The Oxysterol 25-Hydroxycholesterol Inhibits Replication of Murine Norovirus
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Lines and Viruses
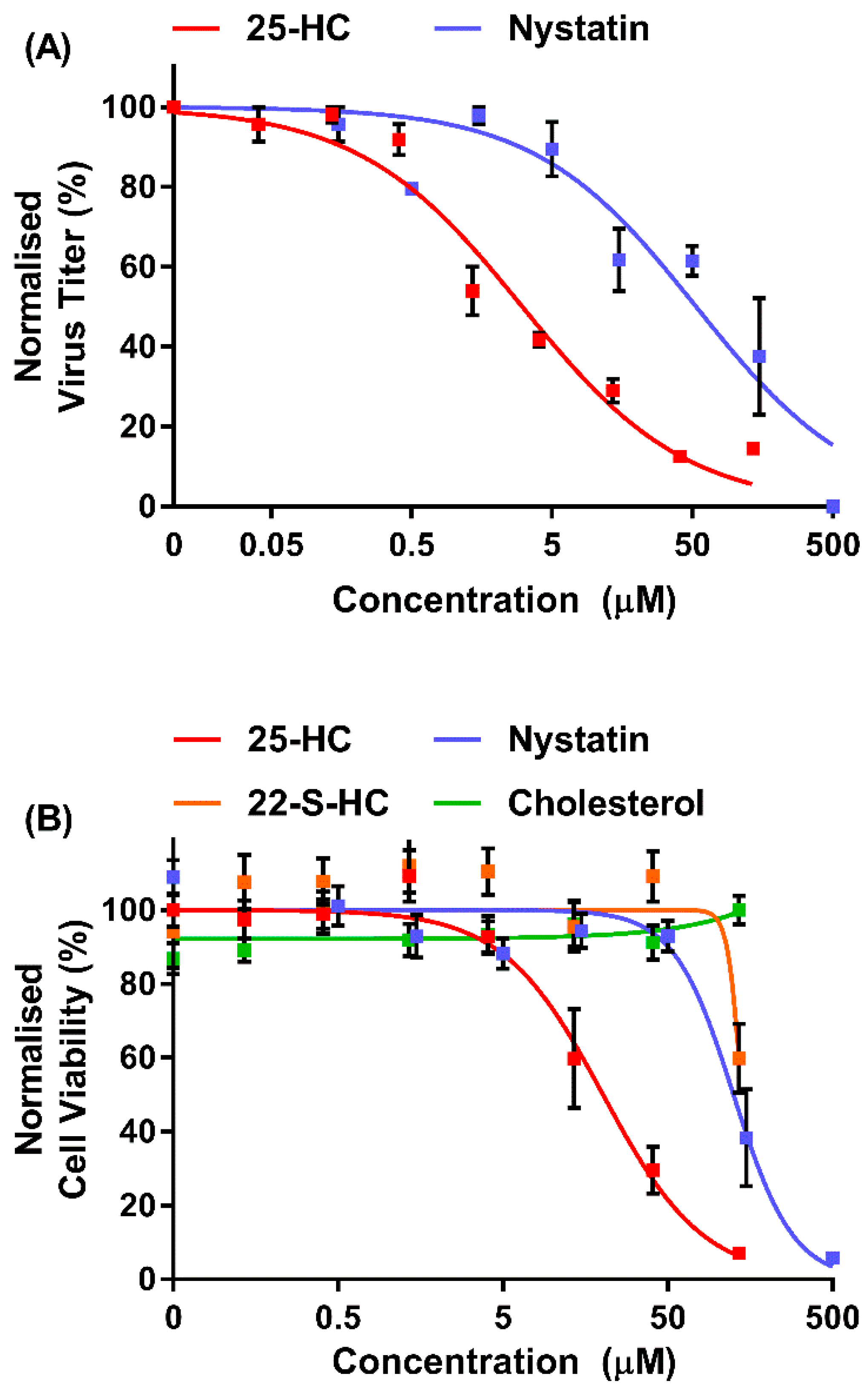
2.3. Assessment of Cell Viability
2.4. MNV Dose–Response Curves
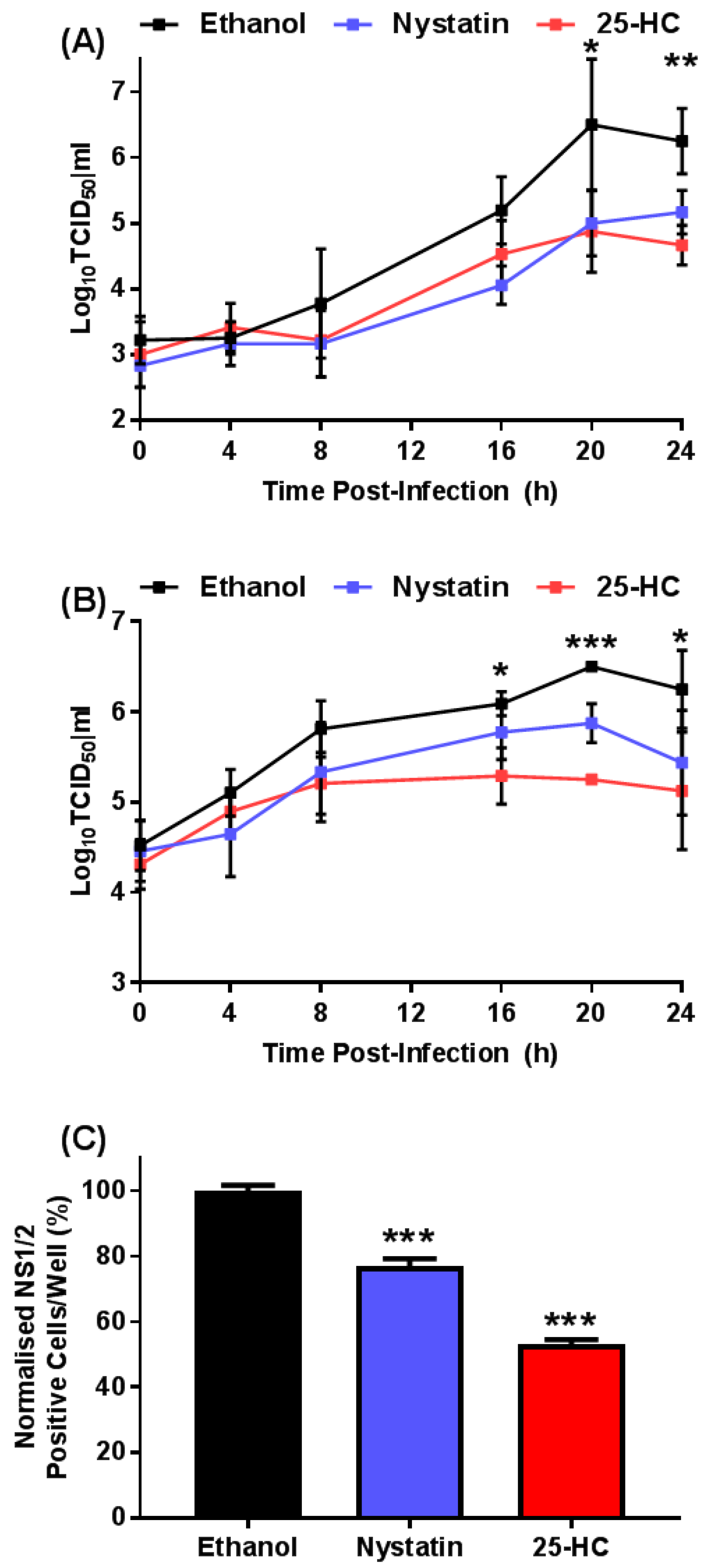
2.5. Single Cycle Growth Analysis
2.6. Fluorescent Focus Assay
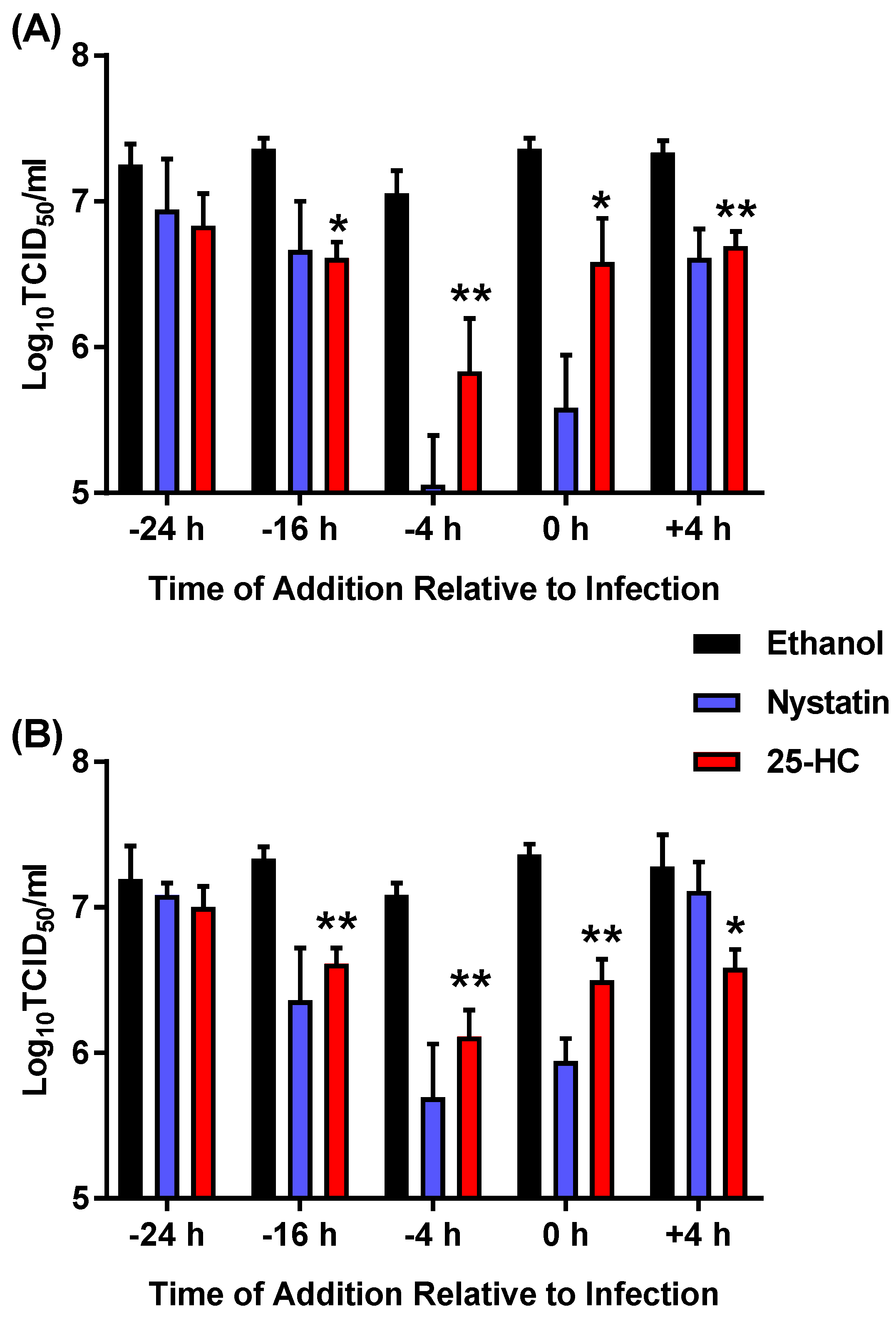
2.7. Time-of-Addition Assays
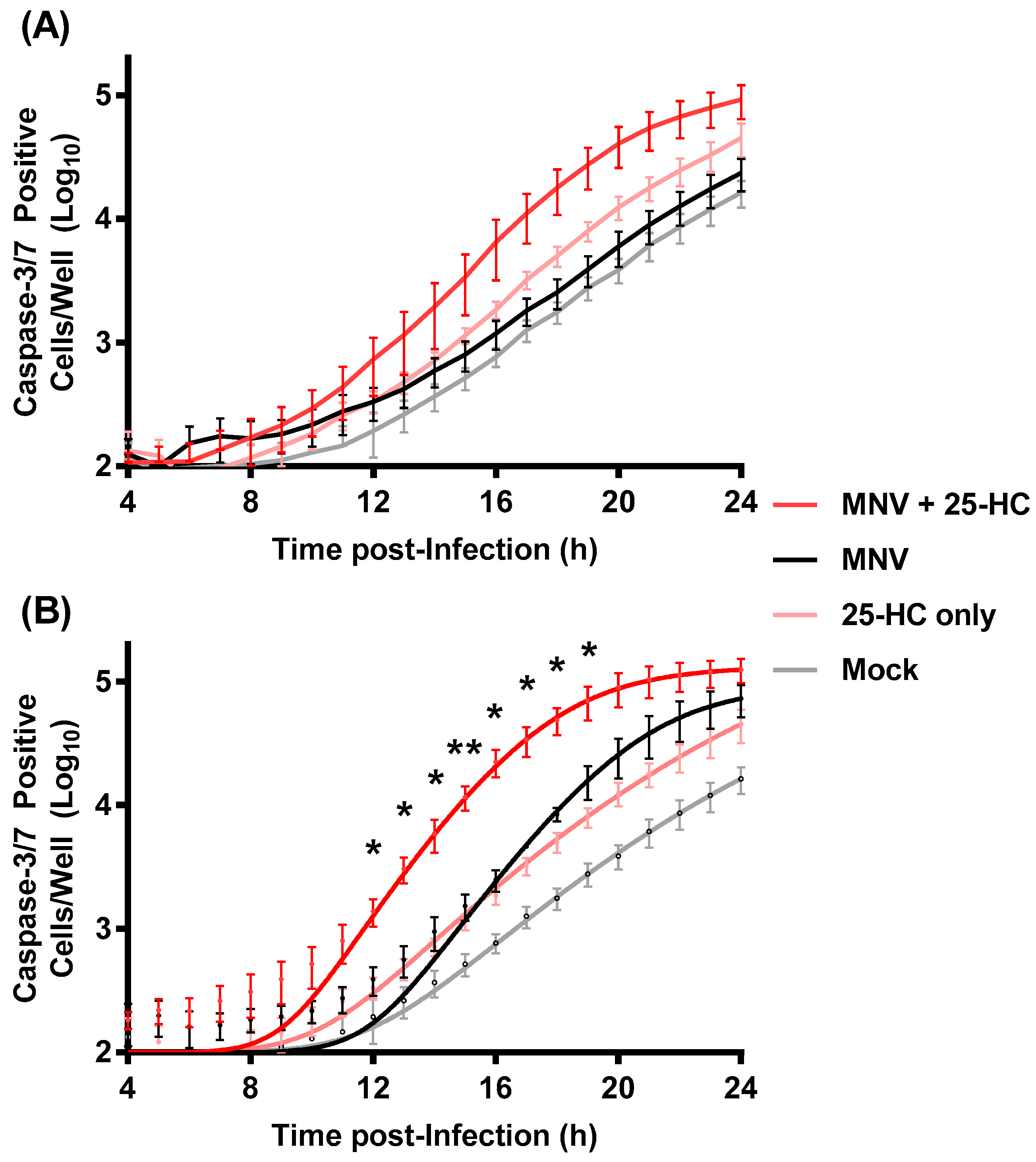
2.8. Measuring Caspase 3/7 Activity
2.9. Measuring HSV-1–GFP Replication
2.10. Cytotoxicity of MNV in the Presence of Oxysterols
2.11. Statistical Analysis
3. Results
3.1. 25-HC Reduced MNV Titer in a Single-Cycle Growth Curve
3.2. Pre-Treatment with 25-HC Prior to Virus Infection Reduced MNV Replication
3.3. 25-HC Stimulates Caspase Activation and Cell Death in MNV-Infected Cells
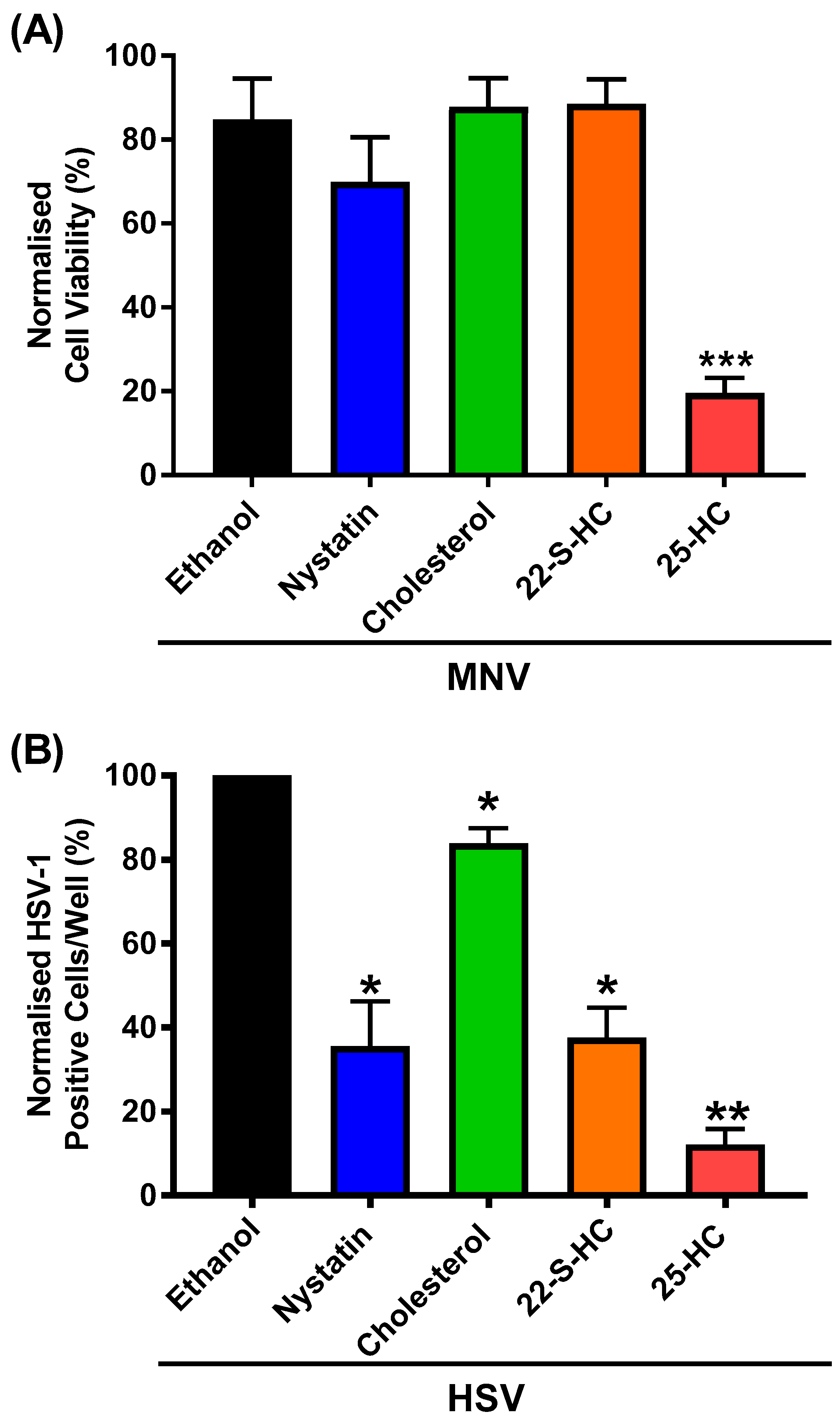
3.4. 25-HC Inhibits HSV-1 Replication in RAW264.7 Macrophages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Lysén, M.; Thorhagen, M.; Brytting, M.; Hjertqvist, M.; Andersson, Y.; Hedlund, K.-O. Genetic diversity among food-borne and waterborne norovirus strains causing outbreaks in Sweden. J. Clin. Microbiol. 2009, 47, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.; Vipond, I.; Carlisle, D.; Deakin, D.; Fey, R.; Caul, E. Evidence for airborne transmission of Norwalk-like virus (NLV) in a hotel restaurant. Epidemiol. Infect. 2000, 124, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Green, K. Chapter 20—Caliciviridae: The noroviruses. In Fields Virology, 6th ed.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2013. [Google Scholar]
- Glass, R.I.; Parashar, U.D.; Estes, M.K. Norovirus Gastroenteritis. N. Engl. J. Med. 2009, 361. [Google Scholar] [CrossRef] [PubMed]
