The Role of Marek’s Disease Virus UL12 and UL29 in DNA Recombination and the Virus Lifecycle
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Generation of Recombinant Viruses
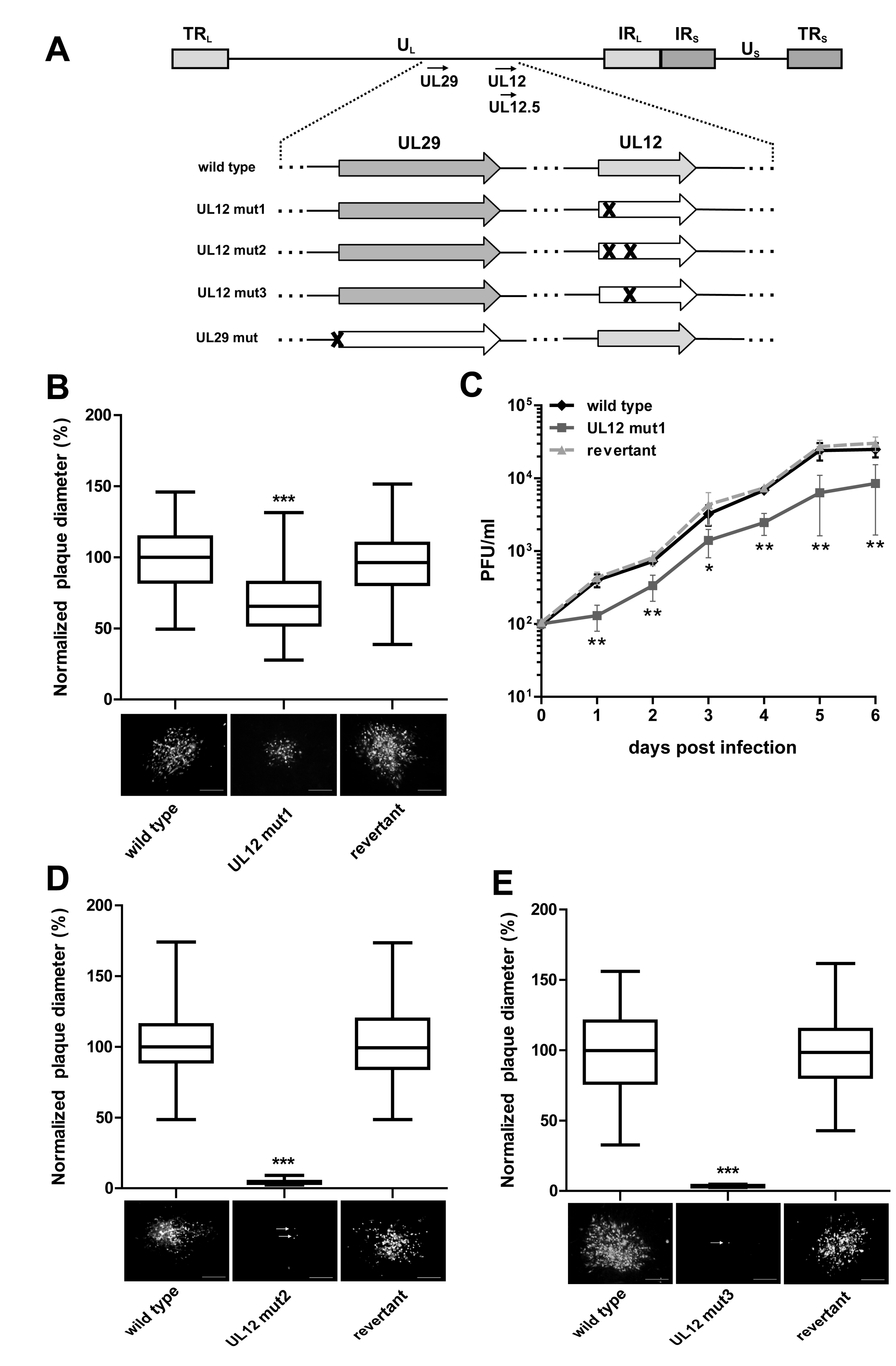
2.3. Plaque Size Assays and Growth Kinetics
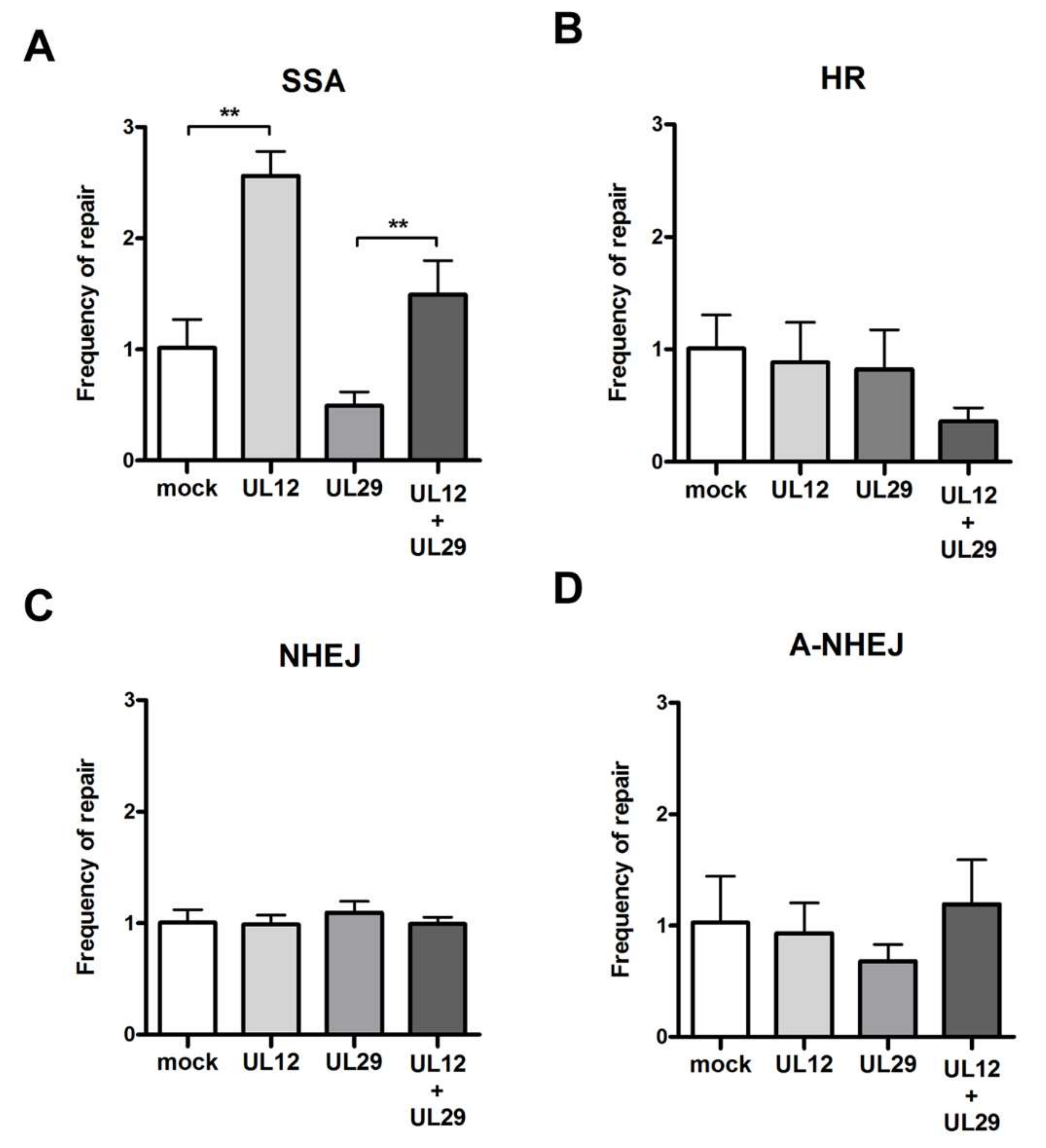
2.4. DNA Repair Assays
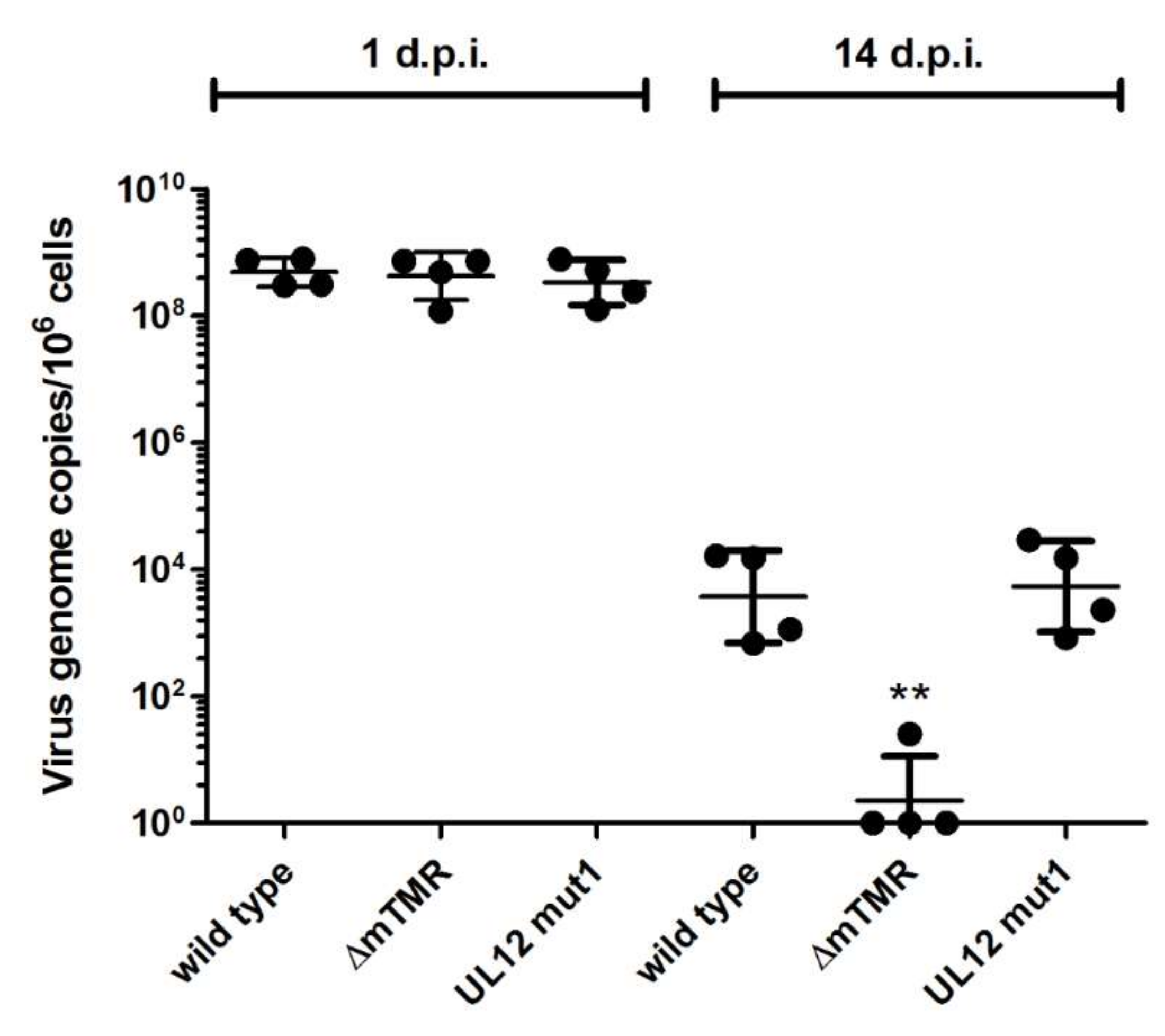
2.5. In Vitro Latency Assay
2.6. shRNA Knockdown of UL12 and UL29
2.7. Statistical Analyses
3. Results
3.1. Role of UL12 and UL29 in MDV Lytic Replication
3.2. Role of UL12 and UL29 in Double Strand Break Repair
3.3. Role of UL12 and UL29 MDV Integration and the Maintenance of the Latent Virus Genome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davison, F.; Nair, V. Marek’s Disease: An Evolving Problem; Elsevier: London, UK, 2004. [Google Scholar]
- Osterrieder, N.; Kamil, J.P.; Schumacher, D.; Tischer, B.K.; Trapp, S. Marek’s disease virus: From miasma to model. Nat. Rev. Microbiol. 2006, 4, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Parcells, M.S.; Burnside, J.; Morgan, R.W. Marek’s Disease Virus-Induced T-Cell Lymphomas. In Current Cancer Research; Springer: New York, USA, 2012; pp. 307–335. [Google Scholar]
- Jarosinski, K.W.; Tischer, B.K.; Trapp, S.; Osterrieder, N. Marek’s disease virus: Lytic replication, oncogenesis and control. Expert Rev. Vaccines 2006, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Bertzbach, L.D.; Laparidou, M.; Hartle, S.; Etches, R.J.; Kaspers, B.; Schusser, B.; Kaufer, B.B. Unraveling the role of B cells in the pathogenesis of an oncogenic avian herpesvirus. Proc. Natl. Acad. Sci. USA 2018, 115, 11603–11607. [Google Scholar] [CrossRef] [PubMed]
