The Clinical Features, Pathogenesis and Methotrexate Therapy of Chronic Chikungunya Arthritis
Abstract
1. Introduction
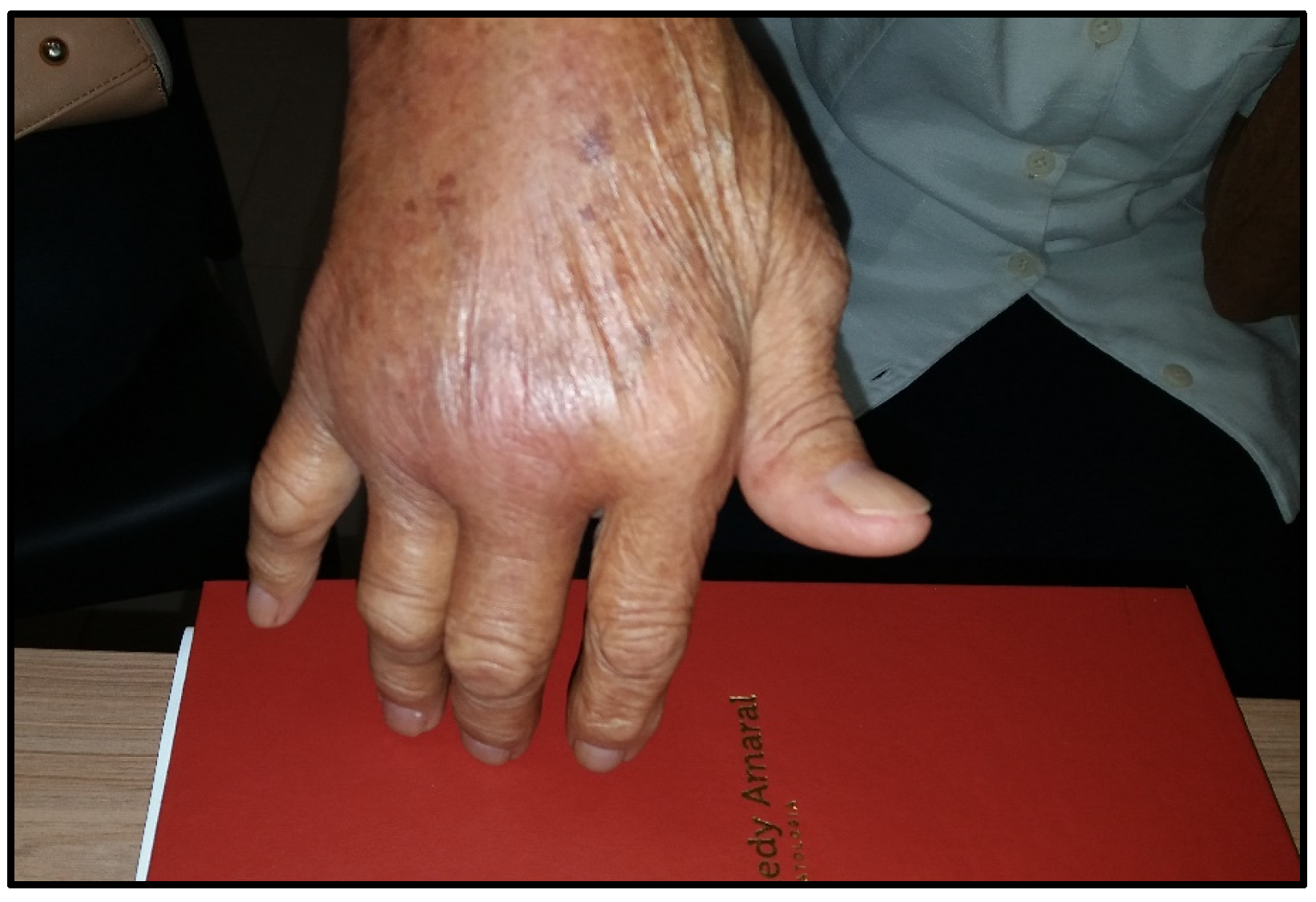
2. Chronic Chikungunya Arthritis
3. Replication Cycle of Chikungunya Virus
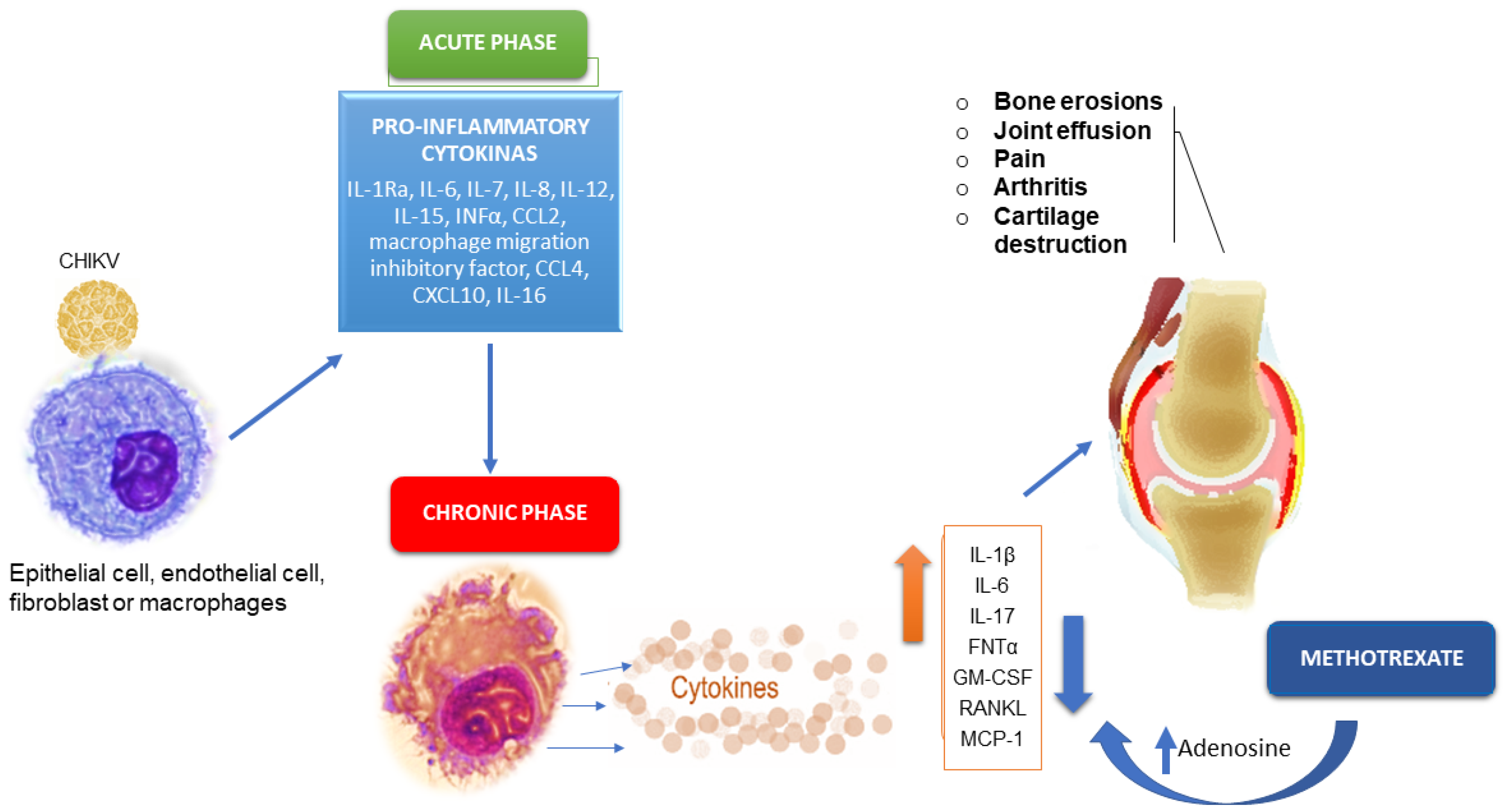
4. Pathogenesis of Chronic Chikungunya Arthritis
4.1. Cytokine/Chemokine Responses
4.2. Cellular Response
4.3. Autoimmunity
5. Methotrexate Therapy of Chronic Chikungunya Arthritis
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Solignat, M.; Gay, B.; Higgs, S.; Briant, L.; Devaux, C. Replication cycle of chikungunya: A re-emerging arbovirus. Virology 2009, 393, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Reyes, E.; Núñez-Avellaneda, D.; Alonso-Palomares, L.A.; Salazar, M.I. Chikungunya: Molecular Aspects, Clinical Outcomes and Pathogenesis. Rev. Invest. Clin. 2017, 69. [Google Scholar] [CrossRef] [PubMed]
- Staples, J.; Breiman, R.; Powers, A. Chikungunya Fever: An Epidemiological Review of a Re-Emerging Infectious Disease. Clin. Infect. Dis. 2009, 49, 942–948. [Google Scholar] [CrossRef]
- Thiberville, S.; Moyen, N.; Dupuis-Maguiraga, L.; Nougairede, A.; Gould, E.A.; Roques, P.; De Lamballerie, X. Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013, 99, 345–370. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.W. The Newala epidemic: III. The virus: Isolation, pathogenic properties and relationship to the epidemic. J. Hyg (Lond). 1956, 54, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Yactoyo, S.; Staples, J.E.; Millot, V.; Cibrelus, L.; Ramon-Pardo, P. Epidemiology of chikungunya in the Americas. J. Infect. Dis. 2016, 214, 441–445. [Google Scholar] [CrossRef]
- Wahid, B.; Ali, A.; Rafique, S.; Idrees, M. Global expansion of chikungunya virus: Mapping the 64-year history. Int. J. Infect. Dis. 2017, 58, 69–76. [Google Scholar] [CrossRef]
- Amaral, J.K.; Schoen, R.T. Chikungunya in Brazil: Rheumatologists on the Front Line. J. Rheumatol. 2018, 45, 1491–1492. [Google Scholar] [CrossRef]
- Simon, F.W.; Javelle, E.; Oliver, M.; Leparc-Goffart, I.; Marimoutou, C. Chikungunya Virus Infection. Curr. Infect. Dis. Rep. 2011, 13, 218–228. [Google Scholar] [CrossRef]
- Gay, N.; Rousset, D.; Huc, P.; Matheus, S.; Rosine, J.; Ledrans, M.; Noël, H. Seroprevalence of Asian Lineage Chikungunya Virus Infection on Saint Martin Island, 7 Months After the 2013 Emergence. Am. J. Trop. Med. Hyg. 2016, 94, 393–396. [Google Scholar] [CrossRef]
