The Roles of Ebola Virus Soluble Glycoprotein in Replication, Pathogenesis, and Countermeasure Development
Abstract
:1. Introduction
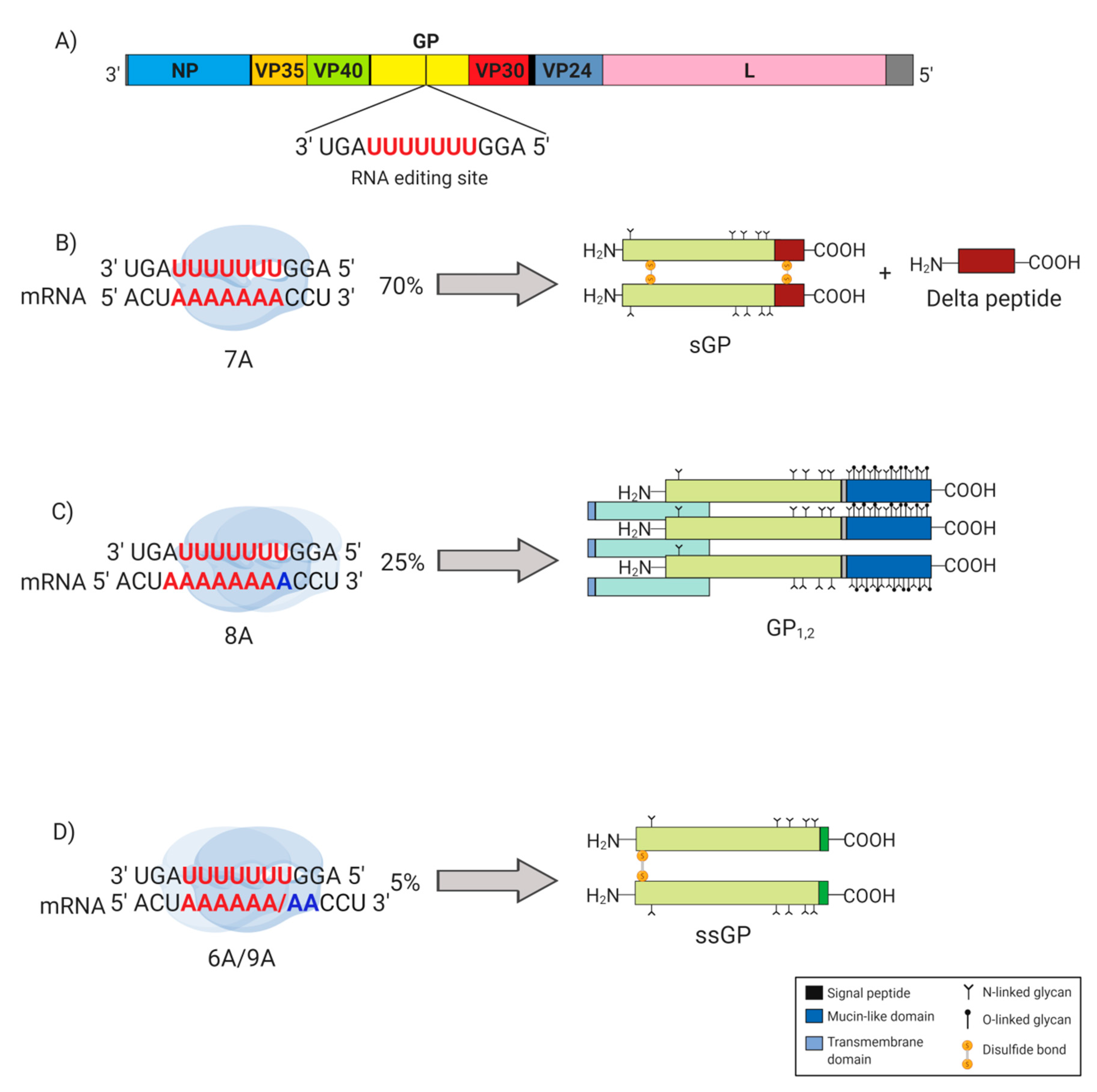
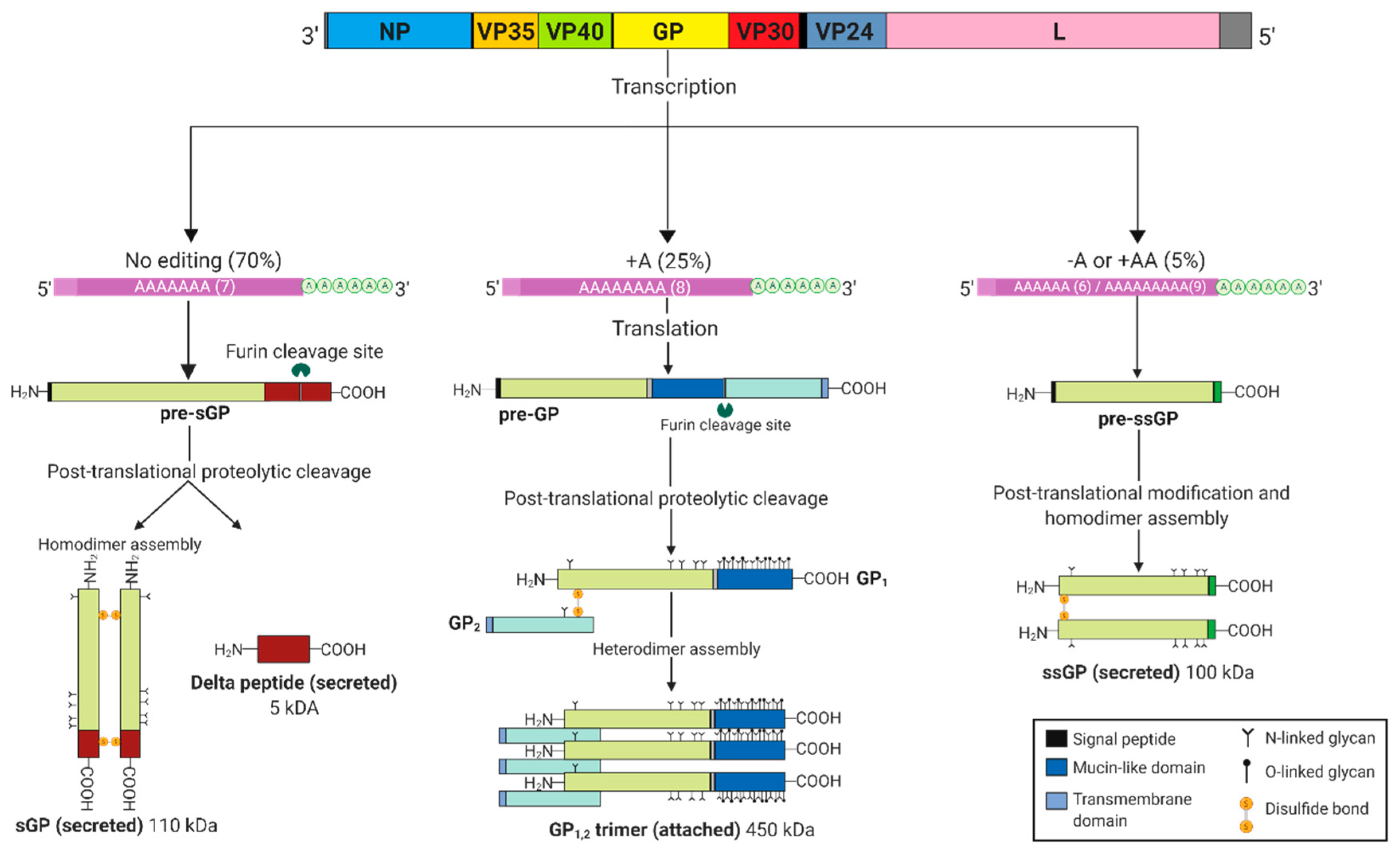
2. EBOV GP Gene Products
3. sGP May Substitute as a Structural Protein
4. sGP Serves as a Virulence Factor
5. sGP Alters the Immune Response
6. sGP Is an Important Target for the Immune Response
7. Δ-Peptide May Have a Role in Viral Replication and Pathogenicity
8. The Roles of ssGP in Pathogenesis Remain Unknown
9. Perspectives on the Role of sGP as a Biomarker for Diagnosis and a Target for Antiviral Therapy
10. Conclusions
Funding
Conflicts of Interest
References
- Kuhn, J.H.; Amarasinghe, G.K.; Basler, C.F.; Bavari, S.; Bukreyev, A.; Chandran, K.; Crozier, I.; Dolnik, O.; Dye, J.M.; Formenty, P.B.H.; et al. ICTV Virus Taxonomy Profile: Filoviridae. J. Gen. Virol. 2019, 100, 911–912. [Google Scholar] [CrossRef] [PubMed]
- Forbes, K.M.; Webala, P.W.; Jaaskelainen, A.J.; Abdurahman, S.; Ogola, J.; Masika, M.M.; Kivisto, I.; Alburkat, H.; Plyusnin, I.; Levanov, L.; et al. Bombali Virus in Mops condylurus Bat, Kenya. Emerg. Infect. Dis. 2019, 25, 955–957. [Google Scholar] [CrossRef] [PubMed]
- Baseler, L.; Chertow, D.S.; Johnson, K.M.; Feldmann, H.; Morens, D.M. The Pathogenesis of Ebola Virus Disease. Annu. Rev. Pathol. 2017, 12, 387–418. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, H.; Sanchez, A.; Geisbert, T.W. Fields Virology. In Filoviridae: Marburg and Ebola Viruses; Wolters Kluwer: Philadelphia, PA, USA, 2013; Chapter 32; pp. 923–956. [Google Scholar]
- Chertow, D.S.; Kleine, C.; Edwards, J.K.; Scaini, R.; Giuliani, R.; Sprecher, A. Ebola virus disease in West Africa—Clinical manifestations and management. N. Engl. J. Med. 2014, 371, 2054–2057. [Google Scholar] [CrossRef]
- Bwaka, M.A.; Bonnet, M.J.; Calain, P.; Colebunders, R.; De Roo, A.; Guimard, Y.; Katwiki, K.R.; Kibadi, K.; Kipasa, M.A.; Kuvula, K.J.; et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: Clinical observations in 103 patients. J. Infect. Dis. 1999, 179 (Suppl. 1), S1–S7. [Google Scholar] [CrossRef]
