C.N.; Hiramatsu, H.; Sugawara, K.E.; et al. Antigenic and Receptor Binding Properties of Enterovirus 68. J. Virol. 2014, 88, 2374. [Google Scholar] [CrossRef] [PubMed]
- Baggen, J.; Thibaut, H.J.; Staring, J.; Jae, L.T.; Liu, Y.; Guo, H.; Slager, J.J.; de Bruin, J.W.; van Vliet, A.L.W.; Blomen, V.A.; et al. Enterovirus D68 receptor requirements unveiled by haploid genetics. Proc. Nat. Acad. Sci. USA 2016, 113, 1399–1404. [Google Scholar] [CrossRef]
- Wei, W.; Guo, H.; Chang, J.; Yu, Y.; Liu, G.; Zhang, N.; Willard, S.H.; Zheng, S.; Yu, X.-F. ICAM-5/Telencephalin Is a Functional Entry Receptor for Enterovirus D68. Cell Host Microbe 2016, 20, 631–641. [Google Scholar] [CrossRef]
- Yang, H. Structure, Expression, and Function of ICAM-5. Comp. Funct. Genomics 2012, 2012, 368938. [Google Scholar] [CrossRef][Green Version]
- Takeuchi, O.; Akira, S. Innate immunity to virus infection. Immunol. Rev. 2009, 227, 75–86. [Google Scholar] [CrossRef]
- Yan, N.; Chen, Z.J. Intrinsic antiviral immunity. Nat. Immunol. 2012, 13, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Devasthanam, A.S. Mechanisms underlying the inhibition of interferon signaling by viruses. Virulence 2014, 5, 270–277. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Sem. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-kappaB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Lei, X.; Sun, Z.; Liu, X.; Jin, Q.; He, B.; Wang, J. Cleavage of the adaptor protein TRIF by enterovirus 71 3C inhibits antiviral responses mediated by Toll-like receptor 3. J. Virol. 2011, 85, 8811–8818. [Google Scholar] [CrossRef]
- Feng, Q.; Langereis, M.A.; Lork, M.; Nguyen, M.; Hato, S.V.; Lanke, K.; Emdad, L.; Bhoopathi, P.; Fisher, P.B.; Lloyd, R.E.; et al. Enterovirus 2Apro targets MDA5 and MAVS in infected cells. J. Virol. 2014, 88, 3369–3378. [Google Scholar] [CrossRef]
- Barral, P.M.; Sarkar, D.; Fisher, P.B.; Racaniello, V.R. RIG-I is cleaved during picornavirus infection. Virology 2009, 391, 171–176. [Google Scholar] [CrossRef]
- Xu, C.; He, X.; Zheng, Z.; Zhang, Z.; Wei, C.; Guan, K.; Hou, L.; Zhang, B.; Zhu, L.; Cao, Y.; et al. Downregulation of microRNA miR-526a by enterovirus inhibits RIG-I-dependent innate immune response. J. Virol. 2014, 88, 11356–11368. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lei, X.; Xiao, X.; Yang, C.; Lu, W.; Huang, Z.; Leng, Q.; Jin, Q.; He, B.; Meng, G.; et al. Reciprocal Regulation between Enterovirus 71 and the NLRP3 Inflammasome. Cell Rep. 2015, 12, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.J.; Langereis, M.A.; Rabouw, H.H.; Wahedi, M.; Muntjewerff, E.M.; de Groot, R.J.; van Kuppeveld, F.J.M. Essential role of enterovirus 2A protease in counteracting stress granule formation and the induction of type I interferon. J. Virol. 2019, JVI.00222-19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Zhong, T.; Wang, Y.; Song, F.-M.; Yu, X.-F.; Xing, L.-P.; Zhang, W.-Y.; Yu, J.-H.; Hua, S.-C.; Yu, X.-F. Human Enterovirus 68 Interferes with the Host Cell Cycle to Facilitate Viral Production. Front. Cellular Infect. Microbiol. 2017, 7, 29. [Google Scholar] [CrossRef]
