Effect of Pullet Vaccination on Development and Longevity of Immunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Experimental Design
2.3. Ciliostasis Test
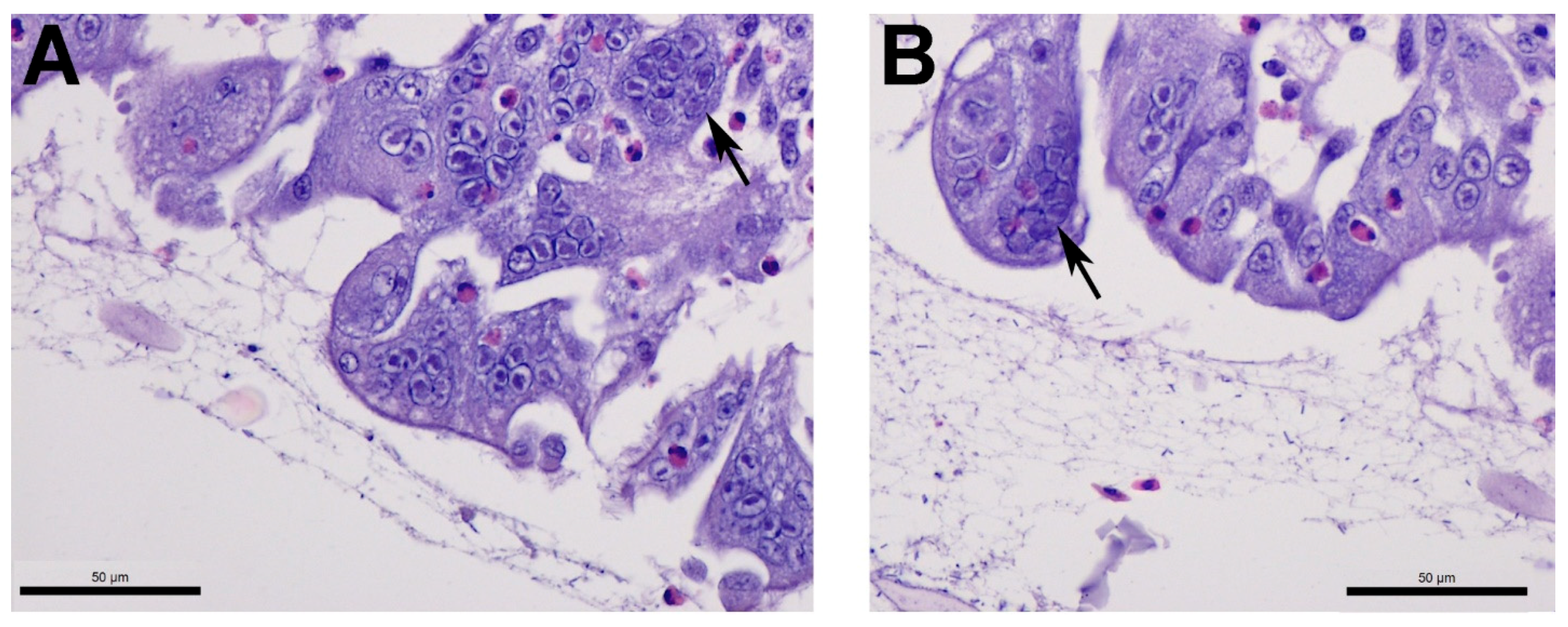
2.4. Tracheal Histopathology
2.5. RNA Extraction and Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
2.6. DNA Extraction
2.7. qPCR
2.8. Serum Infectious Bronchitis Virus (IBV)-Specific IgG Antibody Titers
2.9. Tear-Secreted IBV-Specific IgA Antibody Titers
2.10. Statistical Analysis
3. Results
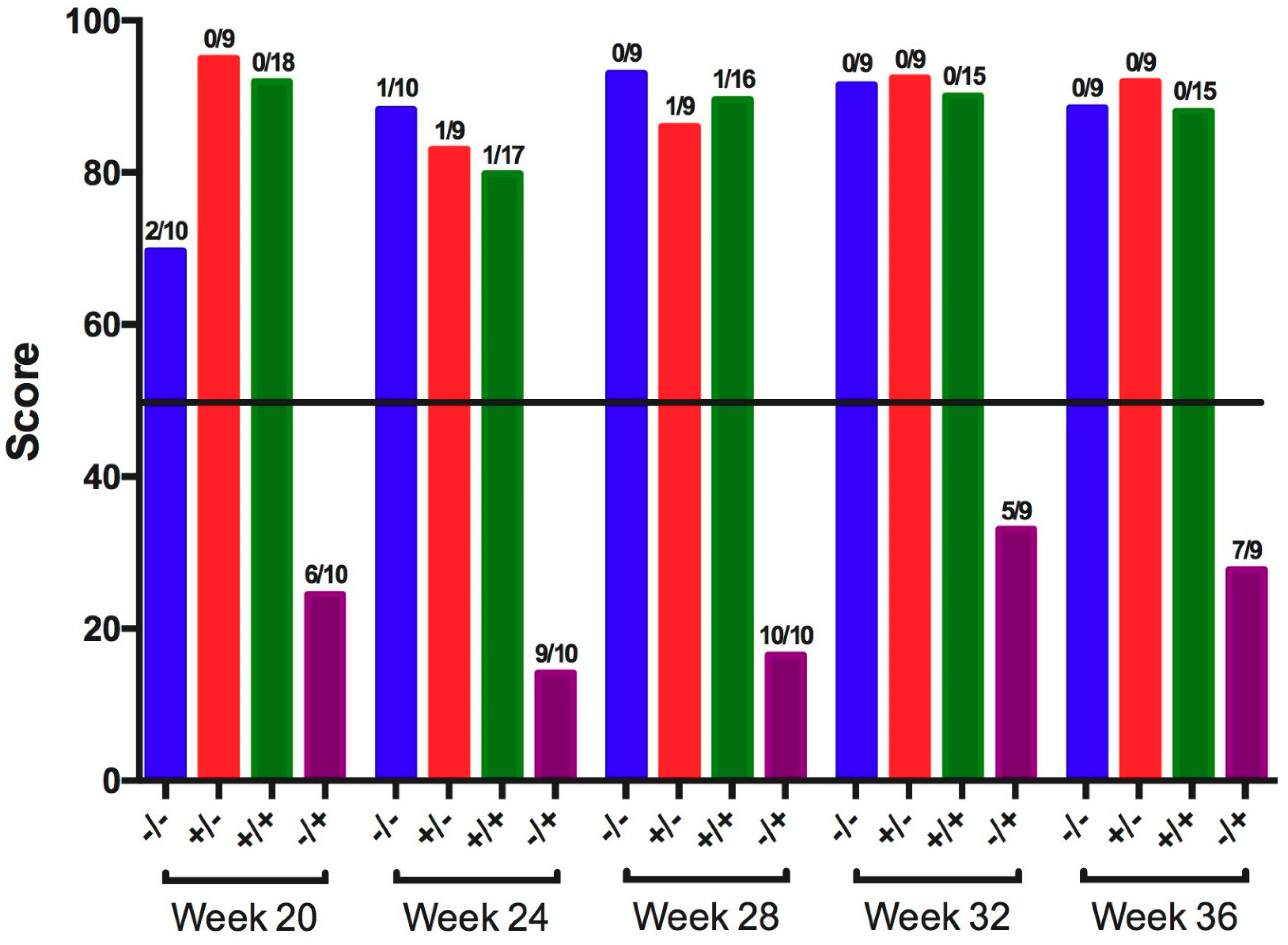
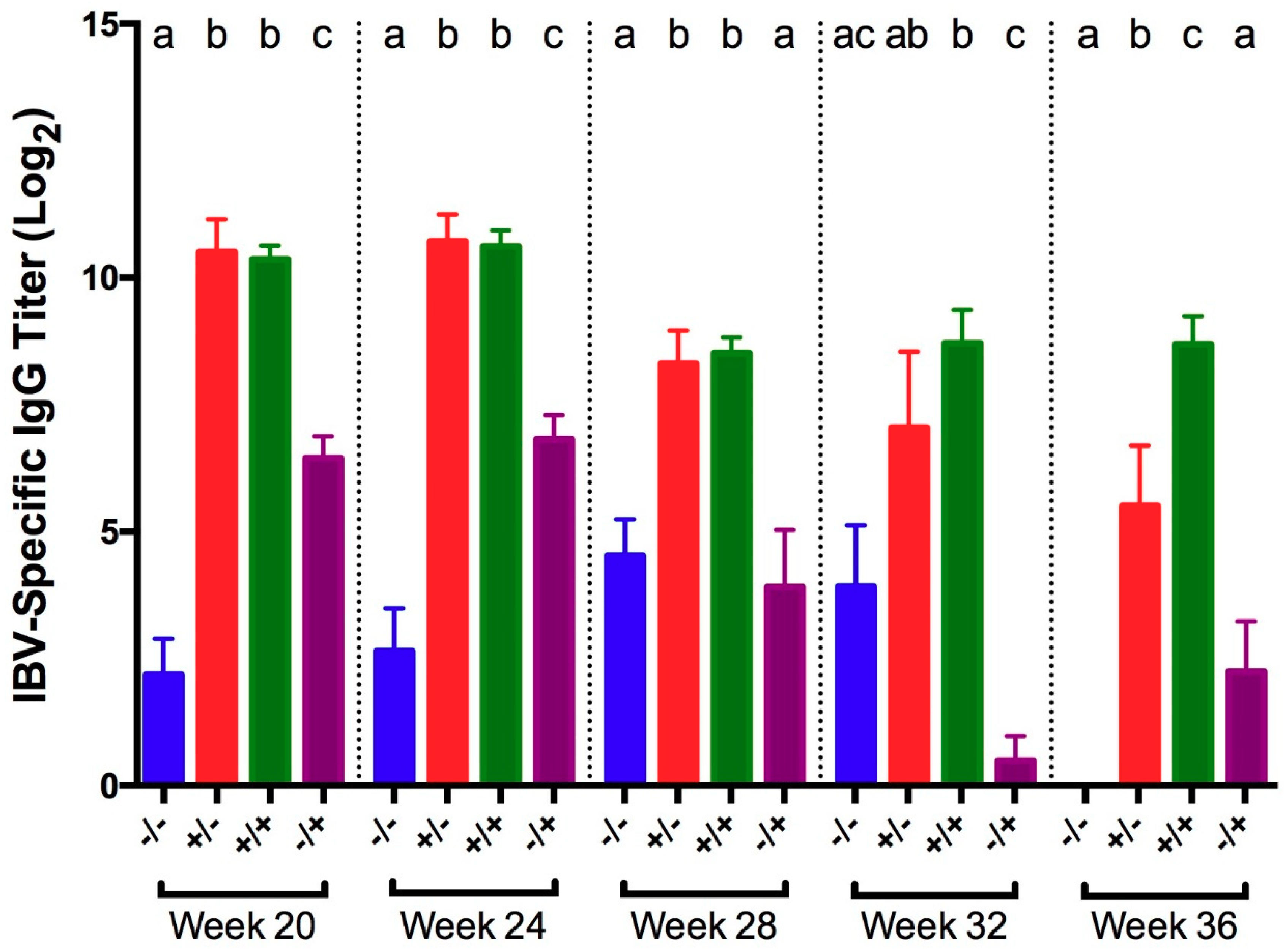
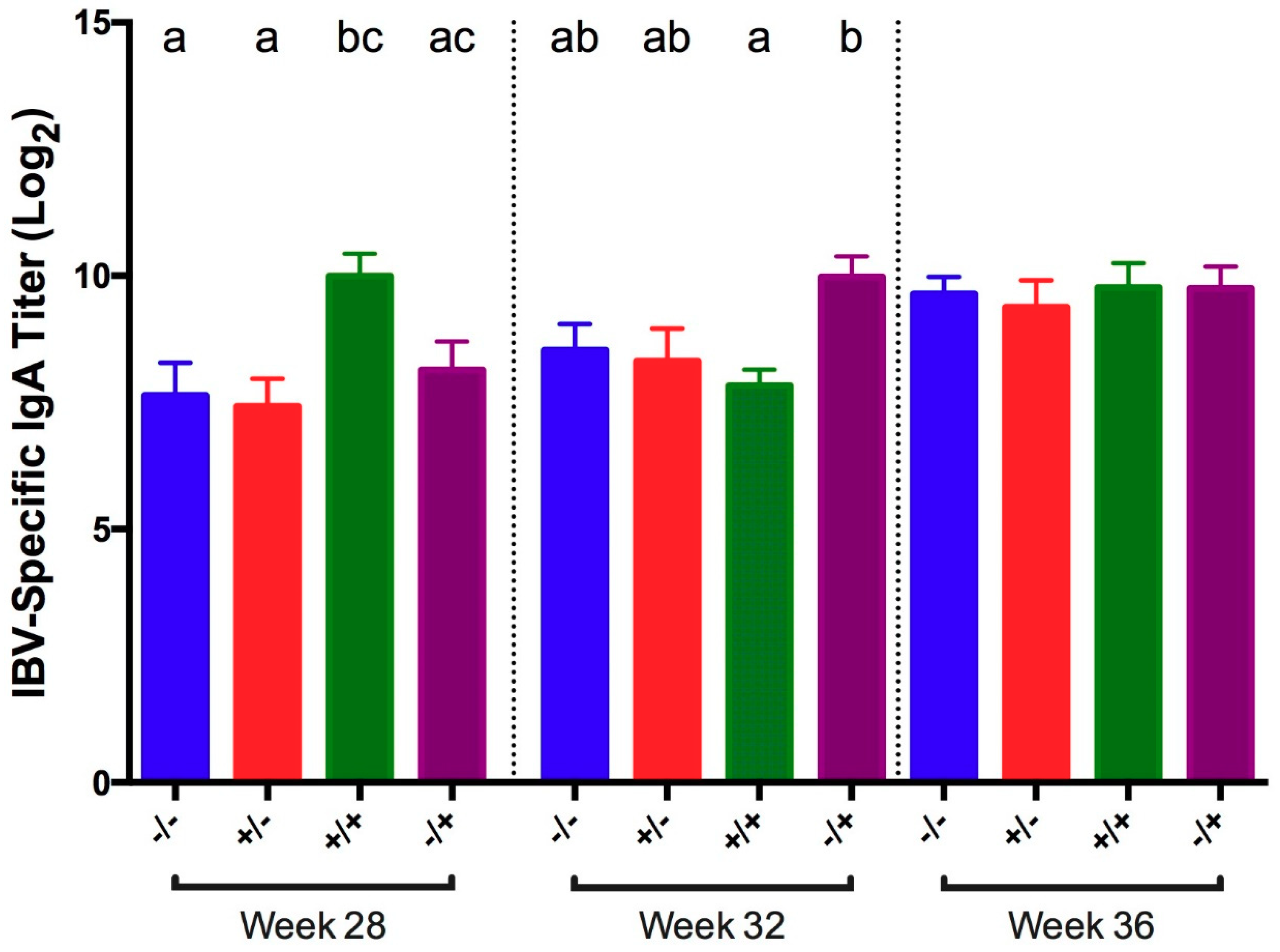
3.1. Infectious Bronchitis Virus
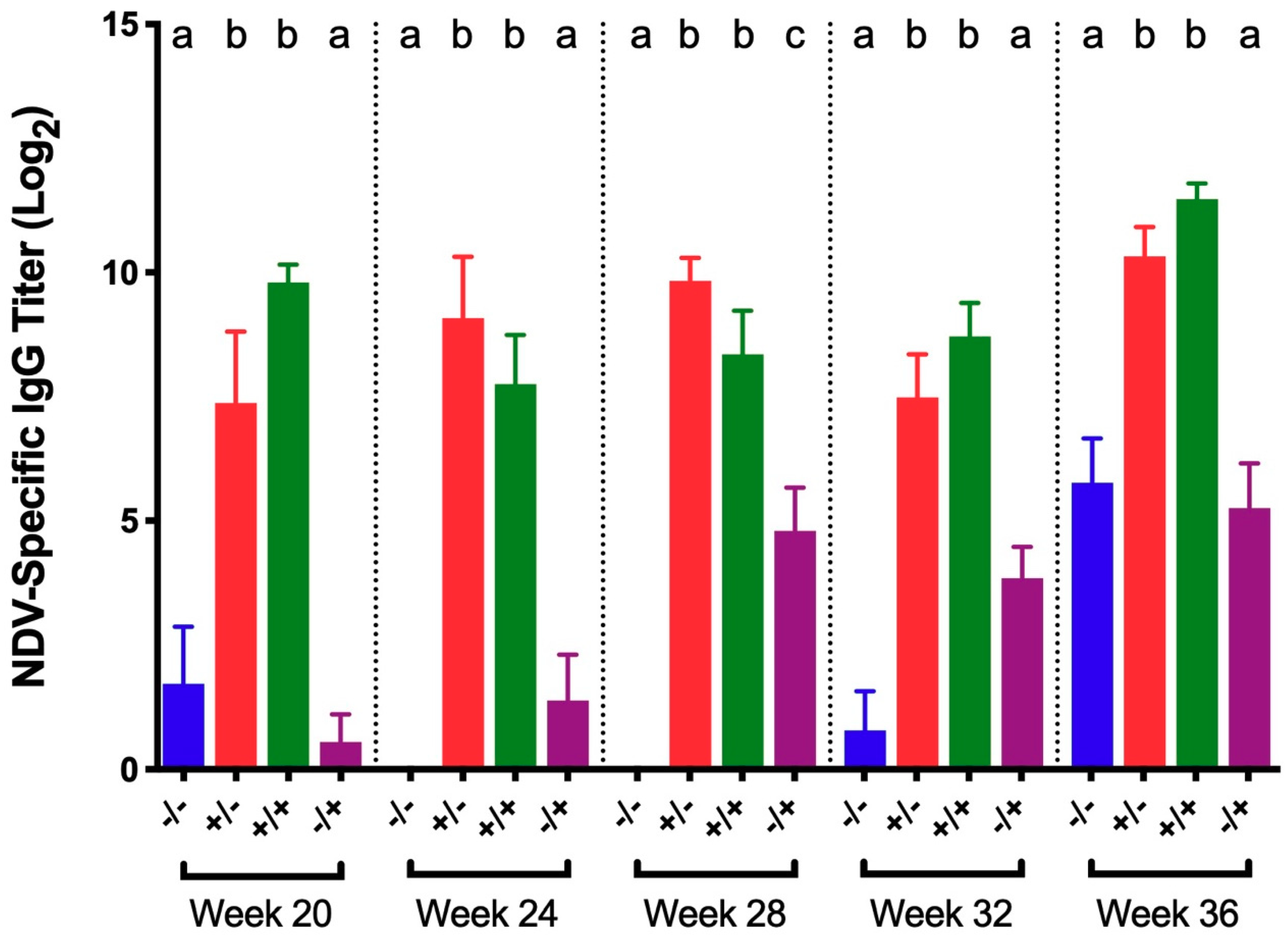
3.2. Newcastle Disease Virus (NDV)
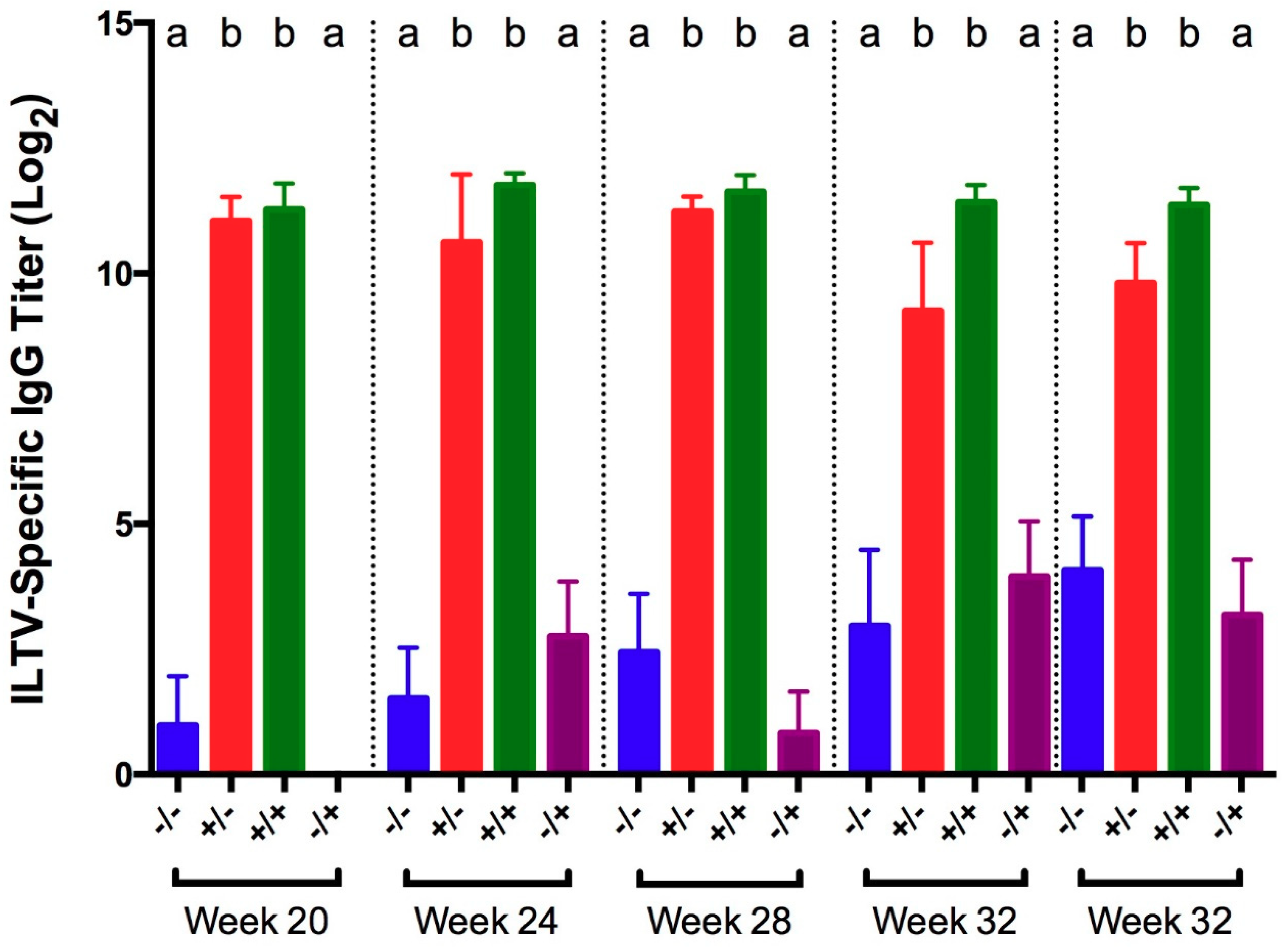
3.3. Infectious Laryngotracheitis Virus (ILTV)
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bermudez, A.J.; Stewart-Brown, B. Disease prevention and diagnosis. In Diseases of Poultry, 12th ed.; Saif, Y.M., Ed.; Blackwell Publishing: Ames, IA, USA, 2008; pp. 5–42. [Google Scholar]
- De Wit, J.J.; Cook, J.K.A.; Van der Heijden, H.M.J.F. Infectious bronchitis virus variants: A review of the history, current situation and control measures. Avian Pathol. 2011, 40, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Mayers, J.; Mansfield, K.L.; Brown, I.H. The role of vaccination in risk mitigation and control of Newcastle disease in poultry. Vaccine 2017, 35, 5974–5980. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, D.; Gelb, J., Jr. Infectious bronchitis. In Diseases of Poultry, 12th ed.; Saif, Y.M., Ed.; Blackwell Publishing: Ames, IA, USA, 2008; pp. 117–136. [Google Scholar]
- Amarasinghe, G.K.; Bào, Y.; Basler, C.F.; Bavari, S.; Beer, M.; Bejerman, N.; Blasdell, K.R.; Bochnowski, A.; Briese, T.; Bukreyev, A.; et al. Taxonomy of the order Mononegavirales: Update 2017. Arch. Virol. 2017, 162, 2493–2504. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.P.; Brandly, C.A. Identification of vaccine strains of Newcastle disease virus. Science 1955, 122, 156–157. [Google Scholar] [PubMed]
- Dimitrov, K.M.; Afonso, C.L.; Miller, P.J.; Yu, Q. Newcastle disease vaccines-A solved problem or a continuous challenge? Vet. Microbiol. 2017, 206, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M. Current and future vaccines and vaccination strategies against infectious laryngotracheitis (ILT) respiratory disease of poultry. Vet. Microbiol. 2017, 206, 157–162. [Google Scholar] [CrossRef] [PubMed]
