Emerging Connections of S1P-Metabolizing Enzymes with Host Defense and Immunity During Virus Infections
Abstract
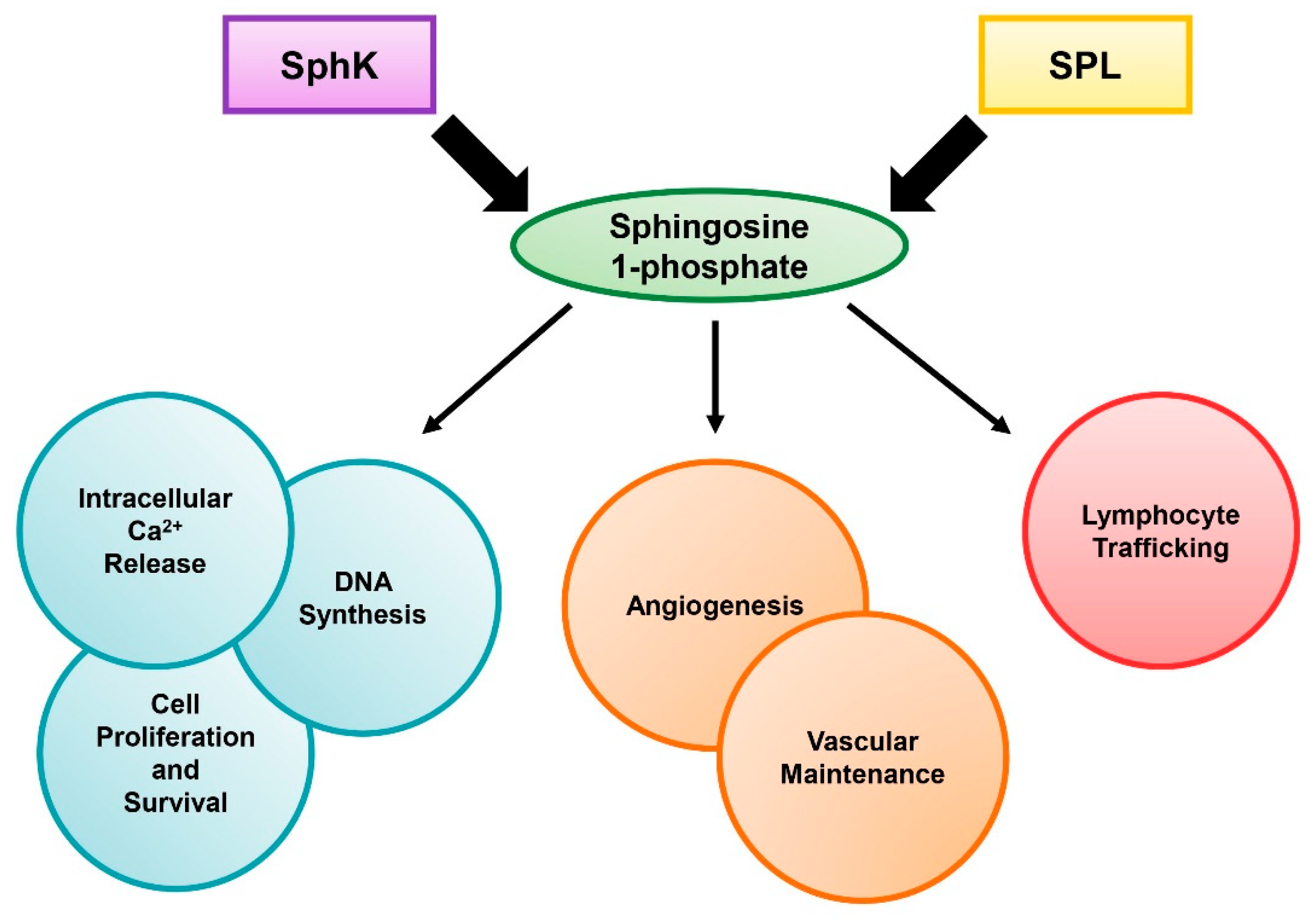
1. Introduction
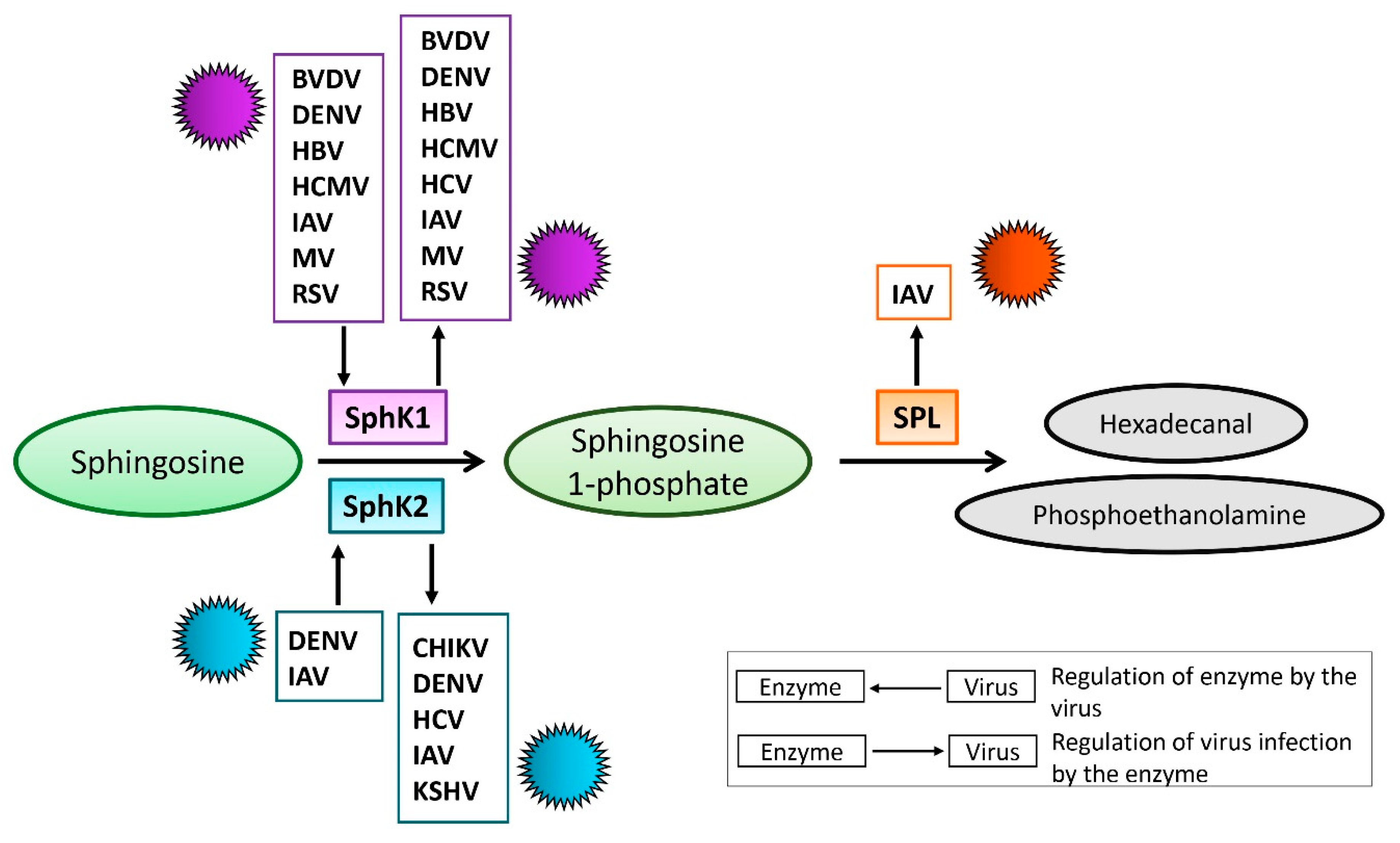
2. S1P-Metabolizing Enzymes During Virus Infections
2.1. SphK During Virus Infection
2.1.1. SphK1 in Virus Replication
2.1.2. SphK/S1PR in Virus Infection
2.1.3. SphK2-Virus Interaction
2.2. S1P Lyase During Virus Infection
3. Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tiper, I.V.; East, J.E.; Subrahmanyam, P.B.; Webb, T.J. Sphingosine 1-phosphate signaling impacts lymphocyte migration, inflammation and infection. Pathog. Dis. 2016, 74, ftw063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Desai, N.N.; Olivera, A.; Seki, T.; Brooker, G.; Spiegel, S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J. Cell Biol. 1991, 114, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Mattie, M.; Brooker, G.; Spiegel, S. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J. Biol. Chem. 1994, 269, 3181–3188. [Google Scholar] [PubMed]
- Shu, X.; Wu, W.; Mosteller, R.D.; Broek, D. Sphingosine Kinase Mediates Vascular Endothelial Growth Factor-Induced Activation of Ras and Mitogen-Activated Protein Kinases. Mol. Cell. Biol. 2002, 22, 7758–7768. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Cosot, O.A.; Gutkind, J.S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine- 1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef]
- Olivera, A.; Spiegel, S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 1993, 365, 557–560. [Google Scholar] [CrossRef]
- Hobson, J.P.; Rosenfeldt, H.M.; Barak, L.S.; Olivera, A.; Poulton, S.; Caron, M.G.; Milstien, S.; Spiegel, S. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science 2001, 291, 1800–1803. [Google Scholar] [CrossRef]
- Liu, Y.; Wada, R.; Yamashita, T.; Mi, Y.; Deng, C.X.; Hobson, J.P.; Rosenfeldt, H.M.; Nava, V.E.; Chae, S.S.; Lee, M.J.; et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Investig. 2000, 106, 951–961. [Google Scholar] [CrossRef]
- Lee, M.J.; Thangada, S.; Claffey, K.P.; Ancellin, N.; Liu, C.H.; Kluk, M.; Volpi, M.; Sha’afi, R.I.; Hla, T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999, 99, 301–312. [Google Scholar] [CrossRef]
- Camerer, E.; Regard, J.B.; Cornelissen, I.; Srinivasan, Y.; Duong, D.N.; Palmer, D.; Pham, T.H.; Wong, J.S.; Pappu, R.; Coughlin, S.R. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Investig. 2009, 119, 1871–1879. [Google Scholar] [CrossRef]
- Mandala, S.; Hajdu, R.; Bergstrom, J.; Quackenbush, E.; Xie, J.; Milligan, J.; Thornton, R.; Shei, G.J.; Card, D.; Keohane, C.A.; et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002, 296, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Davis, M.D.; Heise, C.E.; Albert, R.; Cottens, S.; Hof, R.; Bruns, C.; Prieschl, E.; Baumruker, T.; Hiestand, P.; et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002, 277, 21453–21457. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.R.; Pereira, J.P.; Matloubian, M.; Xu, Y.; Huang, Y.; Cyster, J.G. Immunology: Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 2005, 309, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Billich, A.; Baumruker, T.; Heining, P.; Schmouder, R.; Francis, G.; Aradhye, S.; Burtin, P. Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010, 9, 883–897. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.; Comi, G.; Montalban, X.; Antel, J.; Radue, E.W.; de Vera, A.; Pohlmann, H.; Kappos, L. FTY720 D2201 Study Group Oral fingolimod (FTY720) in multiple sclerosis: Two-year results of a phase II extension study. Neurology 2009, 72, 73–79. [Google Scholar]
- Ziemssen, T.; Medin, J.; Couto, C.A.-M.; Mitchell, C.R. Multiple sclerosis in the real world: A systematic review of fingolimod as a case study. Autoimmun. Rev. 2017, 16, 355–376. [Google Scholar] [CrossRef]
- Walker, S.; Brew, B. Kaposi sarcoma in a fingolimod-treated patient with multiple sclerosis. J. Clin. Neurosci. 2016, 31, 217–218. [Google Scholar] [CrossRef]
- Tagawa, A.; Ogawa, T.; Tetsuka, S.; Otsuka, M.; Hashimoto, R.; Kato, H.; Ando, K.; Tanabe, H. Hepatitis C virus (HCV) reactivation during fingolimod treatment for relapsing and remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 9, 155–157. [Google Scholar] [CrossRef]
- Benedetti, M.D.; Marangi, A.; Bozzetti, S.; Gobbin, F.; Turatti, M.; Pea, M.; Gajofatto, A.; Mocella, S. HPV-related papillary squamous cell carcinoma of the tonsil during treatment with fingolimod. Mult. Scler. Relat. Disord. 2018, 23, 24–26. [Google Scholar] [CrossRef]
- Neubauer, H.A.; Pham, D.H.; Zebol, J.R.; Moretti, P.A.B.; Peterson, A.L.; Leclercq, T.M.; Chan, H.; Powell, J.A.; Pitman, M.R.; Samuel, M.S.; et al. An oncogenic role for sphingosine kinase 2. Oncotarget 2016, 7, 64886–64899. [Google Scholar] [CrossRef]
- Hasanifard, L.; Sheervalilou, R.; Majidinia, M.; Yousefi, B. New insights into the roles and regulation of SphK2 as a therapeutic target in cancer chemoresistance. J. Cell. Physiol. 2019, 234, 8162–8181. [Google Scholar] [CrossRef] [PubMed]
- Alshaker, H.; Sauer, L.; Monteil, D.; Ottaviani, S.; Srivats, S.; Böhler, T.; Pchejetski, D. Therapeutic Potential of Targeting SK1 in Human Cancers. In Advances in Cancer Research; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 117, pp. 143–200. ISBN 9780123942746. [Google Scholar]
- Pyne, N.J.; Pyne, S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 2010, 10, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Rohrbach, T.; Milstien, S.; Spiegel, S. Role of Sphingosine Kinase 1 and Sphingosine-1-Phosphate Axis in Hepatocellular Carcinoma. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–15. [Google Scholar]
- French, K.J.; Schrecengost, R.S.; Lee, B.D.; Zhuang, Y.; Smith, S.N.; Eberly, J.L.; Yun, J.K.; Smith, C.D. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003, 63, 5962–5969. [Google Scholar] [PubMed]
- Lewis, C.S.; Voelkel-Johnson, C.; Smith, C.D. Targeting Sphingosine Kinases for the Treatment of Cancer. Adv. Cancer Res. 2018, 140, 295–325. [Google Scholar] [PubMed]