- Mehta, R.; Gerardin, P.; de Brito, C.; Soares, C.N.; Ferreira, M.; Solomon, T. The neurological complications of chikungunya virus: A. systematic review. Rev. Medical. Virol. 2018, 28, e1978. [Google Scholar] [CrossRef]
- Rodríguez-Morales, A.J.; Cardona-Ospina, J.A.; Fernanda Urbano-Garzón, S.; Sebastian Hurtado-Zapata, J. Prevalence of Post-Chikungunya Infection Chronic Inflammatory Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2016, 68, 1849–1858. [Google Scholar] [CrossRef]
- Javelle, E.; Ribera, A.; Degasne, I.; Gaüzère, B.; Marimoutou, C.; Simon, F. Specific Management of Post-Chikungunya Rheumatic Disorders: A Retrospective Study of 159 Cases in Reunion Island from 2006–2012. PLoS Negl. Trop Dis. 2015, 9, e0003603. [Google Scholar] [CrossRef]
- Tharmarajah, K.; Mahalingam, S.; Zaid, A. Chikungunya: Vaccines and therapeutics. F1000Research 2017, 6, 2114. [Google Scholar] [CrossRef]
- Powers, A.M. Vaccine and Therapeutic Options To Control Chikungunya Virus. Clin. Microbiol. Rev. 2017, 31, e00104-16. [Google Scholar] [CrossRef]
- Reisinger, E.C.; Tschismarov, R.; Beubler, E.; Wiedermann, U.; Firbas, C.; Loebermann, M.; Ramsauer, K. Immunogenicity, safety, and tolerability of the measles-vectored chikungunya virus vaccine MV-CHIK: A double-blind, randomised, placebo-controlled and active-controlled phase 2 trial. Lancet 2018, 392, 2718–2727. [Google Scholar] [CrossRef]
- Zaid, A.M.; Gérardin, P.; Taylor, A.; Mostafavi, H.; Malvy, D.; Mahalingam, S. Chikungunya Virus Arthritis: Implications of Acute and Chronic Inflammation Mechanisms on Patient Management. Arthritis Rheumatol. 2017. [Google Scholar] [CrossRef]
- Simon, F.; Javelle, E.; Cabie, A.; Bouquillard, E.; Troisgros, O.; Gentile, G.; Leparc-Goffart, I.; Hoen, B.; Gandjbakhch, F.; Rene-Corail, P.; et al. French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med. Mal. Infect. 2015, 45, 243–263. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.; Ramon-Pardo, P.; Javelle, E.; Simon, F.; Aldighieri, S.; Horvath, H.; Reveiz, L. Interventions for treating patients with chikungunya virus infection-related rheumatic and musculoskeletal disorders: A systematic review. PLoS ONE 2017, 12, e0179028. [Google Scholar] [CrossRef]
- Amaral, J.K.; Sutaria, R.; Schoen, R.T. Treatment of chronic chikungunya arthritis with methotrexate: A systematic review. Arthritis Care Res. 2018. [Google Scholar] [CrossRef]
