Interaction between Two Iridovirus Core Proteins and Their Effects on Ranavirus (RGV) Replication in Cells from Different Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Virus
2.2. Plasmid Construction
2.3. Yeast Two Hybrid (Y2H) Assays
2.4. Co-IP Assays
2.5. Western Blot Analysis
2.6. Fluorescence Microscopy
2.7. Quantitative Analysis of RGV Genomic DNA
3. Results
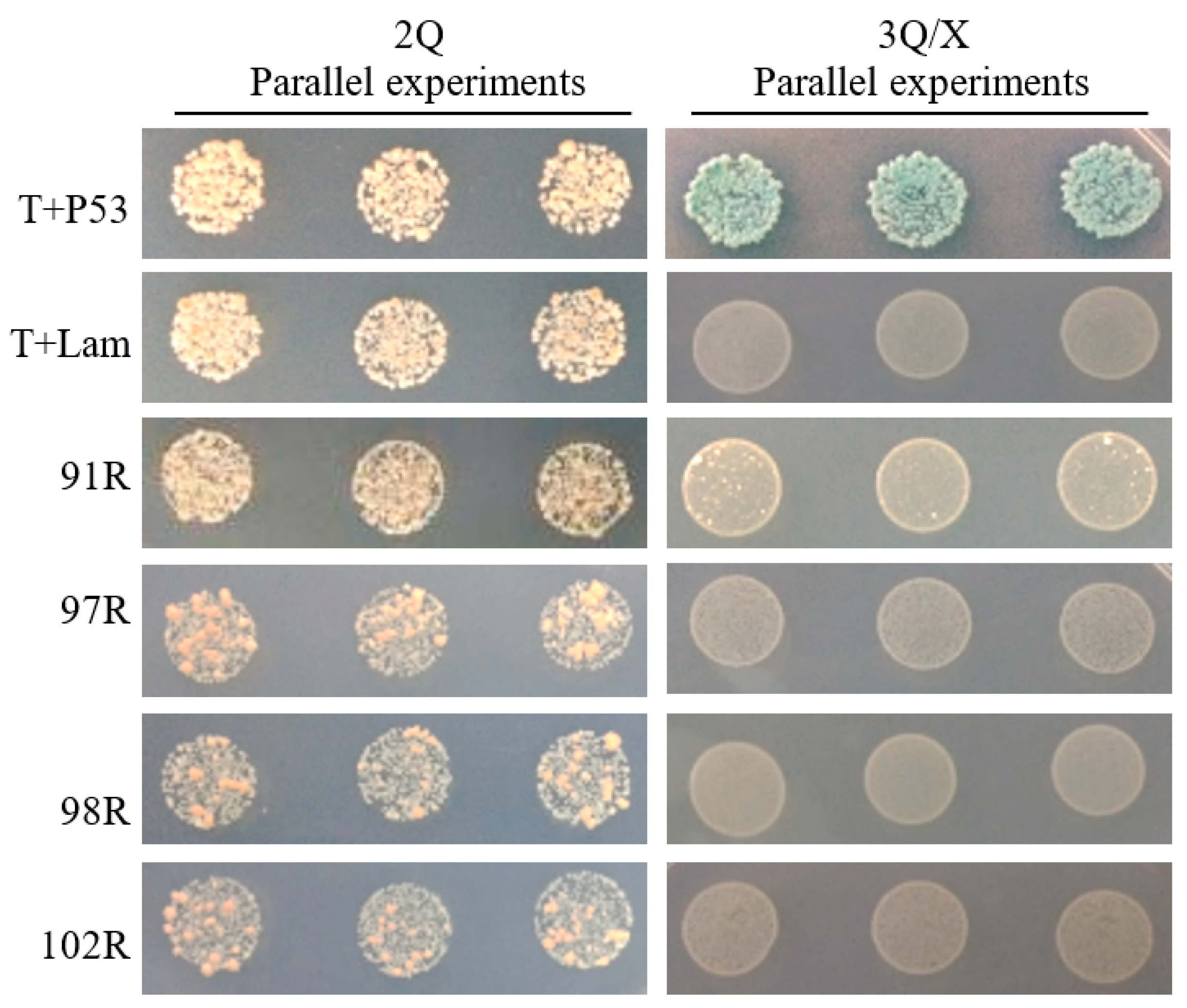
3.1. Interaction between RGV-63R and RGV-91R was First Screened by Y2H
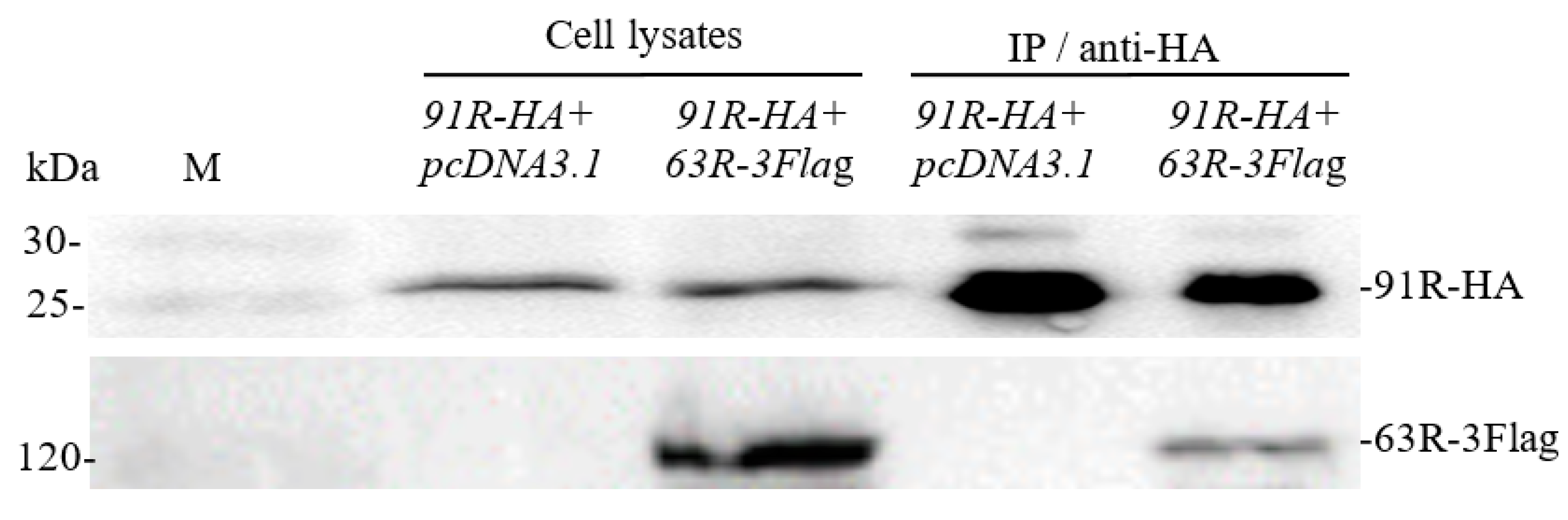
3.2. Confirmation of Interaction between RGV-63R and RGV-91R by Co-IP Followed by Western Blotting
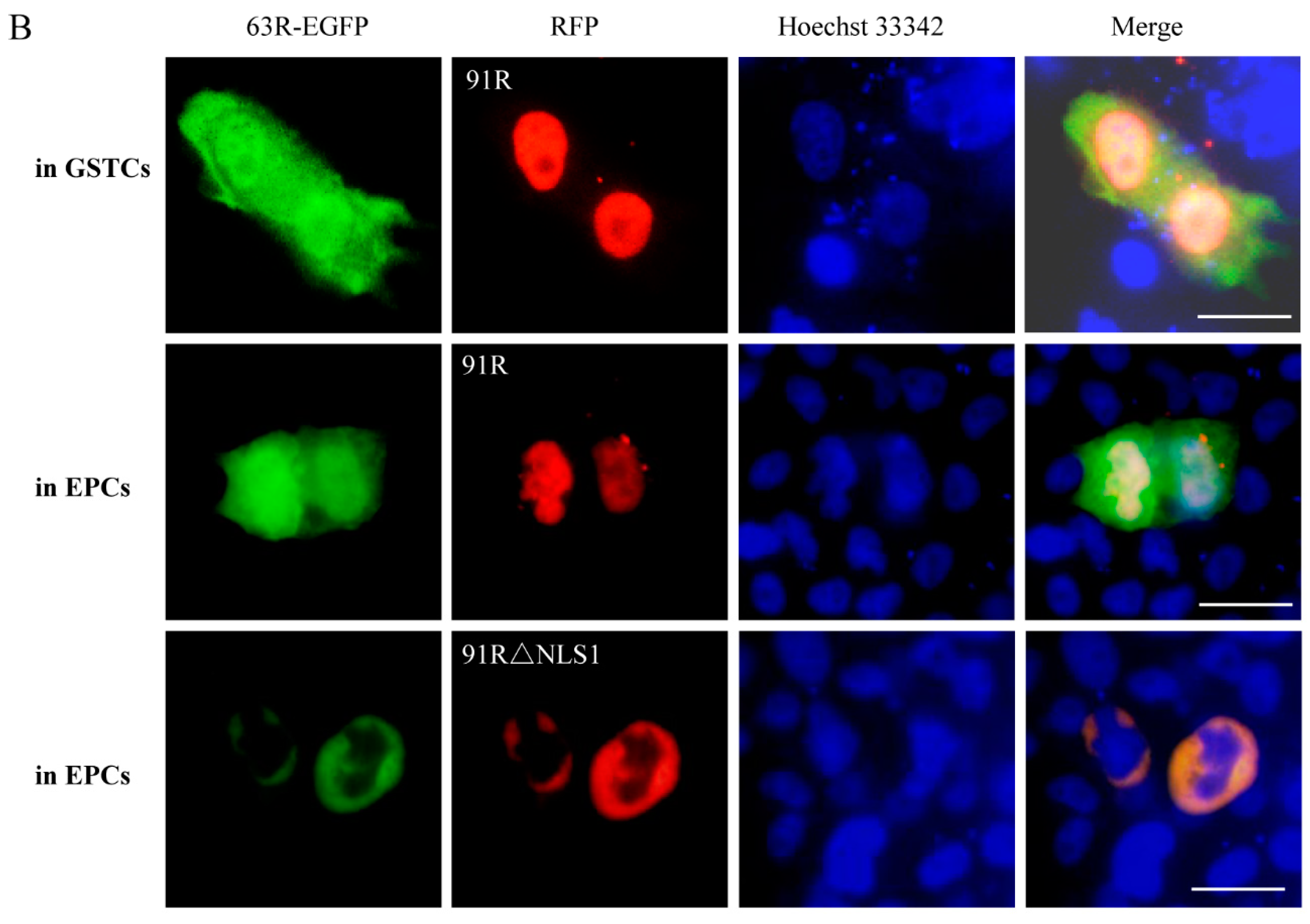
3.3. Localization of RGV-63R and RGV-91R in Cells of Different Species
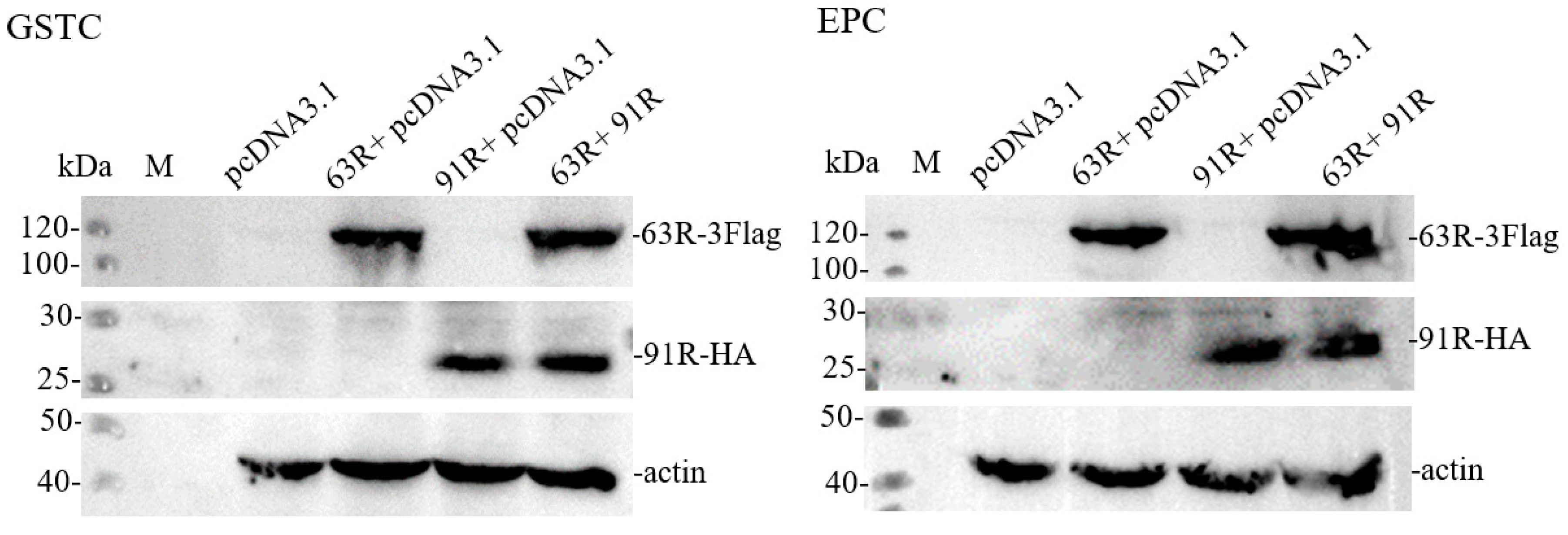
3.4. Detection of RGV-63R and RGV-91R Expressions by Western Blotting
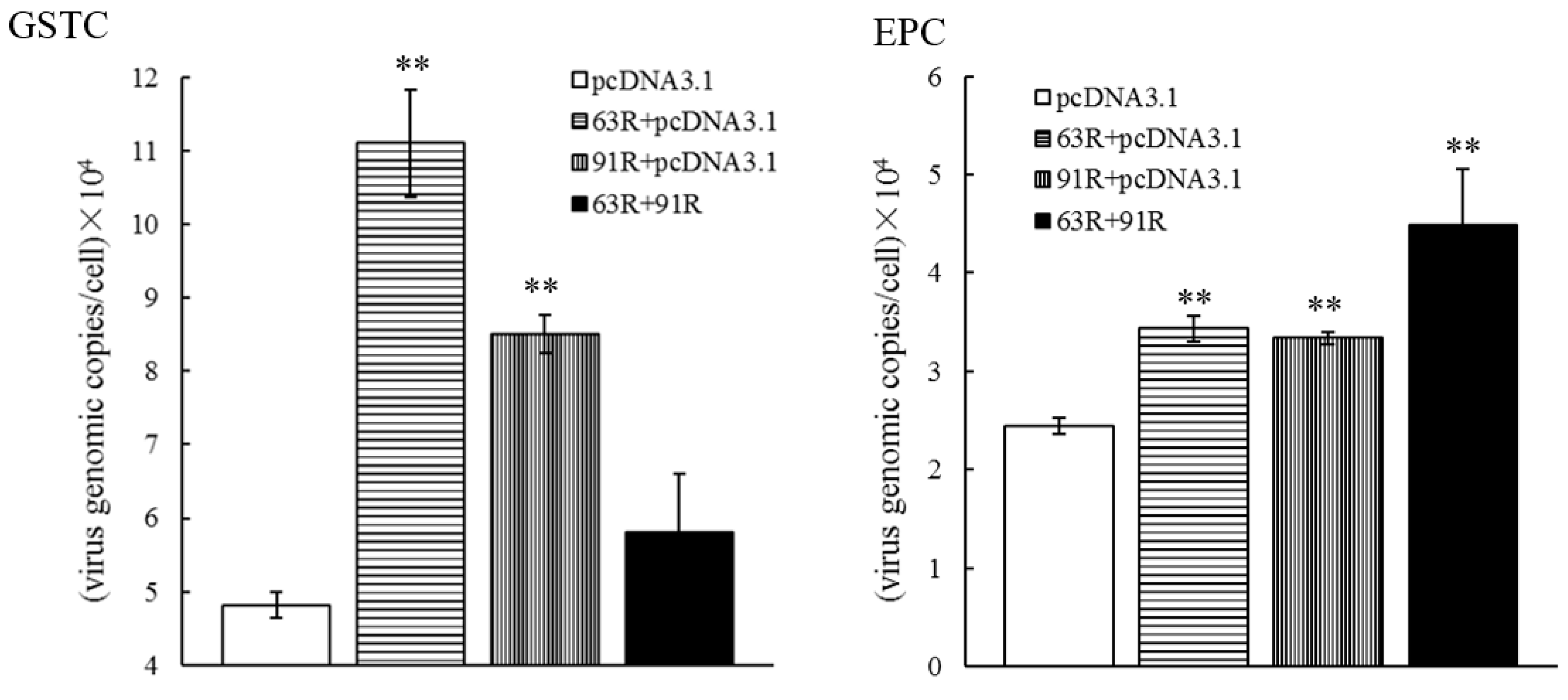
3.5. RGV-63R and RGV-91R Promote RGV Genome Replication
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Q.Y.; Gui, J.F. Diversity, evolutionary contribution and ecological roles of aquatic viruses. Sci. China Life Sci. 2018, 61, 1486–1502. [Google Scholar] [CrossRef]
- Chinchar, V.G.; Hick, P.; Ince, I.A.; Jancovich, J.K.; Marschang, R.; Qin, Q.W.; Subramaniam, K.; Waltzek, T.B.; Whittington, R.; Williams, T.; Zhang, Q.Y. ICTV Report Consortium. ICTV Virus Taxonomy Profile: Iridoviridae. J. Gen. Virol. 2017, 98, 890–891. [Google Scholar] [PubMed]
- Zhang, Q.Y.; Gui, J.F. Virus genomes and virus-host interactions in aquaculture animals. Sci. China Life Sci. 2015, 58, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Gui, J.F. Atlas of Aquatic Viruses and Viral Diseases; Science Press: Beijing, China, 2012. [Google Scholar]
- Gui, L.; Zhang, Q.Y. A brief review on aquatic animal virology researches in China. J. Fisheries China 2019, 43, 168–187. [Google Scholar]