- Esposito, S.; Bosis, S.; Niesters, H.; Principi, N. Enterovirus D68 Infection. Viruses 2015, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Evans, W.J.; Nicolaou, K.C.; Tarbet, E.B.; Day, C.W. Susceptibilities of enterovirus D68, enterovirus 71, and rhinovirus 87 strains to various antiviral compounds. Antiviral Res. 2016, 131, 61–65. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhu, R.; Xu, L.; He, M.; Yan, X.; Liu, D.; Yin, Z.; Wu, Y.; Li, Y.; Yang, L.; et al. Atomic structures of enterovirus D68 in complex with two monoclonal antibodies define distinct mechanisms of viral neutralization. Nat. Microbiol. 2019, 4, 124–133. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, J.; Li, B.; Guo, L.; Li, H.; Song, J.; Yang, Z.; Li, H.; Fan, H.; Huang, X.; et al. A Novel Neutralizing Antibody Specific to the DE Loop of VP1 Can Inhibit EV-D68 Infection in Mice. J. Immunol. 2018, 201, 2557. [Google Scholar] [CrossRef]
- Division of Viral Diseases, CDC; Division of Vector-Borne Diseases, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, CDC; Children’s Hospital Colorado; Council of State and Territorial Epidemiologists. Notes from the field: Acute flaccid myelitis among persons aged ≤21 years—United States, August 1-November 13, 2014. MMWR 2015, 63, 1243–1244. [Google Scholar]
- Van Haren, K.; Ayscue, P.; Waubant, E.; Clayton, A.; Sheriff, H.; Yagi, S.; Glenn-Finer, R.; Padilla, T.; Strober, J.B.; Aldrovandi, G.; et al. Acute Flaccid Myelitis of Unknown Etiology in California, 2012–2015 Trends in Acute Flaccid Myelitis in California, 2012–2015 Trends in Acute Flaccid Myelitis in California, 2012–2015. JAMA 2015, 314, 2663–2671. [Google Scholar] [CrossRef]
- Wang, G.; Zhuge, J.; Huang, W.; Nolan, S.M.; Gilrane, V.L.; Yin, C.; Dimitrova, N.; Fallon, J.T. Enterovirus D68 Subclade B3 Strain Circulating and Causing an Outbreak in the United States in 2016. Sci. Rep. 2017, 7, 1242. [Google Scholar] [CrossRef]
- Messacar, K.; Robinson, C.C.; Pretty, K.; Yuan, J.; Dominguez, S.R. Surveillance for enterovirus D68 in colorado children reveals continued circulation. J. Clin. Virol. 2017, 92, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Acute Flaccid Myelitis Surveillance. Available online: https://www.cdc.gov/acute-flaccid-myelitis/afm-surveillance.html (accessed on 4 March 2019).
- Sejvar, J.J.; Lopez, A.S.; Cortese, M.M.; Leshem, E.; Pastula, D.M.; Miller, L.; Glaser, C.; Kambhampati, A.; Shioda, K.; Aliabadi, N.; et al. Acute Flaccid Myelitis in the United States, August–December 2014: Results of Nationwide Surveillance. Clin. Infect. Dis. 2016, 63, 737–745. [Google Scholar] [CrossRef]
- Naccache, S.; Bender, J.; Desai, J.; Van, T.; Meyers, L.; Jones, J.; Mongkolrattanothai, K.; Bard, J.D. Acute Flaccid Myelitis Cases Presenting During a Spike in Respiratory Enterovirus D68 Circulation: Case Series From a Single Pediatric Referral Center. Open Forum Infect. Dis. 2017, 4, S305–S306. [Google Scholar] [CrossRef]
- Hurley, D. Rise in Acute Flaccid Myelitis Cases Reported in the US and Europe. Neurol. Today 2017, 17, 5. [Google Scholar] [CrossRef]
- The United Kingdom Acute Flaccid Paralysis (AFP) Task Force. An increase in reports of acute flaccid paralysis (AFP) in the United Kingdom, 1 January 2018–21 January 2019: Early findings. Euro Surv. 2019, 24, 1900093. [Google Scholar] [CrossRef]
