A Diacylglycerol Kinase Inhibitor, R-59-022, Blocks Filovirus Internalization in Host Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Inhibitors and Plasmids
2.3. Virus Pseudotype and Viral-Like Particle Production
2.4. Virus Entry and Cell Viability Assays
2.5. Time of Addition Assay
2.6. Virus Attachment Assay
2.7. Virus Internalization Assay
2.8. Dextran Internalization Assay
2.9. Live-Cell Imaging of VLPs and Actin
2.10. Replication-Competent Virus Growth Assay
2.11. Statistical Analysis
3. Results
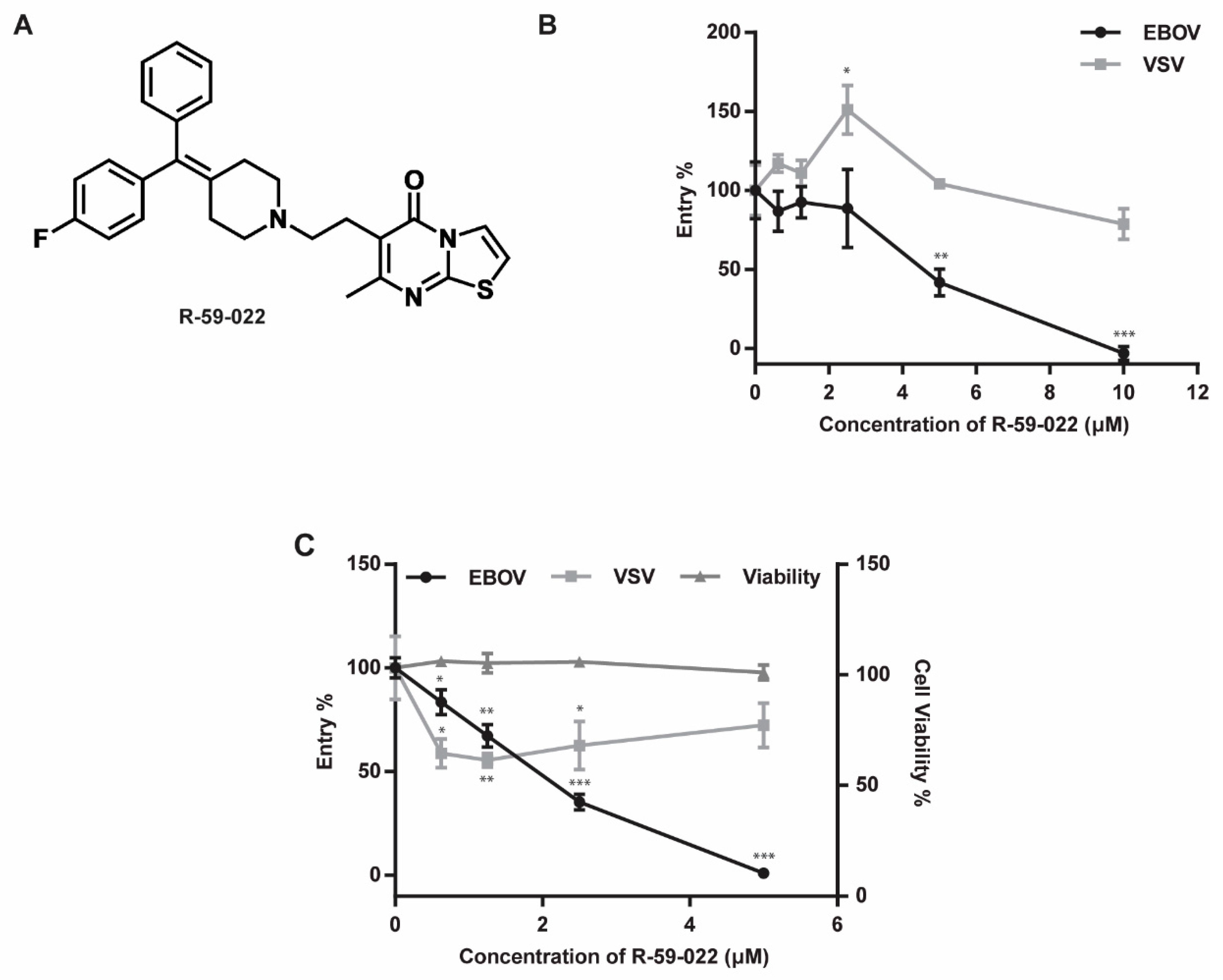
3.1. R-59-022 Efficiently Blocks EBOV GP-Mediated Entry into Vero Cells
3.2. EBOV GP-Mediated Entry in Bone Marrow-Derived Macrophages is Inhibited by R-59-022
3.3. R-59-022 Interferes with Virus Internalization
3.4. R-59-022 Blocks Macropinocytosis in Vero Cells
3.5. R-59-022 Blocks Viral Entry of Pathogenic Filoviruses
3.6. EBOV Growth is Inhibited by R-59-022
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feldmann, H.; Geisbert, T.W. Ebola haemorrhagic fever. Lancet 2011, 377, 849–862. [Google Scholar] [CrossRef]
- Peters, C.J.; Sanchez, A.; Feldmann, H.; Rollin, P.E.; Nichol, S.; Ksiazek, T.G. Filoviruses as Emerging Pathogens. Semin. Virol. 1994, 5, 147–154. [Google Scholar] [CrossRef]
- Bao, Y.; Amarasinghe, G.K.; Basler, C.F.; Bavari, S.; Bukreyev, A.; Chandran, K.; Dolnik, O.; Dye, J.M.; Ebihara, H.; Formenty, P.; et al. Implementation of Objective PASC-Derived Taxon Demarcation Criteria for Official Classification of Filoviruses. Viruses 2017, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Tan, C.W.; Anderson, D.E.; Jiang, R.D.; Li, B.; Zhang, W.; Zhu, Y.; Lim, X.F.; Zhou, P.; Liu, X.L.; et al. Characterization of a filovirus (Mengla virus) from Rousettus bats in China. Nat. Microbiol. 2019, 4, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet 2017, 389, 505–518. [Google Scholar] [CrossRef]
- Wong, G.; Mendoza, E.J.; Plummer, F.A.; Gao, G.F.; Kobinger, G.P.; Qiu, X. From bench to almost bedside: The long road to a licensed Ebola virus vaccine. Expert. Opin. Biol. Ther. 2018, 18, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C. Viral membrane fusion. Virology 2015, 479–480, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Chandran, K.; Sullivan, N.J.; Felbor, U.; Whelan, S.P.; Cunningham, J.M. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 2005, 308, 1643–1645. [Google Scholar] [CrossRef] [PubMed]