- Britten, C.D.; Garrett-Mayer, E.; Chin, S.H.; Shirai, K.; Ogretmen, B.; Bentz, T.A.; Brisendine, A.; Anderton, K.; Cusack, S.L.; Maines, L.W.; et al. A Phase I Study of ABC294640, a First-in-Class Sphingosine Kinase-2 Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 4642–4650. [Google Scholar] [CrossRef]
- Hait, N.C.; Bellamy, A.; Milstien, S.; Kordula, T.; Spiegel, S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J. Biol. Chem. 2007, 282, 12058–12065. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef]
- Saba, J.D.; Hla, T. Point-Counterpoint of Sphingosine 1-Phosphate Metabolism. Circ. Res. 2004, 94, 724–734. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Blake, C.; Alexander, S.; Hahm, B. Sphingosine 1-phosphate-metabolizing enzymes control influenza virus propagation and viral cytopathogenicity. J. Virol. 2010, 84, 8124–8131. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Alexander, S.; Hahm, B. Does cytokine signaling link sphingolipid metabolism to host defense and immunity against virus infections? Cytokine Growth Factor Rev. 2011, 22, 55–61. [Google Scholar] [CrossRef][Green Version]
- Vijayan, M.; Xia, C.; Song, Y.E.; Ngo, H.; Studstill, C.J.; Drews, K.; Fox, T.E.; Johnson, M.C.; Hiscott, J.; Kester, M.; et al. Sphingosine 1-Phosphate Lyase Enhances the Activation of IKKε To Promote Type I IFN–Mediated Innate Immune Responses to Influenza A Virus Infection. J. Immunol. 2017, 199, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-J.; Pritzl, C.J.; Vijayan, M.; Bomb, K.; McClain, M.E.; Alexander, S.; Hahm, B. Sphingosine Kinase 1 Serves as a Pro-Viral Factor by Regulating Viral RNA Synthesis and Nuclear Export of Viral Ribonucleoprotein Complex upon Influenza Virus Infection. PLoS ONE 2013, 8, e75005. [Google Scholar] [CrossRef] [PubMed]
- Wati, S.; Rawlinson, S.M.; Ivanov, R.A.; Dorstyn, L.; Beard, M.R.; Jans, D.A.; Pitson, S.M.; Burrell, C.J.; Li, P.; Carr, J.M. Tumour necrosis factor alpha (TNF-alpha) stimulation of cells with established dengue virus type 2 infection induces cell death that is accompanied by a reduced ability of TNF-alpha to activate nuclear factor kappaB and reduced sphingosine kinase-1 activi. J. Gen. Virol. 2011, 92, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Machesky, N.J.; Zhang, G.; Raghavan, B.; Zimmerman, P.; Kelly, S.L.; Merrill, A.H.; Waldman, W.J.; Van Brocklyn, J.R.; Trgovcich, J. Human cytomegalovirus regulates bioactive sphingolipids. J. Biol. Chem. 2008, 283, 26148–26160. [Google Scholar] [CrossRef] [PubMed]
- Yamane, D.; Zahoor, M.A.; Mohamed, Y.M.; Azab, W.; Kato, K.; Tohya, Y.; Akashi, H. Inhibition of sphingosine kinase by bovine viral diarrhea virus NS3 is crucial for efficient viral replication and cytopathogenesis. J. Biol. Chem. 2009, 284, 13648–13659. [Google Scholar] [CrossRef] [PubMed]
- Monick, M.M.; Cameron, K.; Powers, L.S.; Butler, N.S.; McCoy, D.; Mallampalli, R.K.; Hunninghake, G.W. Sphingosine Kinase Mediates Activation of Extracellular Signal–Related Kinase and Akt by Respiratory Syncytial Virus. Am. J. Respir. Cell Mol. Biol. 2004, 30, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415. [Google Scholar] [CrossRef]