- Marimoutou, C.; Ferraro, J.; Javelle, E.; Deparis, X.; Simon, F. Chikungunya infection: Self-reported rheumatic morbidity and impaired quality of life persist 6 years later. Clin. Microbiol. Infect. 2015, 21, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Ganu, M.A.; Ganu, A.S. Post-chikungunya chronic arthritis—our experience with DMARDs over two year follow up. J. Assoc. Physicians India 2011, 59, 83–86. [Google Scholar]
- Chang, A.Y.; Encinales, L.; Porras, A.; Pacheco, N.; Reid, S.P.; Martins, K.A.; Simon, G.L. Frequency of Chronic Joint Pain Following Chikungunya Virus Infection. Arthritis Rheumatol. 2018. [Google Scholar] [CrossRef]
- Borgherini, G.; Poubeau, P.; Jossaume, A.; Gouix, A.; Cotte, L.; Michault, A.; Paganin, F. Persistent Arthralgia Associated with Chikungunya Virus: A Study of 88 Adult Patients on Reunion Island. Clin. Infect. Dis. 2008, 47, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Schilte, C.; Staikovsky, F.; Couderc, T.; Madec, Y.; Carpentier, F.; Kassab, S.; Michault, A. Chikungunya Virus-associated Long-term Arthralgia: A 36-month Prospective Longitudinal Study. PLoS Negl. Trop Dis. 2013, 7, e2137. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Gil-Restrepo, A.F.; Ramírez-Jaramillo, V.; Montoya-Arias, C.P.; Acevedo-Mendoza, W.F.; Bedoya-Arias, J.E.; Lagos-Grisales, G.J. Post-chikungunya chronic inflammatory rheumatism: Results from a retrospective follow-up study of 283 adult and child cases in La Virginia, Risaralda, Colombia. F1000Research 2016, 5, 360. [Google Scholar] [CrossRef]
- De Andrade, D.C.; Jean, S.; Clavelou, P.; Dallel, R.; Bouhassira, D. Chronic pain associated with the Chikungunya Fever: Long lasting burden of an acute illness. BMC Infect Dis. 2010, 10. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Weerasinghe, S.C.; Gihan, C.; Wickramasinghe, S.; Dharmarathne, S.; Abeyrathna, A.; Jayalath, T. Epidemiology, Clinical Manifestations, and Long-Term Outcomes of a Major Outbreak of Chikungunya in a Hamlet in Sri Lanka, in 2007: A Longitudinal Cohort Study. J. Trop Med. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Mathew, A.J.; Goyal, V.; George, E.; Thekkemuriyil, D.V.; Jayakumar, B.; Chopra, A. Rheumatic-musculoskeletal pain and disorders in a naïve group of individuals 15 months following a Chikungunya viral epidemic in south India: A population based observational study. Int. J. Clin. Pract. 2011, 65, 1306–1312. [Google Scholar] [CrossRef]
- Bouquillard, É.; Combe, B. A report of 21 cases of rheumatoid arthritis following Chikungunya fever. A mean follow-up of two years. Joint. Bone Spine. 2009, 76, 654–657. [Google Scholar] [CrossRef]