- Côté, M.; Misasi, J.; Ren, T.; Bruchez, A.; Lee, K.; Filone, C.M.; Hensley, L.; Li, Q.; Ory, D.; Chandran, K.; Cunningham, J.M. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature 2011, 477, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Carette, J.E.; Raaben, M.; Wong, A.C.; Herbert, A.S.; Obernosterer, G.; Mulherkar, N.; Kuehne, A.I.; Kranzusch, P.J.; Griffin, A.M.; Ruthel, G.; et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 2011, 477, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.H.; Obernosterer, G.; Raaben, M.; Herbert, A.S.; Deffieu, M.S.; Krishnan, A.; Ndungo, E.; Sandesara, R.G.; Carette, J.E.; Kuehne, A.I.; et al. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 2012, 31, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Markosyan, R.M.; Miao, C.; Zheng, Y.M.; Melikyan, G.B.; Liu, S.L.; Cohen, F.S. Induction of Cell-Cell Fusion by Ebola Virus Glycoprotein: Low pH Is Not a Trigger. PLoS Pathog. 2016, 12, e1005373. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.S.; Higgins, C.D.; Chandran, K.; Lai, J.R. Designed protein mimics of the Ebola virus glycoprotein GP2 alpha-helical bundle: stability and pH effects. Protein Sci. 2011, 20, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.F.; Kolokoltsov, A.A.; Albrecht, T.; Davey, R.A. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 2010, 6, e1001110. [Google Scholar] [CrossRef] [PubMed]
- Nanbo, A.; Imai, M.; Watanabe, S.; Noda, T.; Takahashi, K.; Neumann, G.; Halfmann, P.; Kawaoka, Y. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog. 2010, 6, e1001121. [Google Scholar] [CrossRef] [PubMed]
- Mulherkar, N.; Raaben, M.; de la Torre, J.C.; Whelan, S.P.; Chandran, K. The Ebola virus glycoprotein mediates entry via a non-classical dynamin-dependent macropinocytic pathway. Virology 2011, 419, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.; Brindley, M.A.; Weller, M.L.; Kaludov, N.; Kondratowicz, A.; Hunt, C.L.; Sinn, P.L.; McCray, P.B., Jr.; Stein, C.S.; Davidson, B.L.; et al. Rho GTPases modulate entry of Ebola virus and vesicular stomatitis virus pseudotyped vectors. J. Virol. 2009, 83, 10176–10186. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.A. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell. Biol. 2008, 9, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.L.; Kolokoltsov, A.A.; Davey, R.A.; Maury, W. The Tyro3 receptor kinase Axl enhances macropinocytosis of Zaire ebolavirus. J. Virol. 2011, 85, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Condon, N.D.; Heddleston, J.M.; Chew, T.L.; Luo, L.; McPherson, P.S.; Ioannou, M.S.; Hodgson, L.; Stow, J.L.; Wall, A.A. Macropinosome formation by tent pole ruffling in macrophages. J. Cell Biol. 2018, 217, 3873–3885. [Google Scholar] [CrossRef] [PubMed]