- Sagui, E.; Janvier, F.; Baize, S.; Foissaud, V.; Koulibaly, F.; Savini, H.; Maugey, N.; Aletti, M.; Granier, H.; Carmoi, T. Severe Ebola Virus Infection With Encephalopathy: Evidence for Direct Virus Involvement. Clin. Infect. Dis. 2015, 61, 1627–1628. [Google Scholar] [CrossRef] [Green Version]
- Kirchdoerfer, R.N.; Wasserman, H.; Amarasinghe, G.K.; Saphire, E.O. Filovirus Structural Biology: The Molecules in the Machine. Curr. Top. Microbiol. Immunol. 2017, 411, 381–417. [Google Scholar]
- Davey, R.A.; Shtanko, O.; Anantpadma, M.; Sakurai, Y.; Chandran, K.; Maury, W. Mechanisms of Filovirus Entry. Curr. Top. Microbiol. Immunol. 2017, 411, 323–352. [Google Scholar]
- Mehedi, M.; Falzarano, D.; Seebach, J.; Hu, X.; Carpenter, M.S.; Schnittler, H.J.; Feldmann, H. A new Ebola virus nonstructural glycoprotein expressed through RNA editing. J. Virol. 2011, 85, 5406–5414. [Google Scholar] [CrossRef]
- Volchkov, V.E.; Becker, S.; Volchkova, V.A.; Ternovoj, V.A.; Kotov, A.N.; Netesov, S.V.; Klenk, H.D. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology 1995, 214, 421–430. [Google Scholar] [CrossRef]
- Sanchez, A.; Trappier, S.G.; Mahy, B.W.; Peters, C.J.; Nichol, S.T. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc. Natl. Acad. Sci. USA 1996, 93, 3602–3607. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, A.; Shimojima, M.; Kawaoka, Y. sGP serves as a structural protein in Ebola virus infection. J. Infect. Dis. 2011, 204 (Suppl. 3), S897–S903. [Google Scholar] [CrossRef] [PubMed]
- Volchkov, V.E.; Volchkova, V.A.; Muhlberger, E.; Kolesnikova, L.V.; Weik, M.; Dolnik, O.; Klenk, H.D. Recovery of infectious Ebola virus from complementary DNA: RNA editing of the GP gene and viral cytotoxicity. Science 2001, 291, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Volchkova, V.A.; Dolnik, O.; Martinez, M.J.; Reynard, O.; Volchkov, V.E. RNA Editing of the GP Gene of Ebola Virus is an Important Pathogenicity Factor. J. Infect. Dis. 2015, 212 (Suppl. 2), S226–S233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallesen, J.; Murin, C.D.; de Val, N.; Cottrell, C.A.; Hastie, K.M.; Turner, H.L.; Fusco, M.L.; Flyak, A.I.; Zeitlin, L.; Crowe, J.E., Jr.; et al. Structures of Ebola virus GP and sGP in complex with therapeutic antibodies. Nat. Microbiol. 2016, 1, 16128. [Google Scholar] [CrossRef]