- DaPalma, T.; Doonan, B.P.; Trager, N.M.; Kasman, L.M. Review: A systematic approach to virus–virus interactions. Virus Res. 2010, 149, 1–9. [Google Scholar] [CrossRef]
- Pinky, L.; Dobrovolny, H.M. Coinfections of the respiratory tract: Viral competition for resources. PLoS ONE 2016, 11, e0155589. [Google Scholar] [CrossRef]
- Costa-Hurtado, M.; Afonso, C.L.; Miller, P.J.; Spackman, E.; Kapczynski, D.R.; Swayne, D.E.; Shepherd, E.; Smith, D.; Zsak, A.; Pantin-Jackwood, M. Virus interference between H7N2 low pathogenic avian influenza virus and lentogenic Newcastle disease virus in experimental co-infections in chickens and turkeys. Vet. Res. 2014, 45, 1. [Google Scholar] [CrossRef]
- Villegas, P. Newcastle disease virus vaccine titration. In Avian Virus Diseases: Laboratory Manual; College of Veterinary Medicine, University of Georgia: Athens, GA, USA, 2004; p. 62. [Google Scholar]
- Vagnozzi, A.; Riblet, S.M.; Williams, S.M.; Garcia, M.; Zavala, G. Infection of broilers with two virulent strains of infectious laryngotracheitis virus: Criteria for evaluation of experimental infections. Avian Dis. 2015, 59, 394–399. [Google Scholar] [CrossRef]
- Rodríguez-Avila, A.; Oldoni, I.; Riblet, S.; Garcia, M. Evaluation of the protection elicited by direct and indirect exposure to live attenuated infectious laryngotracheitis virus vaccines against a recent challenge strain from the United States. Avian Pathol. J. W.V.P.A 2008, 37, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Toro, H.; Pennington, D.; Gallardo, R.A.; van Santen, V.L.; van Ginkel, F.W.; Zhang, J.; Joiner, K.S. Infectious bronchitis virus subpopulations in vaccinated chickens after challenge. Avian Dis. 2012, 56, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Oldoni, I.; Rodriguez-Avila, A.; Riblet, S.M.; Zavala, G.; Garcia, M. Pathogenicity and growth characteristics of selected infectious laryngotracheitis virus strains from the United States. Avian Pathol. 2009, 38, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.K.A.; Orbell, S.J.; Woods, M.A.; Huggins, M.B. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999, 28, 477–485. [Google Scholar] [CrossRef] [PubMed]