- Stohr, A.C.; Lopez-Bueno, A.; Blahak, S.; Caeiro, M.F.; Rosa, G.M.; Alves de Matos, A.P.; Martel, A.; Alejo, A.; Marschang, R.E. Phylogeny and differentiation of reptilian and amphibian ranaviruses detected in Europe. PLoS ONE 2015, 10, e0118633. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Gui, J.F.; Chen, Z.Y.; Li, T.; Lei, C.K.; Wang, Z.H.; Zhang, Q.Y. Divergent transcriptomic responses underlying the ranaviruses-amphibian interaction processes on interspecies infection of Chinese giant salamander. BMC Genomics 2018, 19, 211. [Google Scholar] [CrossRef]
- Robert, J.; Jancovich, J.K. Recombinant ranaviruses for studying evolution of host-pathogen interactions in ectothermic vertebrates. Viruses 2016, 8, 187. [Google Scholar] [CrossRef]
- Ke, F.; Zhang, Q.Y. Aquatic animal viruses mediated immune evasion in their host. Fish. Shellfish Immunol. 2019, 86, 1096–1105. [Google Scholar] [CrossRef]
- Gui, L.; Chinchar, V.G.; Zhang, Q.Y. Molecular basis of pathogenesis of emerging viruses infecting aquatic animals. Aquaculture Fisheries 2018, 3, 1–5. [Google Scholar] [CrossRef]
- Liu, J.; Yu, C.; Gui, J.F.; Pang, D.W.; Zhang, Q.Y. Real-time dissecting the entry and intracellular dynamics of single reovirus particle. Front. Microbiol. 2018, 9, 2797. [Google Scholar] [CrossRef] [PubMed]
- Corthell, J.T. Basic Molecular Protocols in Neuroscience: Tips, Tricks, and Pitfalls; Elsevier Science: Amsterdam, The Netherland, 2014. [Google Scholar]
- Striebinger, H.; Koegl, M.; Bailer, S.M. A high-throughput yeast two-hybrid protocol to determine virus-host protein interactions. In Virus-Host Interactions. Methods in Molecular Biology (Methods and Protocols); Bailer, S., Lieber, D., Eds.; Humana Press: Totowa, NJ, USA, 2013; vol 1064, pp. 1–15. [Google Scholar]