- Wahl-Jensen, V.M.; Afanasieva, T.A.; Seebach, J.; Stroher, U.; Feldmann, H.; Schnittler, H.J. Effects of Ebola virus glycoproteins on endothelial cell activation and barrier function. J. Virol. 2005, 79, 10442–10450. [Google Scholar] [CrossRef]
- de La Vega, M.A.; Wong, G.; Kobinger, G.P.; Qiu, X. The multiple roles of sGP in Ebola pathogenesis. Viral. Immunol. 2015, 28, 3–9. [Google Scholar] [CrossRef]
- Anthony, S.M.; Bradfute, S.B. Filoviruses: One of These Things is (not) Like the Other. Viruses 2015, 7, 5172–5190. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.D.; Lee, J.E. The secret life of viral entry glycoproteins: Moonlighting in immune evasion. PLoS Pathog. 2013, 9, e1003258. [Google Scholar] [CrossRef] [PubMed]
- Volchkov, V.E.; Feldmann, H.; Volchkova, V.A.; Klenk, H.D. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc. Natl. Acad. Sci. USA 1998, 95, 5762–5767. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Qian, H.; Zhou, X.; Wu, J.; Wan, T.; Cao, P.; Huang, W.; Zhao, X.; Wang, X.; Wang, P.; et al. Structural Insights into the Niemann-Pick C1 (NPC1)-Mediated Cholesterol Transfer and Ebola Infection. Cell 2016, 165, 1467–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolnik, O.; Volchkova, V.; Garten, W.; Carbonnelle, C.; Becker, S.; Kahnt, J.; Stroher, U.; Klenk, H.D.; Volchkov, V. Ectodomain shedding of the glycoprotein GP of Ebola virus. EMBO J. 2004, 23, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Okumura, A.; Pitha, P.M.; Yoshimura, A.; Harty, R.N. Interaction between Ebola virus glycoprotein and host toll-like receptor 4 leads to induction of proinflammatory cytokines and SOCS1. J. Virol. 2010, 84, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Perez, B.; Volchkova, V.A.; Dolnik, O.; Lawrence, P.; Volchkov, V.E. Shed GP of Ebola virus triggers immune activation and increased vascular permeability. PLoS Pathog. 2014, 10, e1004509. [Google Scholar] [CrossRef]
- Volchkova, V.A.; Klenk, H.D.; Volchkov, V.E. Delta-peptide is the carboxy-terminal cleavage fragment of the nonstructural small glycoprotein sGP of Ebola virus. Virology 1999, 265, 164–171. [Google Scholar] [CrossRef]