- De Wit, J.J.; Boelm, G.J.; van Gerwe, T.J.; Swart, W.A. The required sample size in vaccination-challenge experiments with infectious bronchitis virus, a meta-analysis. Avian Pathol. 2013, 42, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, M.W.; Jordan, B.J.; Roh, H.-J.; Hilt, D.A.; Williams, S.M. Evaluating protection against infectious bronchitis virus by clinical signs, ciliostasis, challenge virus detection, and histopathology. Avian Dis. 2015, 59, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.S.; Barnes, H.J.; Morgan, L.M. Virulence of infectious laryngotracheitis viruses: Comparison of modified-live vaccine viruses and North Carolina field isolates. Avian Dis. 1990, 34, 106–113. [Google Scholar] [CrossRef]
- Callison, S.A.; Hilt, D.A.; Boynton, T.O.; Sample, B.F.; Robison, R.; Swayne, D.E.; Jackwood, M.W. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J. Virol. Methods 2006, 138, 60–65. [Google Scholar] [CrossRef]
- Mia Kim, L.; Suarez, D.L.; Afonso, C.L. Detection of a broad range of class I and II Newcastle disease viruses using a multiplex real-time reverse transcription polymerase chain reaction assay. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc. 2008, 20, 414–425. [Google Scholar] [CrossRef]
- Roh, H.J.; Hilt, D.A.; Jackwood, M.W. Detection of infectious bronchitis virus with the use of real-time quantitative reverse transcriptase-PCR and correlation with virus detection in embryonated eggs. Avian Dis. 2014, 58, 398–403. [Google Scholar] [CrossRef]
- Vagnozzi, A.; García, M.; Riblet, S.M.; Zavala, G. Protection induced by infectious laryngotracheitis virus vaccines alone and combined with Newcastle disease virus and/or infectious bronchitis virus vaccines. Avian Dis. 2010, 54, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Vagnozzi, A.; Riblet, S.M.; Zavala, G.; García, M. Optimization of a duplex real-time PCR method for relative quantitation of infectious laryngotracheitis virus. Avian Dis. 2012, 56, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Callison, S.A.; Riblet, S.M.; Oldoni, I.; Sun, S.; Zavala, G.; Williams, S.; Resurreccion, R.S.; Spackman, E.; Garcia, M. Development and validation of a real-time Taqman PCR assay for the detection and quantitation of infectious laryngotracheitis virus in poultry. J. Virol. Methods 2007, 139, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.