- Kujawski, S.A.; Midgley, C.M.; Rha, B.; Lively, J.Y.; Nix, W.A.; Curns, A.T.; Payne, D.C.; Englund, J.A.; Boom, J.A.; Williams, J.V.; et al. Enterovirus D68-Associated Acute Respiratory Illness—New Vaccine Surveillance Network, United States, July-October, 2017 and 2018. MMWR 2019, 68, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Pérez, G.; Rosanova, M.T.; Freire, M.C.; Paz, M.I.; Ruvinsky, S.; Rugilo, C.; Ruggieri, V.; Cisterna, D.; Martiren, S.; Lema, C.; et al. Unusual increase of cases of myelitis in a pediatric hospital in Argentina. Arch. Argent. Pediatr 2017, 115, 364–369. [Google Scholar]
- Kreuter, J.D.; Barnes, A.; McCarthy, J.E.; Schwartzman, J.D.; Oberste, M.S.; Rhodes, C.H.; Modlin, J.F.; Wright, P.F. A fatal central nervous system enterovirus 68 infection. Arch. Pathol Lab. Med. 2011, 135, 793–796. [Google Scholar] [PubMed]
- Bowers, J.R.; Valentine, M.; Harrison, V.; Fofanov, V.Y.; Gillece, J.; Delisle, J.; Patton, B.; Schupp, J.; Sheridan, K.; Lemmer, D.; et al. Genomic Analyses of Acute Flaccid Myelitis Cases among a Cluster in Arizona Provide Further Evidence of Enterovirus D68 Role. mBio 2019, 10, e02262-18. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Hixon, A.M.; Oldfield, L.M.; Zhang, Y.; Novotny, M.; Wang, W.; Das, S.R.; Shabman, R.S.; Tyler, K.L.; Scheuermann, R.H. Contemporary Circulating Enterovirus D68 Strains Have Acquired the Capacity for Viral Entry and Replication in Human Neuronal Cells. mBio 2018, 9, e01954-18. [Google Scholar] [CrossRef] [PubMed]
- Royston, L.; Essaidi-Laziosi, M.; Pérez-Rodríguez, F.J.; Piuz, I.; Geiser, J.; Krause, K.-H.; Huang, S.; Constant, S.; Kaiser, L.; Garcin, D.; et al. Viral chimeras decrypt the role of enterovirus capsid proteins in viral tropism, acid sensitivity and optimal growth temperature. PLoS Pathog. 2018, 14, e1006962. [Google Scholar] [CrossRef] [PubMed]
- Hixon, A.M.; Yu, G.; Leser, J.S.; Yagi, S.; Clarke, P.; Chiu, C.Y.; Tyler, K.L. A mouse model of paralytic myelitis caused by enterovirus D68. PLoS Pathog. 2017, 13, e1006199. [Google Scholar] [CrossRef]
- Hixon, A.M.; Clarke, P.; Tyler, K.L. Evaluating Treatment Efficacy in a Mouse Model of Enterovirus D68-Associated Paralytic Myelitis. Journal Infect. Dis. 2017, 216, 1245–1253. [Google Scholar] [CrossRef]
- Sun, S.; Bian, L.; Gao, F.; Du, R.; Hu, Y.; Fu, Y.; Su, Y.; Wu, X.; Mao, Q.; Liang, Z. A neonatal mouse model of Enterovirus D68 infection induces both interstitial pneumonia and acute flaccid myelitis. Antiviral Res. 2019, 161, 108–115. [Google Scholar] [CrossRef]
- Gahmberg, C.G.; Tian, L.; Ning, L.; Nyman-Huttunen, H. ICAM-5—A novel two-facetted adhesion molecule in the mammalian brain. Immunol. Lett. 2008, 117, 131–135. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Hu, X.-Y.; Yu, X.-F. Current Understanding of Human Enterovirus D68. Viruses 2019, 11, 490. https://doi.org/10.3390/v11060490
Sun J, Hu X-Y, Yu X-F. Current Understanding of Human Enterovirus D68. Viruses. 2019; 11(6):490. https://doi.org/10.3390/v11060490
Chicago/Turabian StyleSun, Jing, Xiao-Yi Hu, and Xiao-Fang Yu. 2019. "Current Understanding of Human Enterovirus D68" Viruses 11, no. 6: 490. https://doi.org/10.3390/v11060490
APA StyleSun, J., Hu, X.-Y., & Yu, X.-F. (2019). Current Understanding of Human Enterovirus D68. Viruses, 11(6), 490. https://doi.org/10.3390/v11060490



