Viroids as Companions of a Professional Career
Abstract
1. Introduction
2. In Vitro Tissue Culture Approaches
3. Field Assays Supporting Viroid Characterization
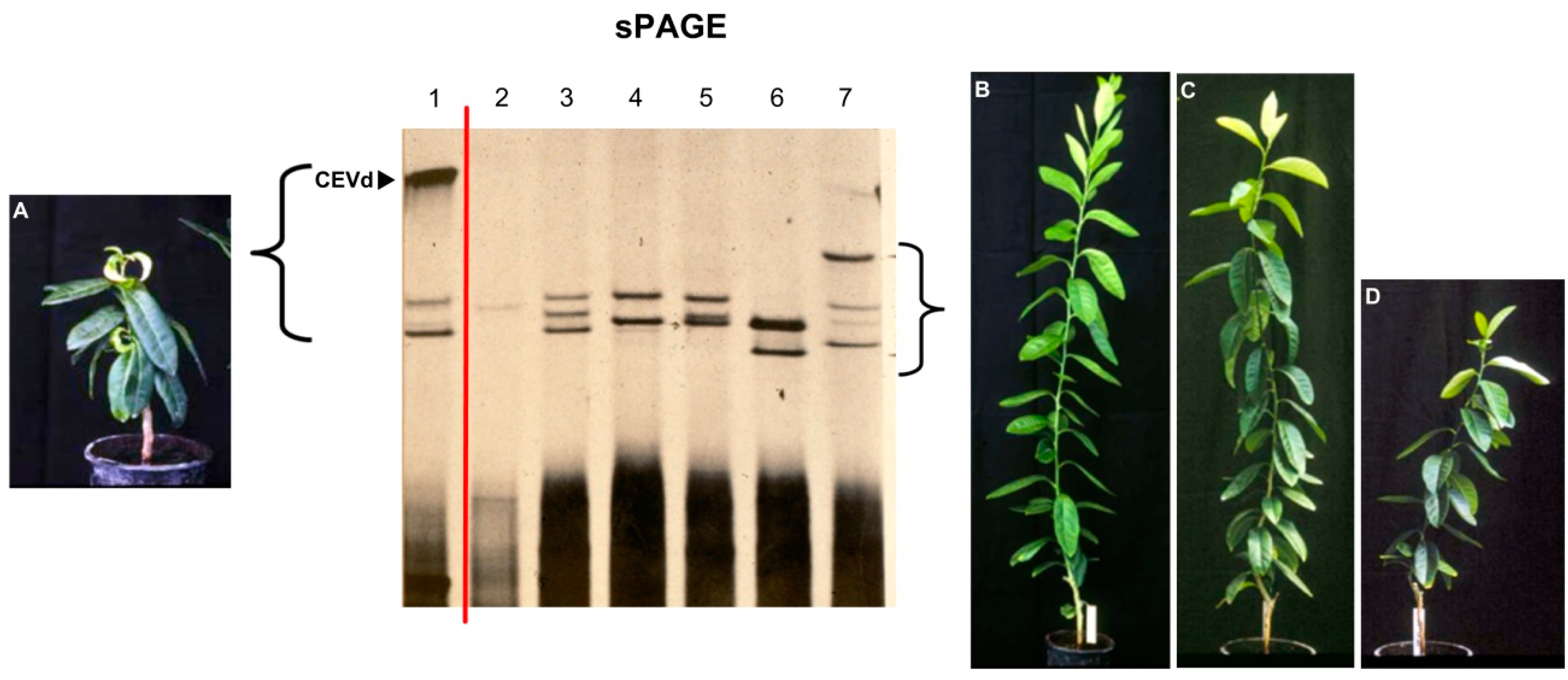
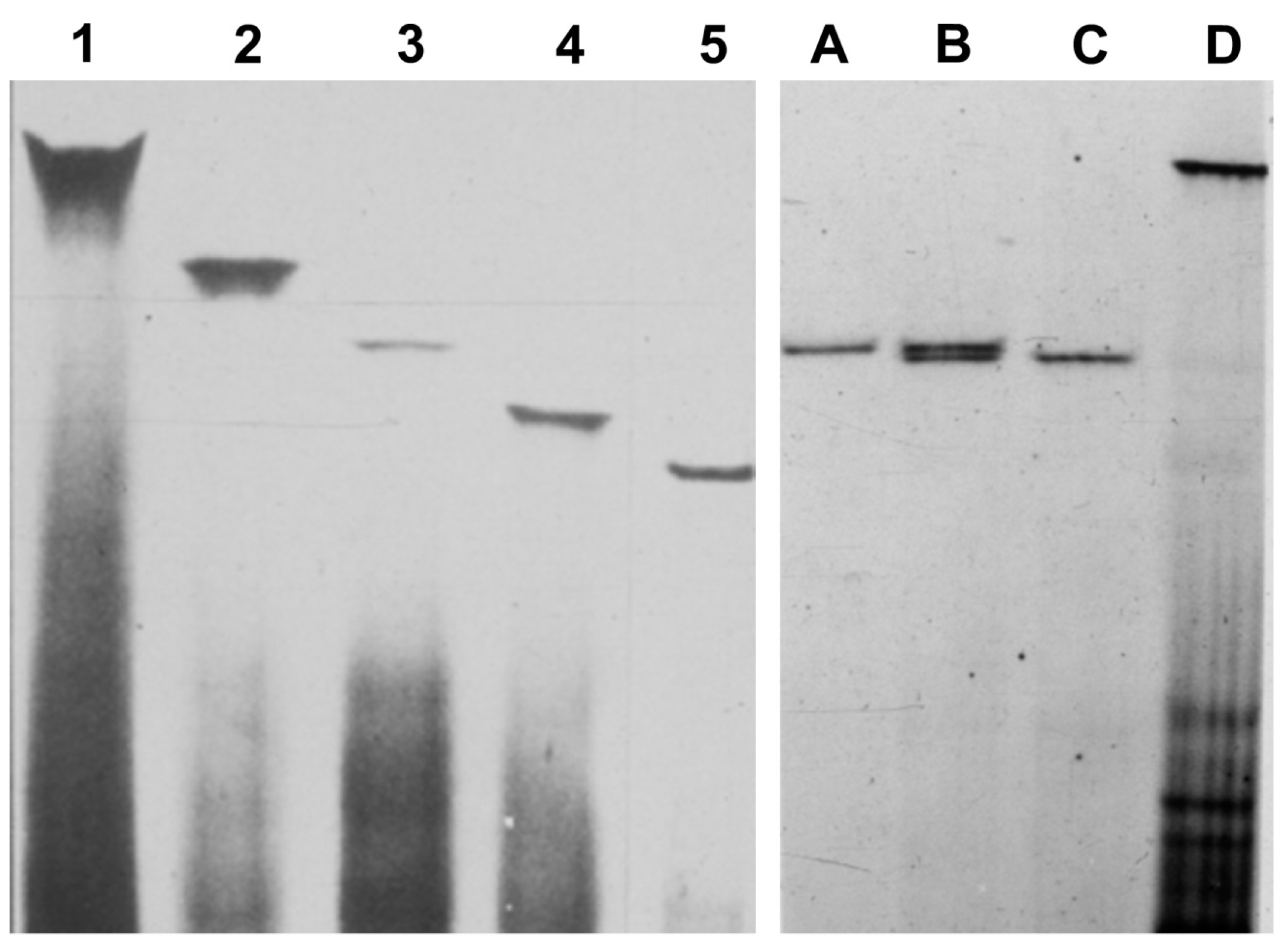
4. Additional Hosts, New Viroids, and Viroid Variants
5. Personality Traits of Scientists: Competition vs. Cooperation
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, W.H. “Spindle tuber”, a new potato trouble. Hints Potato Grow. N. J. State Potato Assoc. 1922, 3, 8. [Google Scholar]
- Schultz, E.S.; Folsom, D. Transmission, variation, and control of certain degeneration diseases of Irish potatoes. J. Agric. Res. 1923, 25, 43–118. [Google Scholar]
- Fawcett, H.S.; Klotz, L.J. Exocortis of trifoliate orange. Citrus Leaves 1948, 28, 8–9. [Google Scholar]
- Benton, R.J.; Bowman, F.T.; Fraser, L.; Kebby, R.G. Selection of citrus budwood to control scaly butt in trifoliata rootstock. Agric. Gaz. N. S. W. 1949, 60, 31–34. [Google Scholar]
- Benton, R.J.; Bowman, F.T.; Fraser, L.; Kebby, R.G. Stunting and scaly butt of citrus associated with Poncirus trifoliata rootstock. N. S. W. Dep. Agric. Sci. Bull. 1950, 61, 20–40. [Google Scholar]
- Weathers, L.G.; Greer, F.C., Jr.; Hartjung, M.K. Transmission of exocortis virus of citrus to herbaceous plants. Plant Dis. Rep. 1967, 51, 868–871. [Google Scholar]
- Semancik, J.S.; Weathers, L.G. Exocortis virus: An infectious free-nucleic acid plant virus with unusual properties. Virology 1972, 46, 456–466. [Google Scholar] [CrossRef]
- Gross, H.J.; Krupp, G.; Domdey, H.; Raba, M.; Jank, P.; Lossow, C.; Alberty, H.; Ramm, K.; Sänger, H.L. Nucleotide sequence and secondary structure of citrus exocortis and chrysanthemum stunt viroid. Eur. J. Biochem. 1982, 121, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Gould, A.; Bruening, G.; Symons, R.H. Citrus esxocortis viroid: Nucleotide sequence and secondary structure of an Australian isolate. FEBS Lett. 1982, 137, 288–292. [Google Scholar] [CrossRef]
- Calavan, E.C.; Frolich, E.F.; Carpenter, J.B.; Roistacher, C.N.; Christiansen, D.W. Rapid indexing for exocortis of citrus. Phytopathology 1964, 54, 1359–1362. [Google Scholar]
- Roistacher, C.N.; Calavan, E.C.; Blue, R.L.; Navarro, L.; González, L. A new more sensitive indicator for detection of mild isolates of citrus exocortis viroid (CEV). Plant Dis. Report. 1977, 61, 135–139. [Google Scholar]