- Javelle, E.; Ribera, A.; Degasne, I.; Marimoutou, C.; Simon, F. Clinical spectrum of post-chikungunya rheumatic musculoskeletal disorders and use of disease-modifying antirheumatic drugs to treat the chronic inflammatory entities: 6-year experience from Reunion Island. BMC Infect Dis. 2014, 14 (Suppl. 2), O20. [Google Scholar] [CrossRef]
- Sissoko, D.; Malvy, D.; Ezzedine, K.; Renault, P.; Moscetti, F.; Ledrans, M.; Pierre, V. Post epidemic chikungunya disease on Reunion Island: Course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl. Trop Dis. 2009, 3, e389. [Google Scholar] [CrossRef]
- Essackjee, K.; Goorah, S.; Ramchurn, S.K.; Cheeneebash, J.; Walker-Bone, K. Prevalence of and risk factors for chronic arthralgia and rheumatoid-like polyarthritis more than 2 years after infection with chikungunya virus. Postgrad. Med. J. 2013, 89, 440–447. [Google Scholar] [CrossRef]
- Manimunda, S.P.; Vijayachari, P.; Uppoor, R.; Sugunan, A.P.; Singh, S.S.; Rai, S.K.; Guruprasad, D.R. Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Trans. R Soc. Trop Med. Hyg. 2010, 104, 392–399. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Villamil-Gomez, W.; Merlano-Espinosa, M.; Simone-Kleber, L. Post-chikungunya chronic arthralgia: A first retrospective follow-up study of 39 cases in Colombia. Clin. Rheumatol. 2015, 35, 831–832. [Google Scholar] [CrossRef]
- Miner, J.J.; Aw Yeang, H.X.; Fox, J.M.; Taffner, S.; Malkova, O.N.; Oh, S.T.; Kim, A.H.J.; Diamond, M.S.; Lenschow, D.J.; Yokoyama, W.M. Brief report: Chikungunya viral arthritis in the united states: A mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol. 2015, 67, 1214–1220. [Google Scholar] [CrossRef]
- Chaaithanya, I.K.; Muruganandam, N.; Raghuraj, U.; Sugunan, A.P.; Rajesh, R.; Anwesh, M.; Vijayachari, P. Chronic inflammatory arthritis with persisting bony erosions in patients following chikungunya infection. Indian J. Med. Res. 2014, 140, 142–145, Retrieved March 10, 2017. [Google Scholar]
- Chopra, A.; Anuradha, V.; Lagoo-Joshi, V.; Kunjir, V.; Salvi, S.; Saluja, M. Chikungunya virus aches and pains: An emerging challenge. Arthritis Rheum. 2008, 58, 2921–2922. [Google Scholar] [CrossRef]
- Horcada, M.L.; Díaz-Calderón, C.; Garrido, L. Chikungunya Fever. Rheumatic Manifestations of an Emerging Disease in Europe. Reumatol. Clin. (English Edition). 2015, 11, 161–164. [Google Scholar] [CrossRef]
- Amaral, J.K.; Schoen, R.T. A Case Report of Chikungunya Fever, Rheumatoid Arthritis, and Felty’s Syndrome. Rheumatol. Ther. 2018. [Google Scholar] [CrossRef]