- Volchkova, V.A.; Feldmann, H.; Klenk, H.D.; Volchkov, V.E. The nonstructural small glycoprotein sGP of Ebola virus is secreted as an antiparallel-orientated homodimer. Virology 1998, 250, 408–414. [Google Scholar] [CrossRef]
- Volchkova, V.A.; Dolnik, O.; Martinez, M.J.; Reynard, O.; Volchkov, V.E. Genomic RNA editing and its impact on Ebola virus adaptation during serial passages in cell culture and infection of guinea pigs. J. Infect. Dis. 2011, 204 (Suppl. 3), S941–S946. [Google Scholar] [CrossRef]
- Tsuda, Y.; Hoenen, T.; Banadyga, L.; Weisend, C.; Ricklefs, S.M.; Porcella, S.F.; Ebihara, H. An Improved Reverse Genetics System to Overcome Cell-Type-Dependent Ebola Virus Genome Plasticity. J. Infect. Dis. 2015, 212 (Suppl. 2), S129–S137. [Google Scholar] [CrossRef]
- Hoenen, T.; Marzi, A.; Scott, D.P.; Feldmann, F.; Callison, J.; Safronetz, D.; Ebihara, H.; Feldmann, H. Soluble Glycoprotein Is Not Required for Ebola Virus Virulence in Guinea Pigs. J. Infect. Dis. 2015, 212 (Suppl. 2), S242–S246. [Google Scholar] [CrossRef] [Green Version]
- Whitmer, S.L.M.; Ladner, J.T.; Wiley, M.R.; Patel, K.; Dudas, G.; Rambaut, A.; Sahr, F.; Prieto, K.; Shepard, S.S.; Carmody, E.; et al. Ebola Virus Persistence Study, G., Active Ebola Virus Replication and Heterogeneous Evolutionary Rates in EVD Survivors. Cell Rep. 2018, 22, 1159–1168. [Google Scholar] [CrossRef]
- Kindzelskii, A.L.; Yang, Z.; Nabel, G.J.; Todd, R.F., 3rd; Petty, H.R. Ebola virus secretory glycoprotein (sGP) diminishes Fc gamma RIIIB-to-CR3 proximity on neutrophils. J. Immunol. 2000, 164, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.H.; Harrison, A.; Corey, A.; Gentry, N.; Gregg, R.K. Ebola virus secreted glycoprotein decreases the anti-viral immunity of macrophages in early inflammatory responses. Cell Immunol. 2018, 324, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Mohan, G.S.; Li, W.; Ye, L.; Compans, R.W.; Yang, C. Antigenic subversion: A novel mechanism of host immune evasion by Ebola virus. PLoS Pathog. 2012, 8, e1003065. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, S.; Das, D.; Qiu, X.; Feldmann, H.; Jones, S.M.; Suresh, M.R. Production and characterization of monoclonal antibodies against different epitopes of Ebola virus antigens. J. Virol. Methods 2007, 143, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Dowling, W.; Thompson, E.; Badger, C.; Mellquist, J.L.; Garrison, A.R.; Smith, J.M.; Paragas, J.; Hogan, R.J.; Schmaljohn, C. Influences of glycosylation on antigenicity, immunogenicity, and protective efficacy of ebola virus GP DNA vaccines. J. Virol. 2007, 81, 1821–1837. [Google Scholar] [CrossRef] [PubMed]
