No Evidence of Mosquito Involvement in the Transmission of Equine Hepacivirus (Flaviviridae) in an Epidemiological Survey of Austrian Horses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Mosquito Collection
2.3. Laboratory Analysis
2.3.1. Detection of EqHV RNA in Mosquito Pools
2.3.2. Detection of EqHV RNA in Horse Serum
2.3.3. Detection of Anti-EqHV Non-Structural Protein 3 (NS3)-Specific Antibodies in Horse Serum
2.3.4. Plasma Biochemistry
2.4. Data Analysis
2.5. Sequencing and Phylogenetic Analyses
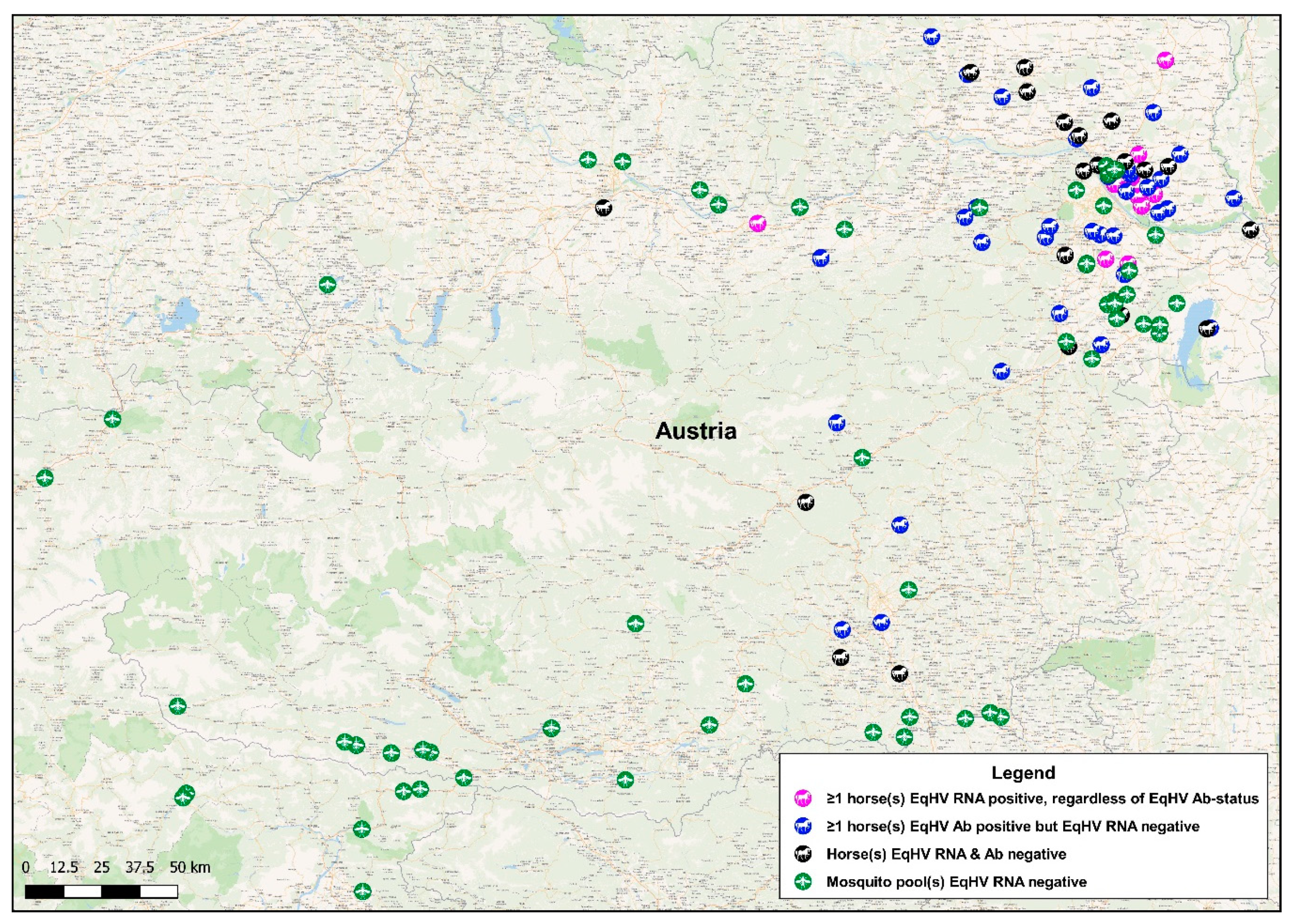
3. Results
3.1. Detection of EqHV RNA in Mosquito Pools
3.2. Detection of EqHV RNA in Horse Serum
3.3. Detection of Anti-EqHV NS3-Specific Antibodies in Horse Serum
3.4. Plasma Biochemistry
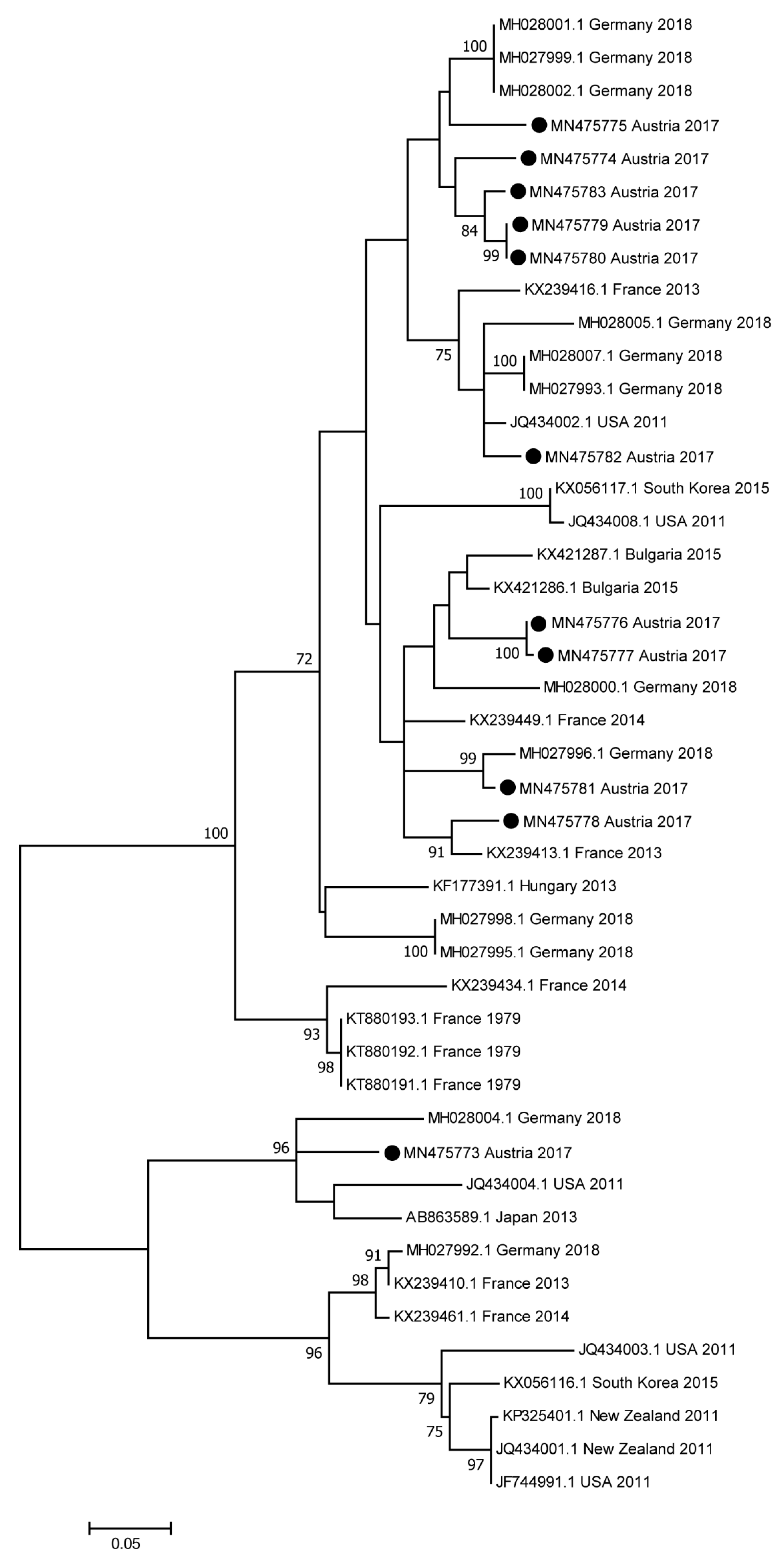
3.5. Sequence and Phylogenetic Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, D.B.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, A.S.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.T.; et al. Proposed update to the taxonomy of the genera Hepacivirus and Pegivirus within the Flaviviridae family. J. Gen. Virol. 2016, 97, 2894–2907. [Google Scholar] [CrossRef] [PubMed]
- Pfaender, S.; Brown, R.J.; Pietschmann, T.; Steinmann, E. Natural reservoirs for homologs of hepatitis C virus. Emerg. Microbes Infect. 2014, 3, e21. [Google Scholar] [CrossRef] [PubMed]