- Amaral, J.K.; Bingham, C.O.; Schoen, R.T. Successful Methotrexate Treatment of Chronic Chikungunya Arthritis. J. Clin. Rheumatol. 2018, 1. [Google Scholar] [CrossRef]
- Zeana, C.; Kelly, P.; Heredia, W.; Cifuentes, A.; Franchin, G.; Purswani, M.; Hagmann, S.H. Post-chikungunya rheumatic disorders in travelers after return from the Caribbean. Travel Med. Infect. Dis. 2016, 14, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Gérardin, P.; Fianu, A.; Michault, A.; Mussard, C.; Boussaïd, K.; Rollot, O.; Grivard, P.; Kassab, S.; Bouquillard, E.; Borgherini, G.; et al. Predictors of chikungunya rheumatism: A prognostic survey ancillary to the TELECHIK cohort study. Arthritis Res. Ther. 2013, 15, R9. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, H.M.; Simon, F.; Deparis, X.; Marimoutou, C. Identification of initial severity determinants to predict arthritis after chikungunya infection in a cohort of French gendarmes. BMC Musculoskelet Disord. 2014, 15. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Fraser, J. Ross river virus infection of human synovial cells in vitro. Aust. J. Exp. Biol. Med. Sci. 1985, 63, 197–204. [Google Scholar] [CrossRef]
- Linn, M.L.; Aaskov, J.G.; Suhrbier, A. Antibody-dependent enhancement and persistence in macrophages of an arbovirus associated with arthritis. J. Gen. Virol. 1996, 77, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Hapuarachchi, H.C.; Chen, K.C.; Hussain, K.M.; Chen, H.; Low, S.L.; Chu, J.J. Mosquito Cellular Factors and Functions in Mediating the Infectious entry of Chikungunya Virus. PLoS Negl. Trop Dis. 2013, 7, e2050. [Google Scholar] [CrossRef]
- Bernard, E.; Solignat, M.; Gay, B.; Chazal, N.; Higgs, S.; Devaux, C.; Briant, L. Endocytosis of Chikungunya Virus into Mammalian Cells: Role of Clathrin and Early Endosomal Compartments. PLoS ONE 2010, 5, e11479. [Google Scholar] [CrossRef]
- Wichit, S.; Hamel, R.; Bernard, E.; Talignani, L.; Diop, F.; Ferraris, P.; Missé, D. Imipramine Inhibits Chikungunya Virus Replication in Human Skin Fibroblasts through Interference with Intracellular Cholesterol Trafficking. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Kielian, M. Membrane fusion And the Alphavirus Life Cycle. Adv. Virus. Res. 1995, 113–151. [Google Scholar] [CrossRef]
- Ozden, S.; Huerre, M.; Riviere, J.; Coffey, L.L.; Afonso, P.V.; Mouly, V.; Ceccaldi, P. Human Muscle Satellite Cells as Targets of Chikungunya Virus Infection. PLoS ONE. 2007, 2, e527. [Google Scholar] [CrossRef]