-J.; Hilt, D.A.; Williams, S.M.; Jackwood, M.W. Evaluation of infectious bronchitis virus Arkansas-type vaccine failure in commercial broilers. Avian Dis. 2013, 57, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Fentie, T.; Dadi, K.; Kassa, T.; Sahle, M.; Cattoli, G. Effect of vaccination on transmission characteristics of highly virulent Newcastle disease virus in experimentally infected chickens. Avian Pathol. J. W.V.P.A 2014, 43, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Williams, R.A.; Gaskell, R.M.; Jordan, F.T.; Bradbury, J.M.; Bennett, M.; Jones, R.C. Latency and reactivation of infectious laryngotracheitis vaccine virus. Arch. Virol. 1991, 121, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Costa-Hurtado, M.; Afonso, C.L.; Miller, P.J.; Shepherd, E.; DeJesus, E.; Smith, D.; Pantin-Jackwood, M.J. Effect of infection with a mesogenic strain of Newcastle disease virus on infection with highly pathogenic avian influenza virus in chickens. Avian Dis. 2016, 60 (Suppl. 1), 269–278. [Google Scholar] [CrossRef]
- Alexander, D.J.; Gough, R.E. Isolation of avian infectious bronchitis virus from experimentally infected chickens. Res. Vet. Sci. 1977, 23, 344–347. [Google Scholar] [CrossRef]
- Chousalkar, K.K.; Roberts, J.R.; Reece, R. Histopathology of two serotypes of infectious bronchitis virus in laying Hens vaccinated in the rearing phase. Poult. Sci. 2007, 86, 59–62. [Google Scholar] [CrossRef]
- Chousalkar, K.K.; Roberts, J.R.; Reece, R. Comparative histopathology of two serotypes of infectious bronchitis virus (T and N1/88) in laying hens and cockerels. Poult. Sci. 2007, 86, 50–58. [Google Scholar] [CrossRef]
- Pharmeuropa. Available online: http://online6.edqm.eu (accessed on 1 May 2018).
- Raj, G.D.; Jones, R.C. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1997, 26, 677–706. [Google Scholar] [CrossRef] [PubMed]
- Raggi, L.G.; Lee, G.G. Lack of correlation between infectivity, serologic response and challenge results in immunization with an avian infectious bronchitis virus vaccine. J. Immunol. 1965, 94, 538–543. [Google Scholar] [PubMed]