- Ozsahin, E.; van Oers, M.M.; Nalcacioglu, R.; Demirbag, Z. Protein–protein interactions among the structural proteins of Chilo iridescent virus. J. Gen. Virol. 2018, 99, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.O.; Tou, C.J.; Wong, E.B.; Modavi, C.; Schaffer, D.V.; Dueber, J.E. CRISPR-guided DNA polymerases enable diversification of all nucleotides in a tunable window. Nature 2018, 560, 248–252. [Google Scholar] [CrossRef]
- Gardner, A.F.; Kelman, Z. DNA polymerases in biotechnology. Front. Microbiol. 2014, 5, 659. [Google Scholar] [CrossRef]
- Czarnecki, M.W.; Traktman, P. The vaccinia virus DNA polymerase and its processivity factor. Virus Res. 2017, 234, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, K.; Piret, J.; Boivin, G. Herpesvirus DNA polymerases: structures, functions and inhibitors. Virus Res. 2017, 234, 177–192. [Google Scholar] [CrossRef]
- Chinchar, V.G.; Waltzek, T.B. Ranaviruses: not just for frogs. PLoS Pathog. 2014, 10, e1003850. [Google Scholar] [CrossRef]
- Chinchar, V.G.; Waltzek, T.B.; Subramaniam, K. Ranaviruses and other members of the family Iridoviridae: Their place in the virosphere. Virology 2017, 511, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Maga, G.; Villani, G.; Crespan, E.; Wimmer, U.; Ferrari, E.; Bertocci, B.; Hubscher, U. 8-oxo-guanine bypass by human DNA polymerases in the presence of auxiliary proteins. Nature 2007, 447, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Dieckman, L.M.; Freudenthal, B.D.; Washington, M.T. PCNA structure and function: insights from structures of PCNA complexes and post-translationally modified PCNA. In The Eukaryotic Replisome: A Guide to Protein Structure and Function; MacNeill, S., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 281–299. [Google Scholar]