- Botelho, R.J.; Teruel, M.; Dierckman, R.; Anderson, R.; Wells, A.; York, J.D.; Meyer, T.; Grinstein, S. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 2000, 151, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K.L.; Ueyama, T.; Michaud, T.M.; Kashiwagi, K.; Wang, D.; Flax, L.A.; Shirai, Y.; Loegering, D.J.; Saito, N.; Lennartz, M.R. Targeting of protein kinase C-epsilon during Fcgamma receptor-dependent phagocytosis requires the epsilonC1B domain and phospholipase C-gamma1. Mol. Biol. Cell 2006, 17, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Shulga, Y.V.; Topham, M.K.; Epand, R.M. Regulation and functions of diacylglycerol kinases. Chem. Rev. 2011, 111, 6186–6208. [Google Scholar] [CrossRef] [PubMed]
- Abramovici, H.; Mojtabaie, P.; Parks, R.J.; Zhong, X.P.; Koretzky, G.A.; Topham, M.K.; Gee, S.H. Diacylglycerol kinase zeta regulates actin cytoskeleton reorganization through dissociation of Rac1 from RhoGDI. Mol. Biol. Cell 2009, 20, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Ard, R.; Mulatz, K.; Pomoransky, J.L.; Parks, R.J.; Trinkle-Mulcahy, L.; Bell, J.C.; Gee, S.H. Regulation of Macropinocytosis by Diacylglycerol Kinase zeta. PLoS ONE 2015, 10, e0144942. [Google Scholar] [CrossRef] [PubMed]
- LeBlond, N.D.; Fullerton, M.D. Methods to Evaluate AMPK Regulation of Macrophage Cholesterol Homeostasis. Methods Mol. Biol. 2018, 1732, 477–493. [Google Scholar] [PubMed]
- Misasi, J.; Chandran, K.; Yang, J.Y.; Considine, B.; Filone, C.M.; Côté, M.; Sullivan, N.; Fabozzi, G.; Hensley, L.; Cunningham, J. Filoviruses require endosomal cysteine proteases for entry but exhibit distinct protease preferences. J. Virol. 2012, 86, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, C.J.; Schornberg, K.L.; Delos, S.E.; Scully, C.; Pajouhesh, H.; Olinger, G.G.; Johansen, L.M.; White, J.M. Multiple cationic amphiphiles induce a Niemann-Pick C phenotype and inhibit Ebola virus entry and infection. PLoS ONE 2013, 8, e56265. [Google Scholar] [CrossRef]
- Burkel, B.M.; von Dassow, G.; Bement, W.M. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil. Cytoskeleton 2007, 64, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Moller-Tank, S.; Maury, W. Ebola virus entry: A curious and complex series of events. PLoS Pathog. 2015, 11, e1004731. [Google Scholar] [CrossRef] [PubMed]