- Kaufer, B.B.; Jarosinski, K.W.; Osterrieder, N. Herpesvirus telomeric repeats facilitate genomic integration into host telomeres and mobilization of viral DNA during reactivation. J. Exp. Med. 2011, 208, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Fester, N.; Engel, A.T.; Kaufer, B.B. Role of the short telomeric repeat region in Marek’s disease virus replication, genomic integration, and lymphomagenesis. J. Virol. 2014, 88, 14138–14147. [Google Scholar] [CrossRef] [PubMed]
- Kheimar, A.; Previdelli, R.L.; Wight, D.J.; Kaufer, B.B. Telomeres and Telomerase: Role in Marek’s Disease Virus Pathogenesis, Integration and Tumorigenesis. Viruses 2017, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.M.; Cheng, H.H.; Delany, M.E. Temporal Kinetics of Marek’s Disease Herpesvirus: Integration Occurs Early after Infection in Both B and T Cells. Cytogenet. Genome Res. 2014, 144, 142–154. [Google Scholar] [CrossRef]
- Kaufer, B.B. Detection of Integrated Herpesvirus Genomes by Fluorescence In Situ Hybridization (FISH). Methods Mol. Biol. 2013, 1064, 141–152. [Google Scholar]
- Wallaschek, N.; Gravel, A.; Flamand, L.; Kaufer, B.B. The putative U94 integrase is dispensable for human herpesvirus 6 (HHV-6) chromosomal integration. J. Gen. Virol. 2016, 97, 1899–1903. [Google Scholar] [CrossRef]
- Arbuckle, J.H.; Medveczky, M.M.; Luka, J.; Hadley, S.H.; Luegmayr, A.; Ablashi, D.; Lund, T.C.; Tolar, J.; De Meirleir, K.; Montoya, J.G.; et al. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2010, 107, 5563–5568. [Google Scholar] [CrossRef]
- Osterrieder, N.; Wallaschek, N.; Kaufer, B.B. Herpesvirus Genome Integration into Telomeric Repeats of Host Cell Chromosomes. Annu. Rev. Virol. 2014, 1, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Wallaschek, N.; Sanyal, A.; Pirzer, F.; Gravel, A.; Mori, Y.; Flamand, L.; Kaufer, B.B. The Telomeric Repeats of Human Herpesvirus 6A (HHV-6A) Are Required for Efficient Virus Integration. PLoS Pathog. 2016, 12, e1005666. [Google Scholar] [CrossRef] [PubMed]