- Duran-Vila, N.; Flores, R.; Semancik, J.S. Characterization of viroid-like RNAs associated with the citrus exocortis syndrome. Virology 1986, 150, 75–84. [Google Scholar] [CrossRef]
- Duran-Vila, N.; Roistacher, C.N.; Rivera-Bustamante, R.; Semancik, J.S. A definition of citrus viroid groups and their relationship to the exocortis disease. J. Gen. Virol. 1988, 69, 3069–3080. [Google Scholar] [CrossRef]
- Serra, P.; Eiras, M.; Bani Hashemian, S.M.; Murcia, N.; Kitajima, E.W.; Daròs, J.A.; Flores, R.; Duran-Vila, N. Citrus viroid V: Occurrence, host range, diagnosis and identification of new variants. Phytopathology 2008, 98, 199–1204. [Google Scholar] [CrossRef] [PubMed]
- Serra, P.; Barbosa, C.J.; Daròs, J.A.; Flores, R.; Duran-Vila, N. Citrus viroid V: Molecular characterization and synergistic interactions with other members of the genus Apscaviroid. Virology 2008, 70, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ieki, H.; Ozaki, K.; Ito, T. Characterization of a new citrus viroid species tentatively termed citrus viroid OS. Arch. Virol. 2001, 146, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wu, Q.; Yang, F.; Wang, X.; Li, R.; Zhou, C.H.; Li, Z. Molecular characterization and phylogenetic analysis of Citrus viroid VI variants from citrus in China. Eur. J. Plant Pathol. 2017, 149, 885–893. [Google Scholar] [CrossRef]
- Chambers, G.A.; Donovan, N.J.; Bodaghi, S.; Jelinek, S.M.; Vidalakis, G. A novel citrus viroid found in Australia, tentatively named citrus viroid VII. Arch. Virol. 2018, 163, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Hataya, T.; Shikata, T. Complete nucleotide sequence of a viroid isolated from Etrog citron, a new member of hop stunt viroid group. Nucl. Acids Res. 1988, 16, 347. [Google Scholar] [CrossRef]
- Semancik, J.S.; Roistacher, C.N.; Rivera-Bustamante, R.; Duran-Vila, N. Citrus cachexia viroid, a new viroid of citrus: Relationship to viroids of the exocortis disease complex. J. Gen. Virol. 1988, 69, 3059–3068. [Google Scholar] [CrossRef]
- Childs, J.F.L. The cachexia disease of Orlando tangelo. Plant Dis. Rep. 1950, 34, 295–298. [Google Scholar]
- Reichert, I.; Perlberger, P. Xyloporosis, the new citrus disease. Jew. Agency Palestine Agr. Exp. Sta. (Rehovot) Bull. 1934, 12, 1–50. [Google Scholar]
- Reanwarakorn, K.; Semancik, J.S. Regulation of pathogenicityin hop stunt viroid-related group II citrus viroids. J. Gen. Virol. 1998, 79, 3163–3171. [Google Scholar] [CrossRef]