- Cureton, D.K.; Massol, R.H.; Saffarian, S.; Kirchhausen, T.L.; Whelan, S.P. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog. 2009, 5, e1000394. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Lovato, K.L.; Wilson, B.S.; Rector, K.D. SERS nanosensors that report pH of endocytic compartments during FcepsilonRI transit. Anal. Bioanal. Chem. 2010, 398, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Spector, I.; Shochet, N.R.; Blasberger, D.; Kashman, Y. Latrunculins--novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil. Cytoskeleton 1989, 13, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.F.; Kolokoltsov, A.A.; Freiberg, A.N.; Holbrook, M.R.; Davey, R.A. Phosphoinositide-3 kinase-Akt pathway controls cellular entry of Ebola virus. PLoS Pathog. 2008, 4, e1000141. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Leung, A.; Bo, Y.; Kozak, R.A.; Anand, S.P.; Warkentin, C.; Salambanga, F.D.R.; Cui, J.; Kobinger, G.; Kobasa, D.; Côté, M. Ebola virus requires phosphatidylinositol (3,5) bisphosphate production for efficient viral entry. Virology 2018, 513, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Kondratowicz, A.S.; Hunt, C.L.; Davey, R.A.; Cherry, S.; Maury, W.J. AMP-activated protein kinase is required for the macropinocytic internalization of ebolavirus. J. Virol. 2013, 87, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Drelich, A.; Judy, B.; He, X.; Chang, Q.; Yu, S.; Li, X.; Lu, F.; Wakamiya, M.; Popov, V.; Zhou, J.; Ksiazek, T.; Gong, B. Exchange Protein Directly Activated by cAMP Modulates Ebola Virus Uptake into Vascular Endothelial Cells. Viruses 2018, 10, 563. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, C.M.; Dorion, S.S.; Ottenbrite, M.A.F.; LeBlond, N.D.; Smith, T.K.T.; Qiu, S.; Fullerton, M.D.; Kobasa, D.; Côté, M. A Diacylglycerol Kinase Inhibitor, R-59-022, Blocks Filovirus Internalization in Host Cells. Viruses 2019, 11, 206. https://doi.org/10.3390/v11030206
Stewart CM, Dorion SS, Ottenbrite MAF, LeBlond ND, Smith TKT, Qiu S, Fullerton MD, Kobasa D, Côté M. A Diacylglycerol Kinase Inhibitor, R-59-022, Blocks Filovirus Internalization in Host Cells. Viruses. 2019; 11(3):206. https://doi.org/10.3390/v11030206
Chicago/Turabian StyleStewart, Corina M., Stephanie S. Dorion, Marie A. F. Ottenbrite, Nicholas D. LeBlond, Tyler K. T. Smith, Shirley Qiu, Morgan D. Fullerton, Darwyn Kobasa, and Marceline Côté. 2019. "A Diacylglycerol Kinase Inhibitor, R-59-022, Blocks Filovirus Internalization in Host Cells" Viruses 11, no. 3: 206. https://doi.org/10.3390/v11030206
APA StyleStewart, C. M., Dorion, S. S., Ottenbrite, M. A. F., LeBlond, N. D., Smith, T. K. T., Qiu, S., Fullerton, M. D., Kobasa, D., & Côté, M. (2019). A Diacylglycerol Kinase Inhibitor, R-59-022, Blocks Filovirus Internalization in Host Cells. Viruses, 11(3), 206. https://doi.org/10.3390/v11030206