- Reuven, N.B.; Staire, A.E.; Myers, R.S.; Weller, S.K. The herpes simplex virus type 1 alkaline nuclease and single-stranded DNA binding protein mediate strand exchange in vitro. J. Virol. 2003, 77, 7425–7433. [Google Scholar] [CrossRef]
- Reuven, N.B.; Antoku, S.; Weller, S.K. The UL12.5 gene product of herpes simplex virus type 1 exhibits nuclease and strand exchange activities but does not localize to the nucleus. J. Virol. 2004, 78, 4599–4608. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.J.; Mohni, K.N.; Kan, Y.; Hendrickson, E.A.; Stark, J.M.; Weller, S.K. The HSV-1 exonuclease, UL12, stimulates recombination by a single strand annealing mechanism. PLoS Pathog. 2012, 8, e1002862. [Google Scholar] [CrossRef] [PubMed]
- Knipe, D.M.; Griffin, D.E.; Lamb, R.A.; Martin, M.A.; Roizman, B.; Straus, S.E. Fields Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Osterrieder, N. Sequence and initial characterization of the U(L)10 (glycoprotein M) and U(L)11 homologous genes of serotype 1 Marek’s Disease Virus. Arch. Virol. 1999, 144, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.T.; Selvaraj, R.K.; Kamil, J.P.; Osterrieder, N.; Kaufer, B.B. Marek’s disease viral interleukin-8 promotes lymphoma formation through targeted recruitment of B cells and CD4+ CD25+ T cells. J. Virol. 2012, 86, 8536–8545. [Google Scholar] [CrossRef] [PubMed]
- Arumugaswami, V.; Kumar, P.M.; Konjufca, V.; Dienglewicz, R.L.; Reddy, S.M.; Parcells, M.S. Latency of Marek’s disease virus (MDV) in a reticuloendotheliosis virus-transformed T-cell line. I: Uptake and structure of the latent MDV genome. Avian Dis. 2009, 53, 149–155. [Google Scholar] [CrossRef]
- Arumugaswami, V.; Kumar, P.M.; Konjufca, V.; Dienglewicz, R.L.; Reddy, S.M.; Parcells, M.S. Latency of Marek’s disease virus (MDV) in a reticuloendotheliosis virus-transformed T-cell line. II: Expression of the latent MDV genome. Avian Dis. 2009, 53, 156–165. [Google Scholar] [CrossRef]