- Oskouian, B.; Saba, J.D. Cancer treatment strategies targeting sphingolipid metabolism. Adv. Exp. Med. Biol. 2010, 688, 185–205. [Google Scholar]
- Pitson, S.M. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem. Sci. 2011, 36, 97–107. [Google Scholar] [CrossRef]
- Maceyka, M.; Sankala, H.; Hait, N.C.; Le Stunff, H.; Liu, H.; Toman, R.; Collier, C.; Zhang, M.; Satin, L.S.; Merrill, A.H.; et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 2005, 280, 37118–37129. [Google Scholar] [CrossRef]
- Min, J.; Traynor, D.; Stegner, A.L.; Zhang, L.; Hanigan, M.H.; Alexander, H.; Alexander, S. Sphingosine kinase regulates the sensitivity of Dictyostelium discoideum cells to the anticancer drug cisplatin. Eukaryot. Cell 2005, 4, 178–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Min, J.; Mesika, A.; Sivaguru, M.; Van Veldhoven, P.P.; Alexander, H.; Futerman, A.H.; Alexander, S. (Dihydro)ceramide synthase 1-regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol. Cancer Res. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, N.; Okada, T.; Hayashi, S.; Fujita, T.; Jahangeer, S.; Nakamura, S.I. Sphingosine Kinase 2 Is a Nuclear Protein and Inhibits DNA Synthesis. J. Biol. Chem. 2003, 278, 46832–46839. [Google Scholar] [CrossRef]
- Taha, T.A.; Hannun, Y.A.; Obeid, L.M. Sphingosine kinase: Biochemical and cellular regulation and role in disease. J. Biochem. Mol. Biol. 2006, 39, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, H.A.; Pitson, S.M. Roles, regulation and inhibitors of sphingosine kinase 2. FEBS J. 2013, 280, 5317–5336. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.E.; Harikumar, K.B.; Hait, N.C.; Allegood, J.; Strub, G.M.; Kim, E.Y.; MacEyka, M.; Jiang, H.; Luo, C.; Kordula, T.; et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 2010, 465, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Don, A.S.; Martinez-Lamenca, C.; Webb, W.R.; Proia, R.L.; Roberts, E.; Rosen, H. Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogues. J. Biol. Chem. 2007, 282, 15833–15842. [Google Scholar] [CrossRef]
- Kharel, Y.; Lee, S.; Snyder, A.H.; Sheasley-O’neill, S.L.; Morris, M.A.; Setiady, Y.; Zhu, R.; Zigler, M.A.; Burcin, T.L.; Ley, K.; et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J. Biol. Chem. 2005, 280, 36865–36872. [Google Scholar] [CrossRef]
- Zemann, B.; Kinzel, B.; Müller, M.; Reuschel, R.; Mechtcheriakova, D.; Urtz, N.; Bornancin, F.; Baumruker, T.; Billich, A. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood 2006, 107, 1454–1458. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J. Biol. Chem. 2007, 282, 2125–2129. [Google Scholar] [CrossRef]