- Baple, E.L.; Chambers, H.; Cross, H.E.; Fawcett, H.; Nakazawa, Y.; Chioza, B.A.; Harlalka, G.V.; Mansour, S.; Sreekantan-Nair, A.; Patton, M.A.; et al. Hypomorphic PCNA mutation underlies a human DNA repair disorder. J. Clin. Invest. 2014, 124, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Eaton, H.E.; Metcalf, J.; Penny, E.; Tcherepanov, V.; Upton, C.; Brunetti, C.R. Comparative genomic analysis of the family Iridoviridae: re-annotating and defining the core set of iridovirus genes. Virol. J. 2007, 4, 11. [Google Scholar] [CrossRef]
- Zhao, Z.; Ke, F.; Gui, J.F.; Zhang, Q.Y. Characterization of an early gene encoding for dUTPase in Rana grylio virus. Virus Res. 2007, 123, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Zhao, Z.; Zhang, Q.Y. Cloning, expression and subcellular distribution of a Rana grylio virus late gene encoding ERV1 homologue. Mol. Biol. Rep. 2009, 36, 1651–1659. [Google Scholar] [CrossRef]
- Lei, X.Y.; Ou, T.; Zhu, R.L.; Zhang, Q.Y. Sequencing and analysis of the complete genome of Rana grylio virus (RGV). Arch. Virol. 2012a, 157, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.Y.; Ou, T.; Zhang, Q.Y. Rana grylio virus (RGV) 50L is associated with viral matrix and exhibited two distribution patterns. PLoS ONE 2012b, 7, e43033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, J.D.; Chen, Z.Y.; Huang, X.; Gao, X.C.; Zhang, Q.Y. Establishment of three cell lines from Chinese giant salamander and their sensitivities to the wild-type and recombinant ranavirus. Vet. Res. 2015, 46, 58. [Google Scholar] [CrossRef]
- Zeng, X.T.; Gao, X.C.; Zhang, Q.Y. Rana grylio virus 43R encodes an envelope protein involved in virus entry. Virus Genes 2018, 54, 779–791. [Google Scholar] [CrossRef]