- Palacio-Bielsa, A.; Romero-Durbán, J.; Duran-Vila, N. Characterization of citrus HSVd isolates. Arch. Virol. 2004, 149, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Shikata, E.; Sano, T.; Uyeda, T. An infectious low molecular weight RNA was detected in grapevines by molecular hybridization with hop stunt viroid cDN A. Proc. Jpn. Acad. Sci. 1984, 60B, 202–205. [Google Scholar] [CrossRef]
- Sano, T.; Uyeda, I.; Shikata, E.; Meshi, T.; Ohano, T.; Okada, Y. A viroid-like RNA isolated from grapevine has high sequence homology with hop stunt viroid. J. Gen. Virol. 1985, 66, 333–338. [Google Scholar] [CrossRef]
- Flores, R.; Duran-Vila, N.; Pallás, V.; Semancik, J.S. Detection of viroid and viroid-like RNAs from grapevine. J. Gen. Virol. 1985, 66, 2095–2102. [Google Scholar] [CrossRef]
- Takebe, I.; Otsuki, Y.; Aoki, S. Isolation of tobacco mesophyll cells in intact and active state. Plant Cell Physiol. 1968, 9, 115–124. [Google Scholar]
- Schenk, R.V.; Hildebrandt, A.C. Production of protoplasts from plant cells in liquid media using purified commercialcellulases. Crop Sci. 1969, 9, 629–631. [Google Scholar] [CrossRef]
- Duran-Vila, N.; Semancik, J.S. Effects of exogenous auxins on tomato tissues infected with the citrus exocortis viroid. Phytopathology 1982, 72, 777–781. [Google Scholar] [CrossRef]
- Rodriguez, J.L.; García-Martinez, J.L.; Flores, R. The relationship between plant growth substances content and infection of Gynura aurantiaca DC by Citrus Exocortis Viroid. Plant Pathol. 1978, 13, 355–363. [Google Scholar] [CrossRef]
- Marton, L.; Duran-Vila, N.; Lin, J.J.; Semancik, J.S. Properties of cell cultures containing the citrus exocortis viroid. Virology 1982, 122, 229–238. [Google Scholar] [CrossRef]
- Wang, M.C.; Lin, J.J.; Duran-Vila, N.; Semancik, J.S. Alteration in cell wall composition and structure in viroid infected cells. Physiol. Mol. Plant Pathol. 1986, 28, 107–124. [Google Scholar] [CrossRef]
- Duran-Vila, N.; Carbonell, E.A.; Pérez Boada, S.; Semancik, J.S. Growth of healthy and viroid-infected tomato cells in vitro. Plant Sci. 1995, 105, 111–120. [Google Scholar] [CrossRef]
- Navarro, L.; Roistacher, C.N.; Murashige, T. Improvement of shoot-tip grafting in vitro for virus-free citrus. J. Am. Soc. Hort. Sci. 1975, 100, 461–479. [Google Scholar]
- Duran-Vila, N.; Semancik, J.S. Tomato shoot tip culture and the elimination of viroid RNA. Sci. Hortic. 1986, 29, 199–203. [Google Scholar] [CrossRef]