- Lu, Z.P.; Xiao, Z.L.; Yang, Z.; Li, J.; Feng, G.X.; Chen, F.Q.; Li, Y.H.; Feng, J.Y.; Gao, Y.E.; Ye, L.H.; et al. Hepatitis B virus X protein promotes human hepatoma cell growth via upregulation of transcription factor AP2α and sphingosine kinase 1. Acta Pharmacol. Sin. 2015, 36, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Yamane, D.; McGivern, D.R.; Wauthier, E.; Yi, M.; Madden, V.J.; Welsch, C.; Antes, I.; Wen, Y.; Chugh, P.E.; McGee, C.E.; et al. Regulation of the hepatitis C virus RNA replicase by endogenous lipid peroxidation. Nat. Med. 2014, 20, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.M.; Kua, T.; Clarke, J.N.; Calvert, J.K.; Zebol, J.R.; Beard, M.R.; Pitson, S.M. Reduced sphingosine kinase 1 activity in dengue virus type-2 infected cells can be mediated by the 3′ untranslated region of dengue virus type-2 RNA. J. Gen. Virol. 2013, 94, 2437–2448. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.K.; Helbig, K.J.; Dimasi, D.; Cockshell, M.; Beard, M.R.; Pitson, S.M.; Bonder, C.S.; Carr, J.M. Dengue Virus Infection of Primary Endothelial Cells Induces Innate Immune Responses, Changes in Endothelial Cells Function and Is Restricted by Interferon-Stimulated Responses. J. Interferon Cytokine Res. 2015, 35, 654–665. [Google Scholar] [CrossRef]
- Calvert, J.K.; Pitson, S.M.; Davies, L.K.; Beard, M.R.; Clarke, J.N.; Helbig, K.J.; Carr, J.M.; Aloia, A.L.; Gliddon, B.L.; Shujari, W.H. Al Reduction in sphingosine kinase 1 influences the susceptibility to dengue virus infection by altering antiviral responses. J. Gen. Virol. 2016, 97, 95–109. [Google Scholar]
- Vijayan, M.; Seo, Y.-J.; Pritzl, C.J.; Squires, S.A.; Alexander, S.; Hahm, B. Sphingosine kinase 1 regulates measles virus replication. Virology 2014, 450–451, 55–63. [Google Scholar] [CrossRef]
- Derakhshani, S.; Kurz, A.; Japtok, L.; Schumacher, F.; Pilgram, L.; Steinke, M.; Kleuser, B.; Sauer, M.; Schneider-Schaulies, S.; Avota, E. Measles Virus Infection Fosters Dendritic Cell Motility in a 3D Environment to Enhance Transmission to Target Cells in the Respiratory Epithelium. Front. Immunol. 2019, 10, 1294. [Google Scholar] [CrossRef]
- Xia, C.; Seo, Y.-J.; Studstill, C.J.; Vijayan, M.; Wolf, J.J.; Hahm, B. Transient inhibition of sphingosine kinases confers protection to influenza A virus infected mice. Antivir. Res. 2018, 158, 171–177. [Google Scholar] [CrossRef]
- Vijayan, M.; Hahm, B. Influenza viral manipulation of sphingolipid metabolism and signaling to modulate host defense system. Scientifica 2014, 2014, 793815. [Google Scholar] [CrossRef]
- Zilch, A.; Rien, C.; Weigel, C.; Huskobla, S.; Glück, B.; Spengler, K.; Sauerbrei, A.; Heller, R.; Gräler, M.; Henke, A. Influence of sphingosine-1-phosphate signaling on HCMV replication in human embryonal lung fibroblasts. Med. Microbiol. Immunol. 2018, 207, 227–242. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Walsh, K.B.; Cahalan, S.; Fremgen, D.M.; Roberts, E.; Scott, F.; Martinborough, E.; Peach, R.; Oldstone, M.B.A.; Rosen, H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 2011, 146, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Marsolais, D.; Hahm, B.; Edelmann, K.H.; Walsh, K.B.; Guerrero, M.; Hatta, Y.; Kawaoka, Y.; Roberts, E.; Oldstone, M.B.A.; Rosen, H. Local not systemic modulation of dendritic cell S1P receptors in lung blunts virus-specific immune responses to influenza. Mol. Pharmacol. 2008, 74, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, M.; Jiang, H.; Shen, S.; Su, X.; Shi, Y. Combination of sphingosine-1-phosphate receptor 1 (S1PR1) agonist and antiviral drug: A potential therapy against pathogenic influenza virus. Sci. Rep. 2019, 9, 5272. [Google Scholar] [CrossRef] [PubMed]