- Wang, J.; Gui, L.; Chen, Z.Y.; Zhang, Q.Y. Mutations in the C-terminal region affect subcellular localization of crucian carp herpesvirus (CaHV) GPCR. Virus Genes 2016, 52, 484–494. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Li, T.; Gao, X.C.; Wang, C.F.; Zhang, Q.Y. Protective immunity induced by DNA vaccination against ranavirus infection in chinese giant salamander Andrias davidianus. Viruses 2018, 10, 52. [Google Scholar] [CrossRef]
- Peters, R. Translocation through the nuclear pore: Kaps pave the way. Bioessays 2009, 31, 466–477. [Google Scholar] [CrossRef]
- Chinchar, V.G.; Yu, K.H.; Jancovich, J.K. The molecular biology of frog virus 3 and other iridoviruses infecting cold-blooded vertebrates. Viruses 2011, 3, 1959–1985. [Google Scholar] [CrossRef]
- Sun, W. Molecular cloning and characterization of two novel viral genes, 3β-HSD and PCNA from Rana grylio virus. Ph.D. Thesis, University of Chinese Academy of Sciences, Wuhan, China, 2007. [Google Scholar]
- Li, W.; Zhang, X.; Weng, S.P.; Zhao, G.X.; He, J.G.; Dong, C.F. Virion-associated viral proteins of a Chinese giant salamander (Andrias davidianus) iridovirus (genus Ranavirus) and functional study of the major capsid protein (MCP). Vet. Microbiol. 2014, 172, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.G.; Guo, M.L.; Ji, H.S.; Yan, Y.; Ouyang, Z.L.; Huang, X.H.; Hang, Y.H.; Qin, Q.W. Grouper translationally controlled tumor protein prevents cell death and inhibits the replication of Singapore grouper iridovirus (SGIV). Fish. Shellfish Immunol. 2012, 33, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Lodermeyer, V.; Ssebyatika, G.; Passos, V.; Ponnurangam, A.; Malassa, A.; Ewald, E.; Stürzel, C.M.; Kirchhoff, F.; Rotger, M.; Falk, C.S.; et al. The antiviral activity of the cellular glycoprotein LGALS3BP/90K is species specific. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Eunhye, K.; Hyunjhung, J.; Joohee, K.; Joohee, K.; Unjoo, P.; Seunghyun, J.; Areum, K.; Sinae, K.; Tan, T.N.; Yongsun, K.; et al. Species specific antiviral activity of porcine interferon-α8 (IFNα8). Immune Netw. 2017, 17, 424–436. [Google Scholar]