- Duran-Vila, N.; Juárez, J.; Arregui, J.M. Production of viroid-free grapevines by shoot-tip culture. Am. J. Enol. Vit. 1988, 39, 217–220. [Google Scholar]
- Juárez, J.; Navarro, L.; Duran-Vila, N. Separation of citrus viroids by shoot tip grafting in vitro. Plant Pathol. 1990, 39, 472–476. [Google Scholar] [CrossRef]
- Vernière, C.; Perrier, X.; Dubois, C.; Dubois, A.; Botella, L.; Chabrier, C.; Bové, J.M.; Duran-Vila, N. Citrus viroids: Symptom expression and effect on vegetative growth and yield on clementine trees grafted on trifoliate orange. Plant Dis. 2004, 88, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Vernière, C.; Perrier, X.; Dubois, C.; Dubois, A.; Botella, L.; Chabrier, C.; Bové, J.M.; Duran-Vila, N. Interactions between citrus viroids affect symptom expression and field performance of clementine trees grafted on trifoliate orange. Phytopathology 2006, 96, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Bani Hashemian, S.M.; Serra, P.; Barbosa, C.J.; Juárez, J.; Aleza, P.; Corvera, J.M.; Lluch, A.; Pina, J.A.; Duran-Vila, N. The effect of a field-source mixture of citrus viroids on the performance of ‘Nules’ clementine and ‘Navelina’ sweet orange trees grafted on Carrizo citrange. Plant Dis. 2009, 93, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Murcia, N.; Bani Hashemian, S.M.; Serra, P.; Pina, J.A.; Duran-Vila, N. Citrus viroids: Symptom expression and performance of Washington navel sweet orange trees grafted on Carrizo citrange. Plant Dis. 2015, 99, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Bani Hashemian, S.M.; Murcia, N.; Trenor, I.; Duran-Vila, N. Low performance of citrus trees grafted on Carrizo citrange is associated with viroid infection. J. Plant Pathol. 2010, 92, 511–517. [Google Scholar]
- Najar, A.; Hamrouni, L.; Bouhlal, R.; Jemmali, A.; Jamoussi, B.; Duran-Vila, N. Viroid infection and rootstocks affect productivity and fruit quality of the Tunisian citrus cultivar Maltaise demi sanguine. Phytopathol. Mediterr. 2017, 56, 409–420. [Google Scholar]
- Najar, A.; Hamdi, I.; Varsani, A.; Duran-Vila, N. Citrus viroids in Tunisia: Prevalence and molecular characterization. J. Plant Pathol. 2017, 99, 787–792. [Google Scholar]
- Mishra, M.D.; Hammond, R.W.; Owens, R.A.; Smith, D.R.; Diener, T.O. Indian bunchy top disease of tomato plants is caused by a distinct strain of citrus exocortis viroid. J. Gen. Virol. 1991, 72, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- García-Arenal, F.; Pallás, V.; Flores, R. The sequence of a viroid from grapevine closely related to severe isolates of citrus exocortis viroid. Nucl. Acids Res. 1987, 15, 4203–4210. [Google Scholar] [CrossRef]