- Wobus, C.E. The Dual Tropism of Noroviruses. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Bartnicki, E.; Cunha, J.B.; Kolawole, A.O.; Wobus, C.E. Recent advances in understanding noroviruses. F1000Research 2017, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.; Bailey, D.; Putics, A.; Goodfellow, I. Model systems for the study of human norovirus biology. Future Virol. 2009, 4, 353–367. [Google Scholar] [CrossRef]
- Wobus, C.E.; Thackray, L.B.; Virgin, H.W. Murine norovirus: A model system to study norovirus biology and pathogenesis. J. Virol. 2006, 80, 5104–5112. [Google Scholar] [CrossRef]
- Holmes, R.S.; VandeBerg, J.L.; Cox, L.A. Genomics and proteomics of vertebrate cholesterol ester lipase (LIPA) and cholesterol 25-hydroxylase (CH25H). 3 Biotech 2011, 1, 99–109. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, L.; Zhang, L.; Guo, Z.-Z.; Lu, S.-F.; Ming, S.-L.; Li, G.-L.; Wan, B.; Tian, K.-G.; Yang, G.-Y.; et al. Cholesterol 25-hydroxylase acts as a host restriction factor on pseudorabies virus replication. J. Gen. Virol. 2017, 98, 1467–1476. [Google Scholar] [CrossRef]
- Liu, S.Y.; Aliyari, R.; Chikere, K.; Li, G.; Marsden, M.D.; Smith, J.K.; Pernet, O.; Guo, H.; Nusbaum, R.; Zack, J.A.; et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 2013, 38, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Blanc, M.; Hsieh, W.Y.; Robertson, K.A.; Kropp, K.A.; Forster, T.; Shui, G.; Lacaze, P.; Watterson, S.; Griffiths, S.J.; Spann, N.J.; et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 2013, 38, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava-Ranjan, P.; Bergeron, É.; Chakrabarti, A.K.; Albariño, C.G.; Flint, M.; Nichol, S.T.; Spiropoulou, C.F. 25-Hydroxycholesterol Inhibition of Lassa Virus Infection through Aberrant GP1 Glycosylation. mBio 2016, 7, e01808-16. [Google Scholar] [CrossRef] [PubMed]