- Oldstone, M.B.A.; Rosen, H. Sphingosine-1-Phosphate Signaling in Immunology and Infectious Diseases; Current Topics in Microbiology and Immunology; Springer International Publishing: New York, NY, USA, 2014; Volume 378, ISBN 978-3-319-05879-5. [Google Scholar]
- Duquenne, C.; Gimenez, S.; Guigues, A.; Viala, B.; Boulouis, C.; Mettling, C.; Maurel, D.; Campos, N.; Doumazane, E.; Comps-Agrar, L.; et al. Reversing HIV latency via sphingosine-1-phosphate receptor 1 signaling. AIDS 2017, 31, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Mudd, J.C.; Murphy, P.; Manion, M.; Debernardo, R.; Hardacre, J.; Ammori, J.; Hardy, G.A.; Harding, C.V.; Mahabaleshwar, G.H.; Jain, M.K.; et al. Impaired T-cell responses to sphingosine-1-phosphate in HIV-1 infected lymph nodes. Blood 2013, 121, 2914–2922. [Google Scholar] [CrossRef][Green Version]
- Kersh, E.N.; Luo, W.; Adams, D.R.; Mitchell, J.; Garcia-Lerma, J.G.; Butera, S.; Folks, T.; Otten, R. Evaluation of the lymphocyte trafficking drug FTY720 in SHIVSF162P3-infected rhesus macaques. J. Antimicrob. Chemother. 2009, 63, 758–762. [Google Scholar] [CrossRef][Green Version]
- Morris, M.; Aubert, R.D.; Butler, K.; Henning, T.; Mitchell, J.; Jenkins, L.; Garber, D.; McNicholl, J.; Kersh, E.N. Preclinical evaluation of the immunomodulatory lymphocyte trafficking drug FTY720 for HIV prevention in the female genital mucosa of macaques. J. Med. Primatol. 2014, 43, 370–373. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Sugiura, M.; Nava, V.E.; Edsall, L.C.; Kono, K.; Poulton, S.; Milstien, S.; Kohama, T.; Spiegel, S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 2000, 275, 19513–19520. [Google Scholar] [CrossRef]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of Histone Acetylation in the Nucleus by Sphingosine-1-Phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef]
- Reid, S.P.; Tritsch, S.R.; Kota, K.; Chiang, C.-Y.; Dong, L.; Kenny, T.; Brueggemann, E.E.; Ward, M.D.; Cazares, L.H.; Bavari, S. Sphingosine kinase 2 is a chikungunya virus host factor co-localized with the viral replication complex. Emerg. Microbes Infect. 2015, 4, e61. [Google Scholar] [CrossRef]
- Morchang, A.; Lee, R.C.H.; Yenchitsomanus, P.T.; Sreekanth, G.P.; Noisakran, S.; Chu, J.J.H.; Limjindaporn, T. RNAi screen reveals a role of SPHK2 in dengue virus–mediated apoptosis in hepatic cell lines. PLoS ONE 2017, 12, e0188121. [Google Scholar] [CrossRef] [PubMed]