- Sourisseau, M.; Schwartz, O. Characterization of reemerging chikungunya virus. Plos Pathog. 2007, 3, 0804–0817. [Google Scholar] [CrossRef]
- Ashok, A.; Atwood, W.J. Contrasting Roles of Endosomal pH and the Cytoskeleton in Infection of Human Glial Cells by JC Virus and Simian Virus 40. J. Virol. 2003, 77, 1347–1356. [Google Scholar] [CrossRef]
- Krejbich-Trotot, P.; Denizot, M.; Hoarau, J.; Jaffar-Bandjee, M.; Das, T.; Gasque, P. Chikungunya virus mobilizes the apoptotic machinery to invade host cell defenses. FASEB J. 2011, 25, 314–325. [Google Scholar] [CrossRef]
- Judith, D.; Mostowy, S.; Bourai, M.; Gangneux, N.; Lelek, M.; Lucas-Hourani, M.; Lecuit, M. Species-specific impact of the autophagy machinery on Chikungunya virus infection. EMBO Rep. 2013, 14, 534–544. [Google Scholar] [CrossRef]
- Krejbich-Trotot, P.; Gay, B.; Li-Pat-Yuen, G.; Hoarau, J.; Jaffar-Bandjee, M.; Briant, L.; Denizot, M. Chikungunya triggers an autophagic process which promotes viral replication. Virol. J. 2011, 8, 432. [Google Scholar] [CrossRef]
- Tang, B.L. The cell biology of Chikungunya virus infection. Cell Microbiol. 2012, 14, 1354–1363. [Google Scholar] [CrossRef]
- Callus, B.A.; Vaux, D.L. Caspase inhibitors: Viral, cellular and chemical. Cell Death Differ. 2006, 14, 73–78. [Google Scholar] [CrossRef]
- Schilte, C.; Couderc, T.; Chretien, F.; Sourisseau, M.; Gangneux, N.; Guivel-Benhassine, F.; Kraxner, A.; Tschopp, J.; Higgs, S.; Michault, A.; et al. Type I IFN controls chikungunya virus via its action on nonhematopoietic cells. J. Exp. Med. 2010, 207, 429–442. [Google Scholar] [CrossRef]
- Her, Z.; Malleret, B.; Chan, M.; Ong, E.K.; Wong, S.C.; Kwek, D.J.; Ng, L.F. Active Infection of Human Blood Monocytes by Chikungunya Virus Triggers an Innate Immune Response. J. Immunol. 2010, 184, 5903–5913. [Google Scholar] [CrossRef]
- Chirathaworn, C.L.; Poovorawan, Y.; Lertmaharit, S.; Wuttirattanakowit, N. Cytokine levels in patients with chikungunya virus infection. Asian Pac. J. Trop Med. 2013, 6, 631–634. [Google Scholar] [CrossRef]
- Miner, J.J.; Lenschow, D.J. Editorial: Lessons Learned From Chikungunya in the Americas. Arthritis Rheumatol. 2018, 70, 477–479. [Google Scholar] [CrossRef]
- Hoarau, J.J.; Jaffar Bandjee, M.C.; Krejbich Trotot, P.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Guichard, E.; Ribera, A.; Henni, T.; Tallet, F.; et al. Persistent chronic inflammation and infection by chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef]