- Pfaender, S.; Cavalleri, J.M.; Walter, S.; Doerrbecker, J.; Campana, B.; Brown, R.J.; Burbelo, P.D.; Postel, A.; Hahn, K.; Anggakusuma Riebesehl, N.; et al. Clinical course of infection and viral tissue tropism of hepatitis C virus-like nonprimate hepaciviruses in horses. Hepatology 2015, 61, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, M.; Tegtmeyer, B.; Todt, D.; Guthrie, A.; Feige, K.; Campe, A.; Steinmann, E.; Cavalleri, J.M.V. First detection and frequent occurrence of Equine Hepacivirus in horses on the African continent. Vet. Microbiol. 2018, 223, 51–58. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Dubovi, E.J.; Simmonds, P.; Medina, J.L.; Henriquez, J.A.; Mishra, N.; Wagner, J.; Tokarz, R.; Cullen, J.M.; Iadarola, M.J.; et al. Serology-enabled discovery of genetically diverse hepaciviruses in a new host. J. Virol. 2012, 86, 6171–6178. [Google Scholar] [CrossRef]
- Elia, G.; Lanave, G.; Lorusso, E.; Parisi, A.; Cavaliere, N.; Patruno, G.; Terregino, C.; Decaro, N.; Martella, V.; Buonavoglia, C. Identification and genetic characterization of equine hepaciviruses in Italy. Vet. Microbiol. 2017, 207, 239–247. [Google Scholar] [CrossRef]
- Gemaque, B.S.; Junior Souza de Souza, A.; do Carmo Pereira Soares, M.; Malheiros, A.P.; Silva, A.L.; Alves, M.M.; Gomes-Gouvea, M.S.; Pinho, J.R.; Ferreira de Figueiredo, H.; Ribeiro, D.B.; et al. Hepacivirus infection in domestic horses, Brazil, 2011–2013. Emerg. Infect. Dis. 2014, 20, 2180–2182. [Google Scholar] [CrossRef]
- Kim, H.-S.; Moon, H.-W.; Sung, H.W.; Kwon, H.M. First identification and phylogenetic analysis of equine hepacivirus in Korea. Infect. Genet. Evol. 2017, 49, 268–272. [Google Scholar] [CrossRef]