- Fagoaga, C.; Semancik, J.S.; Duran-Vila, N. A citrus exocortis viroid variant from broad bean (Vicia faba L.): Infectivity and pathogenesis. J. Gen. Virol. 1995, 76, 2271–2277. [Google Scholar] [CrossRef]
- Fagoaga, C.; Duran-Vila, N. Naturally occurring variants of citrus exocortis viroid in vegetable crops. Plant Pathol. 1996, 45, 45–53. [Google Scholar] [CrossRef]
- Semancik, J.S.; Szychowski, J.A.; Rakowski, A.G.; Symons, R.H. A sTable 463 nucleotide variant of citrus exocortis viroid produced by 324 terminal repeats. J. Gen. Virol. 1994, 75, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Fadda, Z.; Daròs, J.A.; Flores, R.; Duran-Vila, N. Identification in eggplant of a variant of citrus exocortis viroid (CEVd) with a 96 nucleotide duplication in the right terminal region of the rod-like secondary structure. Virus Res. 2003, 97, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Fadda, Z.; Daròs, J.A.; Fagoaga, C.; Flores, R.; Duran-Vila, N. Eggplant Latent Viroid (ELVd): Candidate Type Species for a New Genus Within Family Avsunviroidae (Hammerhead Viroids). J. Virol. 2003, 77, 6528–6532. [Google Scholar] [CrossRef] [PubMed]
- Diener, T.O. Potato spindle tuber “virus” IV. A replicating, low molecular weight RNA. Virology 1971, 45, 411–428. [Google Scholar] [CrossRef]




| Family | Genus | Former Name | Species |
|---|---|---|---|
| Pospiviroidae | Pospiviroid | CEVd | Citrus exocortis viroid (CEVd) |
| Hostuviroid | CVd-II | Hop stunt viroid (HSVd) | |
| Cocadviroid | CVd-IV | Citrus bark cracking viroid (CBCVd) | |
| Apscaviroid | CVd-I | Citrus bent leaf viroid (CBLVd) | |
| Apscaviroid | CVd-III | Citrus dwarfing viroid (CDVd) | |
| Apscaviroid | CVd-V | ||
| Apscaviroid | CVd-VI | ||
| Apscaviroid | CVd-VII |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duran-Vila, N. Viroids as Companions of a Professional Career. Viruses 2019, 11, 245. https://doi.org/10.3390/v11030245
Duran-Vila N. Viroids as Companions of a Professional Career. Viruses. 2019; 11(3):245. https://doi.org/10.3390/v11030245
Chicago/Turabian StyleDuran-Vila, Núria. 2019. "Viroids as Companions of a Professional Career" Viruses 11, no. 3: 245. https://doi.org/10.3390/v11030245
APA StyleDuran-Vila, N. (2019). Viroids as Companions of a Professional Career. Viruses, 11(3), 245. https://doi.org/10.3390/v11030245