- Cagno, V.; Civra, A.; Rossin, D.; Calfapietra, S.; Caccia, C.; Leoni, V.; Dorma, N.; Biasi, F.; Poli, G.; Lembo, D. Inhibition of herpes simplex-1 virus replication by 25-hydroxycholesterol and 27-hydroxycholesterol. Redox Biol. 2017, 12, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Deng, Y.-Q.; Wang, S.; Ma, F.; Aliyari, R.; Huang, X.-Y.; Zhang, N.-N.; Watanabe, M.; Dong, H.-L.; Liu, P.; et al. 25-Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity 2017, 46, 446–456. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Yang, H. Antiviral effect of 25-hydroxycholesterol against porcine reproductive and respiratory syndrome virus in vitro. Antivir. Ther. 2018, 23, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Doms, A.; Sanabria, T.; Hansen, J.N.; Altan-Bonnet, N.; Holm, G.H. 25-Hydroxycholesterol Production by the Cholesterol-25-Hydroxylase Interferon-Stimulated Gene Restricts Mammalian Reovirus Infection. J. Virol. 2018, 92, e01047-18. [Google Scholar] [CrossRef] [PubMed]
- Serquina, A.K.P.; Kambach, D.M.; Sarker, O.; Ziegelbauer, J.M. Viral MicroRNAs Repress the Cholesterol Pathway, and 25-Hydroxycholesterol Inhibits Infection. mBio 2017, 8, e00576-17. [Google Scholar] [CrossRef]
- Civra, A.; Cagno, V.; Donalisio, M.; Biasi, F.; Leonarduzzi, G.; Poli, G.; Lembo, D. Inhibition of pathogenic non-enveloped viruses by 25-hydroxycholesterol and 27-hydroxycholesterol. Sci. Rep. 2014, 4, 7487. [Google Scholar] [CrossRef]
- Arita, M.; Kojima, H.; Nagano, T.; Okabe, T.; Wakita, T.; Shimizu, H. Oxysterol-Binding Protein Family I Is the Target of Minor Enviroxime-Like Compounds. J. Virol. 2013, 87, 4252–4260. [Google Scholar] [CrossRef]