- Orr-Burks, N.; Gulley, S.L.; Gallardo, R.A.; Toro, H.; van Ginkel, F.W. Immunoglobulin A as an early humoral responder after mucosal avian coronavirus vaccination. Avian Dis. 2014, 58, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Aston, E.J.; Jackwood, M.W.; Gogal, R.M., Jr.; Hurley, D.J.; Fairchild, B.D.; Hilt, D.A.; Cheng, S.; Tensa, L.R.; Garcia, M.; Jordan, B.J. Ambient ammonia does not inhibit the immune response to infectious bronchitis virus vaccination and protection from homologous challenge in broiler chickens. Vet. Immunol. Immunopathol. 2018. Under Review. [Google Scholar]
- Alexander, D.J.; Senne, D.A. Newcastle disease, other avian paramyxoviruses, and pneumovirus infections. In Diseases of Poultry; Saif, Y.M., Ed.; Blackwell Publishing: Ames, IA, USA, 2008; pp. 75–115. [Google Scholar]
- Butler, M.; Ellaway, W.J.; Hall, T. Comparative studies on the infectivity of avian respiratory viruses for eggs, cell cultures and tracheal explants. J. Comp. Pathol. 1972, 82, 327–332. [Google Scholar] [CrossRef]
- Malo, A.; de Wit, S.; Swart, W.A.J.M. Alternative methods to compare safety of live-attenuated respiratory Newcastle disease vaccines in young chicks. Vet. Rec. 2017, 181, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.C.S.; Chou, W.K.; Berghman, L.R.; Carey, J.B. Evaluation of the effect of live LaSota Newcastle disease virus vaccine as primary immunization on immune development in broilers. Poult. Sci. 2018, 97, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.S.; Barnes, H.J.; Smith, L.G. Rapid diagnosis of infectious laryngotracheitis using a monoclonal antibody-based immunoperoxidase procedure. Avian Pathol. 1992, 21, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.S.; Garcia, M. Laryngotracheitis. In Diseases of Poultry; Saif, Y.M., Ed.; Blackwell Publishing: Ames, IA, USA, 2008; pp. 137–152. [Google Scholar]
- Gerganov, G.; Surtmadzhiev, K. Cultivation of various avian viruses in pheasant trachea organ cultures and chick embryos. Vet. Meditsinski Nauk. 1982, 19, 18–24. [Google Scholar]
- Hitchner, S.B.; Shea, C.A.; White, P.G. Studies on a serum neutralization test for the diagnosis of laryngotracheitis in chickens. Avian Dis. 1958, 2, 258–269. [Google Scholar] [CrossRef]
| Week | Treatment | Mean ± SEM | ||||
|---|---|---|---|---|---|---|
| Vaccine | Challenge | Choanal Cleft | Harderian Gland | Conjunctiva | Cecal Tonsil | |