| Name | Sequence (5’ to 3’) a | Usage |
|---|---|---|
| 63R-F | GGGAATTCCATATGGATCTCTTTGTGTACCAGTG (NdeI) | pGBKT7-63R/Y2H |
| 63R-R | CCGGAATTCTTACTTTTTCTTGAACGACA (EcoRI) | |
| 1R-F | CCGGAATTCATGGCATTCTCGGCAGAAGA (EcoRI) | pGADT7-1R/Y2H |
| 1R-R | CCGCTCGAGTCATAGGGGGGTAAACTTCC (XhoI) | |
| 2L-F | CCGGAATTCATGTCCATCATCGGAGCGAC (EcoRI) | pGADT7-2L/Y2H |
| 2L-R | CGCGGATCCTTACCATCTCACTGTAGAGA (BamHI) | |
| 9R-F | GGGAATTCCATATGGAAATGTTTGCATCTAAATC (NdeI) | pGADT7-9R/Y2H |
| 9R-R | CCGGAATTCTCATCGCCACTCAAAGGATT (EcoRI) | |
| 10L-F | CCGGAATTCATGGACACATCACCCTACGA (EcoRI) | pGADT7-10L/Y2H |
| 10L-R | CCGCTCGAGTCAGGCAAACTTGCCCCTCC (XhoI) | |
| 13L-F | GGGAATTCCATATGTGCTCCAAACTCGTAGAGAT (NdeI) | pGADT7-13L/Y2H |
| 13L-R | CCGGAATTCTTAGAAACCCATGGTCTCGA (EcoRI) | |
| 16R-F | CCGGAATTCATGGAACAAGTACCCATAAA (EcoRI) | pGADT7-16R/Y2H |
| 16R-R | CCGCTCGAGCTAATCGTCCAAGTCCGACT (XhoI) | |
| 21R-F | GGGAATTCCATATGGCTACAAATTACTGTGACGA (NdeI) | pGADT7-21R/Y2H |
| 21R-R | CCGGAATTCTTACATCGTGAAGCTCTCAA (EcoRI) | |
| 23L-F | CCGGAATTCATGGAAACCATAGTGCTGGT (EcoRI) | pGADT7-23L/Y2H |
| 23L-R | CCGCTCGAGTTACGACGAGGACCCAAATG (XhoI) | |
| 24R-F | CCGGAATTCATGGCTAACGCTACCATAAA (EcoRI) | pGADT7-24R/Y2H |
| 24R-R | CGCGGATCCCTACTCTTGCTGCTCGGCTC (BamHI) | |
| 29R-F | GGGAATTCCATATGGCCAATTTTCTACAAGATGT (NdeI) | pGADT7-29R/Y2H |
| 29R-R | CCGGAATTCTCAATGACGCTCCTTGGCCC (EcoRI) | |
| 40R-F | CCGGAATTCATGCAAGTTTTTCTAGATTT (EcoRI) | pGADT7-40R/Y2H |
| 40R-R | CGCGGATCCTCACCTCCTCCTGCTCCTGC (BamHI) | |
| 44R-F | GGGAATTCCATATGAGAGTCGTGGTAAACGCAAA (NdeI) | pGADT7-44R/Y2H |