- Chaaitanya, I.K.; Muruganandam, N.; Sundaram, S.G.; Kawalekar, O.; Sugunan, A.P.; Manimunda, S.P.; Ghosal, S.R.; Muthumani, K.; Vijayachari, P. Role of pro-inflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral. Immunol. 2011, 24, 265–271. [Google Scholar] [CrossRef]
- Chow, A.; Her, Z.; Ong, E.K.; Chen, J.; Dimatatac, F.; Kwek, D.J.; Ng, L.F. Persistent Arthralgia Induced by Chikungunya Virus Infection is Associated with Interleukin-6 and Granulocyte Macrophage Colony-Stimulating Factor. J. Infect Dis. 2011, 203, 149–157. [Google Scholar] [CrossRef]
- Dupuis-Maguiraga, L.; Noret, M.; Brun, S.; Le Grand, R.; Gras, G.; Roques, P. Chikungunya Disease: Infection-Associated Markers from the Acute to the Chronic Phase of Arbovirus-Induced Arthralgia. PLoS Negl. Trop Dis. 2012, 6, e1446. [Google Scholar] [CrossRef]
- Labadie, K.; Larcher, T.; Joubert, C.; Mannioui, A.; Delache, B.; Brochard, P.; Roques, P. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Invest. 2010, 120, 894–906. [Google Scholar] [CrossRef]
- Ng, L.F.; Chow, A.; Sun, Y.; Kwek, D.J.; Lim, P.; Dimatatac, F.; Leo, Y. IL-1β, IL-6, and RANTES as Biomarkers of Chikungunya Severity. PLoS ONE 2009, 4, e4261. [Google Scholar] [CrossRef]
- Spadaro, A.; Scrivo, R.; Rinaldi, T.; Riccieri, V.; Sili Scavalli, A.; Taccari, E.; Valesini, G. The role of Interleukin-12 in immune-mediated rheumatic diseases. Reumatismo 2011, 54. [Google Scholar] [CrossRef]
- Miossec, P.; Korn, T.; Kuchroo, V.K. Interleukin-17 and Type 17 Helper, T. Cells. N. Engl. J. Med. 2009, 361, 888–898. [Google Scholar] [CrossRef]
- Thanapati, S.; Ganu, M.A.; Tripathy, A.S. Differential inhibitory and activating NK cell receptor levels and NK/NKT-like cell functionality in chronic and recovered stages of chikungunya. PLoS ONE 2017, 12, e0188342. [Google Scholar] [CrossRef]
- Thanapati, S.; Ganu, M.; Giri, P.; Kulkarni, S.; Sharma, M.; Babar, P.; Tripathy, A.S. Impaired NK cell functionality and increased TNF-α production as biomarkers of chronic chikungunya arthritis and rheumatoid arthritis. Hum. Immunol. 2017, 78, 370–374. [Google Scholar] [CrossRef]
- McCarthy, M.K.; Morrison, T.E. Chronic chikungunya virus musculoskeletal disease: What are the underlying mechanisms? Future Microbiol. 2016, 11, 331–334. [Google Scholar] [CrossRef]
- Poo, Y.S.; Rudd, P.A.; Gardner, J.; Wilson, J.A.; Larcher, T.; Suhrbier, A. Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl. Trop Dis. 2014, 8, e3354. [Google Scholar] [CrossRef]