- Civra, A.; Francese, R.; Gamba, P.; Testa, G.; Cagno, V.; Poli, G.; Lembo, D. 25-Hydroxycholesterol and 27-hydroxycholesterol inhibit human rotavirus infection by sequestering viral particles into late endosomes. Redox Biol. 2018, 19, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Watashi, K.; Tsukuda, S.; Aly, H.H.; Fukasawa, M.; Fujimoto, A.; Suzuki, R.; Aizaki, H.; Ito, T.; Koiwai, O. Evaluation and identification of hepatitis B virus entry inhibitors using HepG2 cells overexpressing a membrane transporter NTCP. Biochem. Biophys. Res. Commun. 2014, 443, 808–813. [Google Scholar] [CrossRef]
- Romero-Brey, I.; Berger, C.; Colpitts, C.C.; Boldanova, T.; Engelmann, M.; Todt, D.; Perin, P.M.; Behrendt, P.; Vondran, F.W.; Xu, S.; et al. Interferon-inducible cholesterol-25-hydroxylase restricts hepatitis C virus replication through blockage of membranous web formation. Hepatology 2015, 62, 702–714. [Google Scholar]
- Xiang, Y.; Tang, J.-J.; Tao, W.; Cao, X.; Song, B.-L.; Zhong, J. Identification of cholesterol 25-hydroxylase as a Novel Host restriction factor and a part of the primary innate immune responses against hepatitis C virus infection. J. Virol. 2015, 89, 6805–6816. [Google Scholar] [CrossRef] [PubMed]
- Arita, M. Phosphatidylinositol-4 kinase III beta and oxysterol-binding protein accumulate unesterified cholesterol on poliovirus-induced membrane structure. Microbiol. Immunol. 2014, 58, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Roulin, P.S.; Lotzerich, M.; Torta, F.; Tanner, L.B.; van Kuppeveld, F.J.; Wenk, M.R.; Greber, U.F. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe 2014, 16, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Ward, V.K.; McCormick, C.J.; Clarke, I.N.; Salim, O.; Wobus, C.E.; Thackray, L.B.; Virgin, H.W.; Lambden, P.R. Recovery of infectious murine norovirus using pol II-driven expression of full-length cDNA. Proc. Natl. Acad. Sci. USA 2007, 104, 11050–11055. [Google Scholar] [CrossRef]