- Lu, G.; Sun, L.; Xu, T.; He, D.; Wang, Z.; Ou, S.; Jia, K.; Yuan, L.; Li, S. First Description of Hepacivirus and Pegivirus Infection in Domestic Horses in China: A Study in Guangdong Province, Heilongjiang Province and Hong Kong District. PLoS ONE 2016, 11, e0155662. [Google Scholar] [CrossRef]
- Lyons, S.; Kapoor, A.; Sharp, C.; Schneider, B.S.; Wolfe, N.D.; Culshaw, G.; Corcoran, B.; McGorum, B.C.; Simmonds, P. Nonprimate hepaciviruses in domestic horses, United Kingdom. Emerg. Infect. Dis. 2012, 18, 1976–1982. [Google Scholar] [CrossRef]
- Matsuu, A.; Hobo, S.; Ando, K.; Sanekata, T.; Sato, F.; Endo, Y.; Amaya, T.; Osaki, T.; Horie, M.; Masatani, T.; et al. Genetic and serological surveillance for non-primate hepacivirus in horses in Japan. Vet. Microbiol. 2015, 179, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Pronost, S.; Hue, E.; Fortier, C.; Foursin, M.; Fortier, G.; Desbrosse, F.; Rey, F.A.; Pitel, P.H.; Richard, E.; Saunier, B. Prevalence of Equine Hepacivirus Infections in France and Evidence for Two Viral Subtypes Circulating Worldwide. Transbound. Emerg. Dis. 2017, 64, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.; Campe, A.; Walter, S.; Pfaender, S.; Welsch, K.; Ruddat, I.; Sieme, H.; Feige, K.; Steinmann, E.; Cavalleri, J.M.V. Frequent occurrence of nonprimate hepacivirus infections in Thoroughbred breeding horses—A cross-sectional study for the occurrence of infections and potential risk factors. Vet. Microbiol. 2017, 203, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Reuter, G.; Maza, N.; Pankovics, P.; Boros, A. Non-primate hepacivirus infection with apparent hepatitis in a horse—Short communication. Acta. Vet. Hung. 2014, 62, 422–427. [Google Scholar] [CrossRef]