- Al-Shujairi, W.H.; Clarke, J.N.; Davies, L.T.; Pitman, M.R.; Calvert, J.K.; Aloia, A.L.; Pitson, S.M.; Carr, J.M. In vitro and in vivo roles of sphingosine kinase 2 during dengue virus infection. J. Gen. Virol. 2019, 100, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, Y.; Ye, J. Inhibition of hepatitis C virus replication by peroxidation of arachidonate and restoration by vitamin E. Proc. Natl. Acad. Sci. USA 2007, 104, 18666–18670. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.B.; Chisari, F.V. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc. Natl. Acad. Sci. USA 2005, 102, 2561–2566. [Google Scholar] [CrossRef]
- Qin, Z.; Dai, L.; Trillo-Tinoco, J.; Senkal, C.; Wang, W.; Reske, T.; Bonstaff, K.; Del Valle, L.; Rodriguez, P.; Flemington, E.; et al. Targeting sphingosine kinase induces apoptosis and tumor regression for KSHV-associated primary effusion lymphoma. Mol. Cancer Ther. 2014, 13, 154–164. [Google Scholar] [CrossRef]
- Dai, L.; Plaisance-Bonstaff, K.; Voelkel-Johnson, C.; Smith, C.D.; Ogretmen, B.; Qin, Z.; Parsons, C. Sphingosine Kinase-2 Maintains Viral Latency and Survival for KSHV-Infected Endothelial Cells. PLoS ONE 2014, 9, e102314. [Google Scholar] [CrossRef]
- Grossmann, C.; Ganem, D. Effects of NFκB activation on KSHV latency and lytic reactivation are complex and context-dependent. Virology 2008, 375, 94–102. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, F.; Qin, D.; Zeng, Y.; Lv, Z.; Yao, S.; Lu, C. Human immunodeficiency virus type 1 induces lytic cycle replication of Kaposi’s-sarcoma-associated herpesvirus: Role of Ras/c-Raf/MEK1/2, PI3K/AKT, and NF-κB signaling pathways. J. Mol. Biol. 2011, 410, 1035–1051. [Google Scholar] [CrossRef]
- Dai, L.; Trillo-Tinoco, J.; Bai, A.; Chen, Y.; Bielawski, J.; Del Valle, L.; Smith, C.D.; Ochoa, A.C.; Qin, Z.; Parsons, C. Ceramides promote apoptosis for virus-infected lymphoma cells through induction of ceramide synthases and viral lytic gene expression. Oncotarget 2015, 6, 24246–24260. [Google Scholar] [CrossRef][Green Version]
- Dai, L.; Bai, A.; Smith, C.D.; Rodriguez, P.C.; Yu, F.; Qin, Z. ABC294640, A Novel Sphingosine Kinase 2 Inhibitor, Induces Oncogenic Virus-Infected Cell Autophagic Death and Represses Tumor Growth. Mol. Cancer Ther. 2017, 16, 2724–2734. [Google Scholar] [CrossRef]
- Serra, M.; Saba, J.D. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv. Enzym. Regul. 2010, 50, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Alexander, H.; Schneider, N.; Alexander, S. Molecular basis for resistance to the anticancer drug cisplatin in Dictyostelium. Microbiology 2000, 146 Pt 9, 2219–2227. [Google Scholar] [CrossRef][Green Version]
- Degagné, E.; Pandurangan, A.; Bandhuvula, P.; Kumar, A.; Eltanawy, A.; Zhang, M.; Yoshinaga, Y.; Nefedov, M.; De Jong, P.J.; Fong, L.G.; et al. Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J. Clin. Investig. 2014, 124, 5368–5384. [Google Scholar] [CrossRef] [PubMed]
- Lovric, S.; Goncalves, S.; Gee, H.Y.; Oskouian, B.; Srinivas, H.; Choi, W.-I.; Shril, S.; Ashraf, S.; Tan, W.; Rao, J.; et al. Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. J. Clin. Investig. 2017, 127, 912–928. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Hadjidemetriou, I.; Maharaj, A.; Meimaridou, E.; Buonocore, F.; Saleem, M.; Hurcombe, J.; Bierzynska, A.; Barbagelata, E.; Bergadá, I.; et al. Sphingosine-1-phosphate lyase mutations cause primary adrenal insufficiency and steroid-resistant nephrotic syndrome. J. Clin. Investig. 2017, 127, 942–953. [Google Scholar] [CrossRef]