- Hwang, S.; Alhatlani, B.; Arias, A.; Caddy, S.L.; Christodoulou, C.; Cunha, J.B.; Emmott, E.; Gonzalez-Hernandez, M.; Kolawole, A.; Lu, J.; et al. Murine norovirus: Propagation, quantification, and genetic manipulation. Curr. Protoc. Microbiol. 2014, 33, 15K. [Google Scholar]
- Spearman, C. The method of ‘right and wrong cases’(‘constant stimuli’) without Gauss’s formulae. Br. J. Psychol. 1908, 2, 227–242. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Herod, M.R.; Salim, O.; Skilton, R.J.; Prince, C.A.; Ward, V.K.; Lambden, P.R.; Clarke, I.N. Expression of the murine norovirus (MNV) ORF1 polyprotein is sufficient to induce apoptosis in a virus-free cell model. PLoS ONE 2014, 9, e90679. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.W.; Wobus, C.E. Endocytosis of Murine Norovirus 1 into Murine Macrophages Is Dependent on Dynamin II and Cholesterol. J. Virol. 2010, 84, 6163–6176. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.W.; Taube, S.; Wobus, C.E. Murine norovirus-1 entry into permissive macrophages and dendritic cells is pH-independent. Virus Res. 2009, 143, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Bok, K.; Prikhodko, V.G.; Green, K.Y.; Sosnovtsev, S.V. Apoptosis in murine norovirus-infected RAW264. 7 cells is associated with downregulation of survivin. J. Virol. 2009, 83, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Rusinol, A.E.; Thewke, D.; Liu, J.; Freeman, N.; Panini, S.R.; Sinensky, M.S. AKT/protein kinase B regulation of BCL family members during oxysterol-induced apoptosis. J. Biol. Chem. 2004, 279, 1392–1399. [Google Scholar] [CrossRef]