- Jordan, I.; Vos, A.; Beilfuss, S.; Neubert, A.; Breul, S.; Sandig, V. An avian cell line designed for production of highly attenuated viruses. Vaccine 2009, 27, 748–756. [Google Scholar] [CrossRef]
- Corcoran, J.A.; Saffran, H.A.; Duguay, B.A.; Smiley, J.R. Herpes Simplex Virus UL12.5 Targets Mitochondria through a Mitochondrial Localization Sequence Proximal to the N Terminus. J. Virol. 2009, 83, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.; Tischer, B.K.; Trapp, S.; Osterrieder, N. The protein encoded by the US3 orthologue of Marek’s disease virus is required for efficient de-envelopment of perinuclear virions and involved in actin stress fiber breakdown. J. Virol. 2005, 79, 3987–3997. [Google Scholar] [CrossRef] [PubMed]
- Veiga, I.B.; Jarosinski, K.W.; Kaufer, B.B.; Osterrieder, N. Marek’s disease virus (MDV) ubiquitin-specific protease (USP) performs critical functions beyond its enzymatic activity during virus replication. Virology 2013, 437, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, N.; Cheng, A.; Huang, N.; Stark, J.M. Alternative-NHEJ Is a Mechanistically Distinct Pathway of Mammalian Chromosome Break Repair. PLoS Genet. 2008, 4, e1000110. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.M.; Pierce, A.J.; Oh, J.; Pastink, A.; Jasin, M. Genetic steps of mammalian homologous repair with distinct mutagenic consequences. Mol. Cell. Biol. 2004, 24, 9305–9316. [Google Scholar] [CrossRef] [PubMed]
- Wight, D.; Wallaschek, N.; Sanyal, A.; Weller, S.; Flamand, L.; Kaufer, B. Viral Proteins U41 and U70 of Human Herpesvirus 6A Are Dispensable for Telomere Integration. Viruses 2018, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.; Goldstein, J.N.; Weller, S.K. The product of the UL12.5 gene of herpes simplex virus type 1 is not essential for lytic viral growth and is not specifically associated with capsids. Virology 2002, 298, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Porter, I.M.; Stow, N.D. Virus particles produced by the herpes simplex virus type 1 alkaline nuclease null mutant ambUL12 contain abnormal genomes. J. Gen. Virol. 2004, 85 Pt 3, 583–591. [Google Scholar] [CrossRef]