- Ilinykh, P.A.; Shen, X.; Flyak, A.I.; Kuzmina, N.; Ksiazek, T.G.; Crowe, J.E., Jr.; Bukreyev, A. Chimeric Filoviruses for Identification and Characterization of Monoclonal Antibodies. J. Virol. 2016, 90, 3890–3901. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ye, L.; Lin, F.; Gomaa, Y.; Flyer, D.; Carrion, R., Jr.; Patterson, J.L.; Prausnitz, M.R.; Smith, G.; Glenn, G.; et al. Intradermal Vaccination With Adjuvanted Ebola Virus Soluble Glycoprotein Subunit Vaccine by Microneedle Patches Protects Mice Against Lethal Ebola Virus Challenge. J. Infect. Dis. 2018, 218 (Suppl. 5), S545–S552. [Google Scholar] [CrossRef]
- Saphire, E.O.; Schendel, S.L.; Fusco, M.L.; Gangavarapu, K.; Gunn, B.M.; Wec, A.Z.; Halfmann, P.J.; Brannan, J.M.; Herbert, A.S.; Qiu, X.; et al. Viral Hemorrhagic Fever Immunotherapeutic, C., Systematic Analysis of Monoclonal Antibodies against Ebola Virus GP Defines Features that Contribute to Protection. Cell 2018, 174, 938–952.e13. [Google Scholar] [CrossRef]
- Qiu, X.; Wong, G.; Audet, J.; Bello, A.; Fernando, L.; Alimonti, J.B.; Fausther-Bovendo, H.; Wei, H.; Aviles, J.; Hiatt, E.; et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014, 514, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Gunn, B.M.; Roy, V.; Karim, M.M.; Hartnett, J.N.; Suscovich, T.J.; Goba, A.; Momoh, M.; Sandi, J.D.; Kanneh, L.; Andersen, K.G.; et al. Survivors of Ebola virus disease develop polyfunctional antibody responses. J. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Sakabe, S.; Sullivan, B.M.; Hartnett, J.N.; Robles-Sikisaka, R.; Gangavarapu, K.; Cubitt, B.; Ware, B.C.; Kotliar, D.; Branco, L.M.; Goba, A.; et al. Analysis of CD8(+) T cell response during the 2013-2016 Ebola epidemic in West Africa. Proc. Natl. Acad. Sci. USA 2018, 115, E7578–E7586. [Google Scholar] [CrossRef] [PubMed]