- Sosnovtsev, S.V.; Belliot, G.; Chang, K.O.; Prikhodko, V.G.; Thackray, L.B.; Wobus, C.E.; Karst, S.M.; Virgin, H.W.; Green, K.Y. Cleavage map and proteolytic processing of the murine norovirus nonstructural polyprotein in infected cells. J. Virol. 2006, 80, 7816–7831. [Google Scholar] [CrossRef] [PubMed]
- Nice, T.J.; Baldridge, M.T.; McCune, B.T.; Norman, J.M.; Lazear, H.M.; Artyomov, M.; Diamond, M.S.; Virgin, H.W. Interferon-lambda cures persistent murine norovirus infection in the absence of adaptive immunity. Science 2015, 347, 269–273. [Google Scholar] [CrossRef]
- Nice, T.J.; Strong, D.W.; McCune, B.T.; Pohl, C.S.; Virgin, H.W. A single-amino-acid change in murine norovirus NS1/2 is sufficient for colonic tropism and persistence. J. Virol. 2013, 87, 327–334. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, Y.; Choi, I.; Kim, S.-W.; Kim, W.-K. 25-Hydroxycholesterol induces mitochondria-dependent apoptosis via activation of glycogen synthase kinase-3 β in PC12 cells. Free Radic. Res. 2008, 42, 544–553. [Google Scholar] [CrossRef]
- Bender, F.C.; Whitbeck, J.C.; Ponce de Leon, M.; Lou, H.; Eisenberg, R.J.; Cohen, G.H. Specific Association of Glycoprotein B with Lipid Rafts during Herpes Simplex Virus Entry. J. Virol. 2003, 77, 9542–9552. [Google Scholar] [CrossRef]
- Wudiri, G.A.; Nicola, A.V. Cellular Cholesterol Facilitates the Postentry Replication Cycle of Herpes Simplex Virus 1. J. Virol. 2017, 91, JVI-00445. [Google Scholar] [CrossRef] [PubMed]
- Wudiri, G.A.; Pritchard, S.M.; Li, H.; Liu, J.; Aguilar, H.C.; Gilk, S.D.; Nicola, A.V. Molecular requirement for sterols in herpes simplex virus entry and infectivity. J. Virol. 2014, 88, 13918–13922. [Google Scholar] [CrossRef] [PubMed]