- Weller, S.K.; Seghatoleslami, M.R.; Shao, L.; Rowse, D.; Carmichael, E.P. The herpes simplex virus type 1 alkaline nuclease is not essential for viral DNA synthesis: Isolation and characterization of a lacZ insertion mutant. J. Gen. Virol. 1990, 71 Pt 12, 2941–2952. [Google Scholar] [CrossRef]
- Martinez, R.; Shao, L.; Bronstein, J.C.; Weber, P.C.; Weller, S.K. The product of a 1.9-kb mRNA which overlaps the HSV-1 alkaline nuclease gene (UL12) cannot relieve the growth defects of a null mutant. Virology 1996, 215, 152–164. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Previdelli, R.L.; Bertzbach, L.D.; Wight, D.J.; Vychodil, T.; You, Y.; Arndt, S.; Kaufer, B.B. The Role of Marek’s Disease Virus UL12 and UL29 in DNA Recombination and the Virus Lifecycle. Viruses 2019, 11, 111. https://doi.org/10.3390/v11020111
Previdelli RL, Bertzbach LD, Wight DJ, Vychodil T, You Y, Arndt S, Kaufer BB. The Role of Marek’s Disease Virus UL12 and UL29 in DNA Recombination and the Virus Lifecycle. Viruses. 2019; 11(2):111. https://doi.org/10.3390/v11020111
Chicago/Turabian StylePrevidelli, Renato L., Luca D. Bertzbach, Darren J. Wight, Tereza Vychodil, Yu You, Sina Arndt, and Benedikt B. Kaufer. 2019. "The Role of Marek’s Disease Virus UL12 and UL29 in DNA Recombination and the Virus Lifecycle" Viruses 11, no. 2: 111. https://doi.org/10.3390/v11020111
APA StylePrevidelli, R. L., Bertzbach, L. D., Wight, D. J., Vychodil, T., You, Y., Arndt, S., & Kaufer, B. B. (2019). The Role of Marek’s Disease Virus UL12 and UL29 in DNA Recombination and the Virus Lifecycle. Viruses, 11(2), 111. https://doi.org/10.3390/v11020111