- Bamborschke, D.; Pergande, M.; Becker, K.; Koerber, F.; Dötsch, J.; Vierzig, A.; Weber, L.T.; Cirak, S. A novel mutation in sphingosine-1-phosphate lyase causing congenital brain malformation. Brain Dev. 2018, 40, 480–483. [Google Scholar] [CrossRef]
- Settas, N.; Persky, R.; Faucz, F.R.; Sheanon, N.; Voutetakis, A.; Lodish, M.; Metherell, L.A.; Stratakis, C.A. SGPL1 deficiency: A rare cause of primary adrenal insufficiency. J. Clin. Endocrinol. Metab. 2019, 104, 1484–1490. [Google Scholar] [CrossRef]
- Aguilar, A.; Saba, J.D. Truth and consequences of sphingosine-1-phosphate lyase. Adv. Biol. Regul. 2012, 52, 17–30. [Google Scholar] [CrossRef]
- Bandhuvula, P.; Saba, J.D. Sphingosine-1-phosphate lyase in immunity and cancer: Silencing the siren. Trends Mol. Med. 2007, 13, 210–217. [Google Scholar] [CrossRef]
- Fyrst, H.; Saba, J.D. Sphingosine-1-phosphate lyase in development and disease: Sphingolipid metabolism takes flight. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2008, 1781, 448–458. [Google Scholar] [CrossRef]
- Fyrst, H.; Saba, J.D. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat. Chem. Biol. 2010, 6, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Saba, J.D. Sphingosine phosphate lyase insufficiency syndrome (SPLIS): A novel inborn error of sphingolipid metabolism. Adv. Biol. Regul. 2019, 71, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Tenoever, B.R.; Ng, S.-L.; Chua, M.A.; McWhirter, S.M.; García-Sastre, A.; Maniatis, T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science 2007, 315, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Pineda, J.; Kumar, A.; Suh, J.H.; Zhang, M.; Saba, J.D. Dendritic cell sphingosine-1-phosphate lyase regulates thymic egress. J. Exp. Med. 2016, 213, 2773–2791. [Google Scholar] [CrossRef] [PubMed]
- Triplett, J.; Kermode, A.G.; Corbett, A.; Reddel, S.W. Warts and all: Fingolimod and unusual HPV-associated lesions. Mult. Scler. J. 2018, 1547–1550. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, J.J.; Studstill, C.J.; Hahm, B. Emerging Connections of S1P-Metabolizing Enzymes with Host Defense and Immunity During Virus Infections. Viruses 2019, 11, 1097. https://doi.org/10.3390/v11121097
Wolf JJ, Studstill CJ, Hahm B. Emerging Connections of S1P-Metabolizing Enzymes with Host Defense and Immunity During Virus Infections. Viruses. 2019; 11(12):1097. https://doi.org/10.3390/v11121097
Chicago/Turabian StyleWolf, Jennifer J., Caleb J. Studstill, and Bumsuk Hahm. 2019. "Emerging Connections of S1P-Metabolizing Enzymes with Host Defense and Immunity During Virus Infections" Viruses 11, no. 12: 1097. https://doi.org/10.3390/v11121097
APA StyleWolf, J. J., Studstill, C. J., & Hahm, B. (2019). Emerging Connections of S1P-Metabolizing Enzymes with Host Defense and Immunity During Virus Infections. Viruses, 11(12), 1097. https://doi.org/10.3390/v11121097