- Radoshitzky, S.R.; Warfield, K.L.; Chi, X.; Dong, L.; Kota, K.; Bradfute, S.B.; Gearhart, J.D.; Retterer, C.; Kranzusch, P.J.; Misasi, J.N.; et al. Ebolavirus delta-peptide immunoadhesins inhibit marburgvirus and ebolavirus cell entry. J. Virol. 2011, 85, 8502–8513. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, W.R.; Garry, R.F. Modeling of the Ebola virus delta peptide reveals a potential lytic sequence motif. Viruses 2015, 7, 285–305. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Melnik, L.I.; Komin, A.; Wiedman, G.; Fuselier, T.; Morris, C.F.; Starr, C.G.; Searson, P.C.; Gallaher, W.R.; Hristova, K.; et al. Ebola Virus Delta Peptide Is a Viroporin. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, H.; Volchkov, V.E.; Volchkova, V.A.; Stroher, U.; Klenk, H.D. Biosynthesis and role of filoviral glycoproteins. J. Gen. Virol. 2001, 82 Pt 12, 2839–2848. [Google Scholar] [CrossRef] [Green Version]
- Hoenen, T.; Groseth, A.; Feldmann, H. Therapeutic strategies to target the Ebola virus life cycle. Nat. Rev. Microbiol. 2019, 17, 593–606. [Google Scholar] [CrossRef]
- Lucey, D.R. New treatments for Ebola virus disease. BMJ 2019, 366, l5371. [Google Scholar] [CrossRef]
- Shears, P.; O’Dempsey, T.J. Ebola virus disease in Africa: Epidemiology and nosocomial transmission. J. Hosp. Infect. 2015, 90, 1–9. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Banadyga, L.; Emeterio, K.; Wong, G.; Qiu, X. The Roles of Ebola Virus Soluble Glycoprotein in Replication, Pathogenesis, and Countermeasure Development. Viruses 2019, 11, 999. https://doi.org/10.3390/v11110999
Zhu W, Banadyga L, Emeterio K, Wong G, Qiu X. The Roles of Ebola Virus Soluble Glycoprotein in Replication, Pathogenesis, and Countermeasure Development. Viruses. 2019; 11(11):999. https://doi.org/10.3390/v11110999
Chicago/Turabian StyleZhu, Wenjun, Logan Banadyga, Karla Emeterio, Gary Wong, and Xiangguo Qiu. 2019. "The Roles of Ebola Virus Soluble Glycoprotein in Replication, Pathogenesis, and Countermeasure Development" Viruses 11, no. 11: 999. https://doi.org/10.3390/v11110999
APA StyleZhu, W., Banadyga, L., Emeterio, K., Wong, G., & Qiu, X. (2019). The Roles of Ebola Virus Soluble Glycoprotein in Replication, Pathogenesis, and Countermeasure Development. Viruses, 11(11), 999. https://doi.org/10.3390/v11110999