| 44R-R | CCGGAATTCTCACATCAGAAGAGACACGT (EcoRI) | |
| 53R-F | CCGGAATTCATGGGAGCAGCGGAATCTAT (EcoRI) | pGADT7-53R/Y2H |
| 53R-R | CCGCTCGAGTTAACCCCTGTGGGCCGGAA (XhoI) | |
| 60R-F | GGGAATTCCATATGGCAATGGTTTCCAACGTAAA (NdeI) | pGADT7-60R/Y2H |
| 60R-R | CCGGAATTCCTACAGGCTCTTTAGGATAA (EcoRI) | |
| 63R-F | GGGAATTCCATATGGATCTCTTTGTGTACCAGTG (NdeI) | pGADT7-63R/Y2H |
| 63R-R2 | CCGGAATTCTTACTTTTTCTTGAACGACA (EcoRI) | |
| 65L-F | CGCGGATCCTGTCCAGGGGCATGACTACC (BamHI) | pGADT7-65L/Y2H |
| 65L-R | CCGCTCGAGTCACTTGAAGGCTATGGAAA (XhoI) | |
| 73L-F | CCGGAATTCATGTTTCCTCACGTCACCAT (EcoRI) | pGADT7-73L/Y2H |
| 73L-R | CGCGGATCCTTAGATGTCCAGGGGTTCGT (BamHI) | |
| 87L-F | CCGGAATTCATGGAAGGTTGGTTGGGAAA (EcoRI) | pGADT7-87L/Y2H |
| 87L-R | CCGCTCGAGCTAGACTCCCTTGGCATGAA (XhoI) | |
| 88R-F | CCGGAATTCATGTCTTTTCAGAGAGATTA (EcoRI) | pGADT7-88R/Y2H |
| 88R-R | CCGCTCGAGCTACCTGGTCCACCTCTTGC (XhoI) | |
| 91R-F | GGGAATTCCATATGCTGTGGGAAGCCGTAACAGA (NdeI) | pGADT7-91R/Y2H |
| 91R-R | CCGGAATTCTTAGCCCTCAAAGAGAGTCA (EcoRI) | |
| 92R-F | GGGAATTCCATATGAGCATCCCTACAGTCATAGC (NdeI) | pGADT7-92R/Y2H |
| 92R-R | CCGGAATTCTTACCGCACATTTCTAGACA (EcoRI) | |
| 95R-F | CCGGAATTCATGCACGGTTGCAATTGTAA (EcoRI) | pGADT7-95R/Y2H |
| 95R-R | CCGCTCGAGTCAGTTAAAAGTGCTCGTAT (XhoI) | |
| 97R-F | CCGGAATTCATGTCTTCTGTAACTGGTTC (EcoRI) | pGADT7-97R/Y2H |
| 97R-R | CCGCTCGAGGACCCATGACGGAAAAGACT (XhoI) | |
| 98R-F | CCGGAATTCATGGCAAACTTTGTGACAGA (EcoRI) | pGADT7-98R/Y2H |
| 98R-R | CCGCTCGAGTTAGGCTCTGACCACAAACA (XhoI) | |
| 101L-F | CCGGAATTCATGGATCCAGAAGGAATGAT (EcoRI) | pGADT7-101L/Y2H |
| 101L-R | CCGCTCGAGTCACAGCACCTTTCTCAGGT (XhoI) | |
| 102R-F | CCGGAATTCATGGGCATAAAAGGACTGAA (EcoRI) | pGADT7-102R/Y2H |
| 102R-R | CCGCTCGAGTCACTTGCGCTTGCACTTCT (XhoI) | |
| 63R-F2 | CCCAAGCTTATGGATCTCTTTGTGTACCA (HindIII) | pcDNA3.1-63R-3Flag |