| 20 | − | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 36.5 ± 0.9 b | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 24.9 ± 1.9 b | |
| + | + | 27.9 ± 0.8 c | 33.2 ± 0.6 b | 31.9 ± 0.7 b | 30.2 ± 1.2 c | |
| − | + | 17.5 ± 0.3 d | 26.1 ± 1.3 c | 22.7 ± 0.6 c | 21.6 ± 1.1 b | |
| 24 | − | − | 40.0 ± 0.0 ab | 34.4 ± 1.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 41.1 ± 0.5 a | 39.0 ± 0.7 b | 40.0 ± 0.0 a | 30.7 ± 3.3 b | |
| + | + | 39.6 ± 0.4 b | 33.8 ± 0.5 a | 31.0 ± 0.7 b | 30.5 ± 1.7 b | |
| − | + | 34.3 ± 0.5 c | 21.0 ± 1.5 c | 24.1 ± 0.8 c | 26.1 ± 2.0 b | |
| 28 | − | − | 40.0 ± 0.0 a | 39.2 ± 0.6 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 39.6 ± 0.4 a | |
| + | + | 30.4 ± 1.2 b | 30.9 ± 1.7 b | 31.5 ± 1.0 b | 33.7 ± 1.6 b | |
| − | + | 22.3 ± 0.3 c | 20.4 ± 1.3 c | 22.8 ± 0.5 c | 24.3 ± 1.8 c | |
| 32 | − | − | 40.0 ± 0.0 a | 38.9 ± 0.8 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 40.0 ± 0.0 a | 38.6 ± 0.7 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | |
| + | + | 32.3 ± 0.5 b | 34.1 ± 0.4 b | 34.5 ± 1.6 b | 34.5 ± 2.0 a | |
| − | + | 20.6 ± 0.5 c | 21.8 ± 1.3 c | 24.2 ± 0.7 c | 23.4 ± 1.3 b | |
| 36 | − | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | |
| + | + | 30.0 ± 0.8 b | 30.3 ± 0.9 b | 28.3 ± 1.4 b | 32.5 ± 1.0 b | |
| − | + | 22.2 ± 0.4 c | 21.2 ± 1.4 c | 25.0 ± 0.5 c | 23.1 ± 1.2 c | |
| Week | Treatment | Clinical Signs | Histopathology Lesions | ||
|---|---|---|---|---|---|
| Vaccine | Challenge | Mean ± SEM | Deciliation | Necrosis | |
| 20 | − | − | 0.0 ± 0.0 a | 2/10 a | 0/10 a |
| + | − | 0.0 ± 0.0 a | 1/9 a | 1/9 a | |
| + | + | 0.0 ± 0.0 a | 0/18 a | 0/18 a | |
| − | + | 0.7 ± 0.2 b | 8/10 b | 8/10 b | |
| 24 | − | − | 0.0 ± 0.0 a | 0/10 a | 0/10 a |
| + | − | 0.0 ± 0.0 a | 0/9 a | 1/9 a | |
| + | + | 0.1 ± 0.1 a | 0/16 a | 0/16 a | |
| − | + | 1.0 ± 0.3 b | 6/8 b | 5/8 b | |
| 28 | − | − | 0.0 ± 0.0 | 0/9 a | 0/9 a |
| + | − | 0.0 ± 0.0 | 0/9 a | 0/9 a | |
| + | + | 0.1 ± 0.1 | 1/17 a | 2/17 a | |
| − | + | 0.5 ± 0.3 | 6/10 b | 6/10 b | |
| 32 | − | − | 0.0 ± 0.0 a | 0/0 a | 0/0 a |
| + | − | 0.0 ± 0.0 a | 0/9 a | 0/9 a | |
| + | + | 0.1 ± 0.1 a | 0/16 a | 0/16 a | |
| − | + | 1.2 ± 0.4 b | 7/9 b | 5/9 b | |
| 36 | − | − | 0.0 ± 0.0 a | 0/9 a | 0/9 a |
| + | − | 0.0 ± 0.0 a | 0/9 a | 0/9 a | |