- Lembo, D.; Cagno, V.; Civra, A.; Poli, G. Oxysterols: An emerging class of broad spectrum antiviral effectors. Mol. Asp. Med. 2016, 49, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.; Farasati, N.A.; Huang, Y. BK Virus replication in vitro: Limited effect of drugs interfering with viral uptake and intracellular transport. Antimicrob. Agents Chemother. 2007, 51, 4492–4494. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Yamamuro, D.; Ohshiro, T.; Honda, A.; Takahashi, M.; Kumagai, M.; Sakai, K.; Nagashima, S.; Tomoda, H.; Igarashi, M. Absence of Nceh1 augments 25-hydroxycholesterol-induced ER stress and apoptosis in macrophages. J. Lipid Res. 2014, 55, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Furman, L.M.; Maaty, W.S.; Petersen, L.K.; Ettayebi, K.; Hardy, M.E.; Bothner, B. Cysteine protease activation and apoptosis in Murine norovirus infection. Virol. J. 2009, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Post, R.M.; Kegan, R. Prevention of recurrent affective episodes using extinction training in the reconsolidation window: A testable psychotherapeutic strategy. Psychiatry Res. 2017, 249, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Scott, A.L. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J. Leukoc. Biol. 2010, 88, 1081–1087. [Google Scholar] [CrossRef]
- Karst, S. The role of type I interferon in regulating norovirus infections. J. Clin. Cell Immunol. S 2011, 1, 001. [Google Scholar] [CrossRef]
- Rocha-Pereira, J.; Jacobs, S.; Noppen, S.; Verbeken, E.; Michiels, T.; Neyts, J. Interferon lambda (IFN-λ) efficiently blocks norovirus transmission in a mouse model. Antiv. Res. 2018, 149, 7–15. [Google Scholar] [CrossRef]