- Chang, A.Y.; Martins, K.A.; Encinales, L.; Reid, S.P.; Acuña, M.; Encinales, C.; Firestein, G.S. Chikungunya Arthritis Mechanisms in the Americas. Arthritis Rheumatol. 2018, 70, 585–593. [Google Scholar] [CrossRef]
- McCarthy, M.K.; Morrison, T.E. Persistent RNA virus infections: Do PAMPS drive chronic disease? Curr. Opin. Virol. 2017, 23, 8–15. [Google Scholar] [CrossRef]
- Tian, H.; Cronstein, B. Understanding the mechanisms of action of methotrexate: Implications for the treatment of rheumatoid arthritis. Bull NYU Hosp. Jt. Dis. 2007, 65, 168–173. [Google Scholar]
- Swierkot, J.; Szechinski, J. Methotrexate in rheumatoid arthritis. Pharmacol. Rep. 2006, 58, 473–492. [Google Scholar]
- Cutolo, M. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann. Rheum. Dis. 2001, 60, 729–735. [Google Scholar] [CrossRef]
- Wessels, J.A.; Huizinga, T.W.; Guchelaar, H. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford). 2007, 47, 249–255. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Yap, J.S.; Desai, A.; Posadas, I.; McCrary, C.T.; Cronstein, B.N. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: Evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis Rheum. 2000, 43, 656–663. [Google Scholar] [PubMed]
- Bedoui, Y.; Giry, C.; Jaffar-Bandjee, M.; Selambarom, J.; Guiraud, P.; Gasque, P. Immunomodulatory drug methotrexate used to treat patients with chronic inflammatory rheumatisms post-chikungunya does not impair the synovial antiviral and bone repair responses. PLoS Negl. Trop Dis. 2018, 12, e0006634. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, J.K.; Taylor, P.C.; Teixeira, M.M.; Morrison, T.E.“.; Schoen, R.T. The Clinical Features, Pathogenesis and Methotrexate Therapy of Chronic Chikungunya Arthritis. Viruses 2019, 11, 289. https://doi.org/10.3390/v11030289
Amaral JK, Taylor PC, Teixeira MM, Morrison TE“, Schoen RT. The Clinical Features, Pathogenesis and Methotrexate Therapy of Chronic Chikungunya Arthritis. Viruses. 2019; 11(3):289. https://doi.org/10.3390/v11030289
Chicago/Turabian StyleAmaral, J. Kennedy, Peter C. Taylor, Mauro Martins Teixeira, Thomas E. “Tem” Morrison, and Robert T. Schoen. 2019. "The Clinical Features, Pathogenesis and Methotrexate Therapy of Chronic Chikungunya Arthritis" Viruses 11, no. 3: 289. https://doi.org/10.3390/v11030289
APA StyleAmaral, J. K., Taylor, P. C., Teixeira, M. M., Morrison, T. E. “., & Schoen, R. T. (2019). The Clinical Features, Pathogenesis and Methotrexate Therapy of Chronic Chikungunya Arthritis. Viruses, 11(3), 289. https://doi.org/10.3390/v11030289