| 63R-3Flag-R | CCGGAATTCTTACTTATCGTCGTCATCCTTGTAATCGATCTTATCGTCGTCATCCTTGTAATCTCCCTTATCGTCGTCATCCTTGTAATCCTTTTTCTTGAACGACACAA (EcoRI) | |
| 91R-F2 | CCCAAGCTTATGCTGTGGGAAGCCGTAAC (HindIII) | pcDNA3.1-91R-HA |
| 91R-HA-R | CCGGAATTCTTAAGCGTAATCTGGAACATCGTATGGGTACATGCCCTCAAAGAGAGTCACGG (EcoRI) | |
| 63R-R3 | CCGGAATTCGACTTTTTCTTGAACGACAC (EcoRI) | pEGFP-63R/ colocalization |
| 91R-F3 | CCCAAGCTTCAATGCTGTGGGAAGCCGTA (HindIII) | pDsRed2-91R/ colocalization |
| 91R-R2 | ACGCGTCGACTTAGCCCTCAAAGAGAGTCA (SalI) | |
| 91R△NLS1-R | CCCATGAGCCTCAGCGTCACGTAGCTGGTAAAGACCGATG | pDsRed2-91R△NLS1 |
| 91R△NLS1-F | CATCGGTCTTTACCAGCTACGTGACGCTGAGGCTCATGGG | |
| 91R△NLS2-R | GAAAAGGGTCCCATCTTGACCACTCCTCCGGACGCCACCA | pDsRed2-91R△NLS2 |
| 91R△NLS2-F | TGGTGGCGTCCGGAGGAGTGGTCAAGATGGGACCCTTTTC | |
| MCP-F | ATGGTTGTGGAGCAGGTG | qPCR |
| MCP-R | TGACGCAGGTGTAATTGGAG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.-T.; Zhang, Q.-Y. Interaction between Two Iridovirus Core Proteins and Their Effects on Ranavirus (RGV) Replication in Cells from Different Species. Viruses 2019, 11, 416. https://doi.org/10.3390/v11050416
Zeng X-T, Zhang Q-Y. Interaction between Two Iridovirus Core Proteins and Their Effects on Ranavirus (RGV) Replication in Cells from Different Species. Viruses. 2019; 11(5):416. https://doi.org/10.3390/v11050416
Chicago/Turabian StyleZeng, Xiao-Tao, and Qi-Ya Zhang. 2019. "Interaction between Two Iridovirus Core Proteins and Their Effects on Ranavirus (RGV) Replication in Cells from Different Species" Viruses 11, no. 5: 416. https://doi.org/10.3390/v11050416
APA StyleZeng, X.-T., & Zhang, Q.-Y. (2019). Interaction between Two Iridovirus Core Proteins and Their Effects on Ranavirus (RGV) Replication in Cells from Different Species. Viruses, 11(5), 416. https://doi.org/10.3390/v11050416