| + | + | 0.1 ± 0.1 a | 0/16 a | 0/16 a | |
| − | + | 0.4 ± 0.3 b | 6/9 b | 7/9 b | |
| Week | Treatment | Mean ± SEM | |||
|---|---|---|---|---|---|
| Vaccine | Challenge | Choanal Cleft | Harderian Gland | Conjunctiva | |
| 20 | − | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 39.7 ± 0.4 a |
| + | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | |
| + | + | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 38.2 ± 0.6 a | |
| − | + | 29.3 ± 1.1 b | 28.6 ± 0.9 b | 27.6 ± 1.9 b | |
| 24 | − | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | |
| + | + | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 39.5 ± 0.5 a | |
| − | + | 26.6 ± 1.0 b | 34.2 ± 1.4 b | 27.8 ± 1.0 b | |
| 28 | − | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | |
| + | + | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 37.0 ± 0.7 b | |
| − | + | 24.5 ± 0.7 b | 33.0 ± 1.3 b | 27.9 ± 0.8 c | |
| 32 | − | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | |
| + | + | 39.2 ± 0.6 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | |
| − | + | 27.7 ± 2.2 b | 31.5 ± 1.6 b | 28.6 ± 1.2 b | |
| 36 | − | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | |
| + | + | 38.0 ± 1.1 a | 39.1 ± 0.7 a | 39.6 ± 0.4 a | |
| − | + | 24.9 ± 1.0 b | 33.7 ± 1.8 b | 30.3 ± 0.5 b | |
| Week | Treatment | Clinical Signs | |
|---|---|---|---|
| Vaccine | Challenge | Mean ± SEM | |
| 20 | − | − | 0.0 ± 0.0 a |
| + | − | 0.0 ± 0.0 a | |
| + | + | 0.4 ± 0.2 a | |
| − | + | 1.6 ± 0.2 b | |
| 24 | − | − | 0.0 ± 0.0 |
| + | − | 0.0 ± 0.0 | |
| + | + | 0.0 ± 0.0 | |
| − | + | 0.2 ± 0.1 | |
| 28 | − | − | 0.0 ± 0.0 |
| + | − | 0.0 ± 0.0 | |
| + | + | 0.0 ± 0.0 | |
| − | + | 0.1 ± 0.1 | |
| 32 | − | − | 0.0 ± 0.0 |
| + | − | 0.0 ± 0.0 | |
| + | + | 0.0 ± 0.0 | |
| − | + | 0.0 ± 0.0 | |
| 36 | − | − | 0.0 ± 0.0 |
| + | − | 0.0 ± 0.0 | |
| + | + | 0.0 ± 0.0 | |
| − | + | 0.0 ± 0.0 | |
| Week | Treatment | Mean ± SEM | |||
|---|---|---|---|---|---|
| Vaccine | Challenge | Trachea | Harderian Gland | Conjunctiva | |
| 20 | − | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a | |
| + | + | 40.0 ± 0.0 a | 39.7 ± 0.3 a | 35.4 ± 1.2 b | |
| − | + | 24.2 ± 0.4 b | 22.7 ± 0.4 b | 18.2 ± 1.4 c | |
| 24 | − | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 38.7 ± 0.9 a | |
| + | + | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 34.5 ± 0.9 b | |
| − | + | 25.2 ± 1.1 b | 26.0 ± 1.4 b | 17.3 ± 0.8 c | |
| 28 | − | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 36.8 ± 1.3 b | |
| + | + | 39.5 ± 0.3 a | 40.0 ± 0.0 a | 38.5 ± 0.6 ab | |
| − | + | 23.9 ± 0.9 b | 22.5 ± 1.4 b | 17.0 ± 0.5 c | |
| 32 | − | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 40.0 ± 0.0 a | 39.7 ± 0.4 a | 40.0 ± 0.0 a | |
| + | + | 39.7 ± 0.3 a | 40.0 ± 0.0 a | 27.2 ± 0.6 b | |
| − | + | 24.7 ± 1.0 b | 24.2 ± 1.1 b | 17.7 ± 0.6 c | |
| 36 | − | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 40.0 ± 0.0 a |
| + | − | 40.0 ± 0.0 a | 40.0 ± 0.0 a | 39.5 ± 0.5 a | |
| + | + | 39.8 ± 0.2 a | 38.3 ± 0.8 a | 32.0 ± 0.5 b | |
| − | + | 26.4 ± 1.7 b | 34.9 ± 1.6 b | 18.8 ± 0.9 c | |
| Week | Treatment | Clinical Signs | |
|---|---|---|---|
| Vaccine | Challenge | Mean ± SEM | |
| 20 | − | − | 0.0 ± 0.0 a |
| + | − | 0.0 ± 0.0 a | |
| + | + | 0.6 ± 0.2 a | |
| − | + | 4.7 ± 0.7 b | |
| 24 | − | − | 0.0 ± 0.0 a |
| + | − | 0.0 ± 0.0 a | |
| + | + | 0.0 ± 0.0 a | |
| − | + | 2.5 ± 0.7 b | |
| 28 | − | − | 0.0 ± 0.0 a |
| + | − | 0.0 ± 0.0 a | |
| + | + | 0.1 ± 0.1 a | |
| − | + | 4.9 ± 0.7 b | |
| 32 | − | − | 0.0 ± 0.0 a |
| + | − | 0.0 ± 0.0 a | |
| + | + | 0.4 ± 0.1 a | |
| − | + | 4.0 ± 0.7 b | |
| 36 | − | − | 0.0 ± 0.0 a |
| + | − | 0.0 ± 0.0 a | |
| + | + | 1.1 ± 0.2 b | |
| − | + | 2.9 ± 0.4 b | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aston, E.J.; Jordan, B.J.; Williams, S.M.; García, M.; Jackwood, M.W. Effect of Pullet Vaccination on Development and Longevity of Immunity. Viruses 2019, 11, 135. https://doi.org/10.3390/v11020135
Aston EJ, Jordan BJ, Williams SM, García M, Jackwood MW. Effect of Pullet Vaccination on Development and Longevity of Immunity. Viruses. 2019; 11(2):135. https://doi.org/10.3390/v11020135
Chicago/Turabian StyleAston, Emily J., Brian J. Jordan, Susan M. Williams, Maricarmen García, and Mark W. Jackwood. 2019. "Effect of Pullet Vaccination on Development and Longevity of Immunity" Viruses 11, no. 2: 135. https://doi.org/10.3390/v11020135
APA StyleAston, E. J., Jordan, B. J., Williams, S. M., García, M., & Jackwood, M. W. (2019). Effect of Pullet Vaccination on Development and Longevity of Immunity. Viruses, 11(2), 135. https://doi.org/10.3390/v11020135