- Honda, A.; Yamashita, K.; Hara, T.; Ikegami, T.; Miyazaki, T.; Shirai, M.; Xu, G.; Numazawa, M.; Matsuzaki, Y. Highly sensitive quantification of key regulatory oxysterols in biological samples by LC-ESI-MS/MS. J. Lipid Res. 2009, 50, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Schroepfer, G.J. Oxysterols: Modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000, 80, 361–554. [Google Scholar] [CrossRef] [PubMed]
- Upston, J.M.; Niu, X.; Brown, A.J.; Mashima, R.; Wang, H.; Senthilmohan, R.; Kettle, A.J.; Dean, R.T.; Stocker, R. Disease stage-dependent accumulation of lipid and protein oxidation products in human atherosclerosis. Am. J. Pathol. 2002, 160, 701–710. [Google Scholar] [CrossRef]
- Gold, E.S.; Diercks, A.H.; Podolsky, I.; Podyminogin, R.L.; Askovich, P.S.; Treuting, P.M.; Aderem, A. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 10666–10671. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shawli, G.T.; Adeyemi, O.O.; Stonehouse, N.J.; Herod, M.R. The Oxysterol 25-Hydroxycholesterol Inhibits Replication of Murine Norovirus. Viruses 2019, 11, 97. https://doi.org/10.3390/v11020097
Shawli GT, Adeyemi OO, Stonehouse NJ, Herod MR. The Oxysterol 25-Hydroxycholesterol Inhibits Replication of Murine Norovirus. Viruses. 2019; 11(2):97. https://doi.org/10.3390/v11020097
Chicago/Turabian StyleShawli, Ghada T., Oluwapelumi O. Adeyemi, Nicola J. Stonehouse, and Morgan R. Herod. 2019. "The Oxysterol 25-Hydroxycholesterol Inhibits Replication of Murine Norovirus" Viruses 11, no. 2: 97. https://doi.org/10.3390/v11020097
APA StyleShawli, G. T., Adeyemi, O. O., Stonehouse, N. J., & Herod, M. R. (2019). The Oxysterol 25-Hydroxycholesterol Inhibits Replication of Murine Norovirus. Viruses, 11(2), 97. https://doi.org/10.3390/v11020097