- Schlottau, K.; Fereidouni, S.; Beer, M.; Hoffmann, B. Molecular identification and characterization of nonprimate hepaciviruses in equines. Arch. Virol. 2019, 164, 391–400. [Google Scholar] [CrossRef]
- Tanaka, T.; Kasai, H.; Yamashita, A.; Okuyama-Dobashi, K.; Yasumoto, J.; Maekawa, S.; Enomoto, N.; Okamoto, T.; Matsuura, Y.; Morimatsu, M.; et al. Hallmarks of hepatitis C virus in equine hepacivirus. J. Virol. 2014, 88, 13352–13366. [Google Scholar] [CrossRef]
- Gather, T.; Walter, S.; Todt, D.; Pfaender, S.; Brown, R.J.; Postel, A.; Becher, P.; Moritz, A.; Hansmann, F.; Baumgaertner, W.; et al. Vertical transmission of hepatitis C virus-like non-primate hepacivirus in horses. J. Gen. Virol. 2016, 97, 2540–2551. [Google Scholar] [CrossRef]
- Figueiredo, A.S.; Lampe, E.; de Albuquerque, P.P.L.F.; Chalhoub, F.L.L.; de Filippis, A.M.B.; Villar, L.M.; Cruz, O.G.; Pinto, M.A.; de Oliveira, J.M. Epidemiological investigation and analysis of the NS5B gene and protein variability of non-primate hepacivirus in several horse cohorts in Rio de Janeiro state, Brazil. Infect. Genet. Evol. 2018, 59, 38–47. [Google Scholar] [CrossRef]
- Figueiredo, A.S.; de Moraes, M.V.D.S.; Soares, C.C.; Chalhoub, F.L.L.; de Filippis, A.M.B.; Dos Santos, D.R.L.; de Almeida, F.Q.; Godoi, T.L.O.S.; de Souza, A.M.; Burdman, T.R.; et al. First description of Theiler’s disease-associated virus infection and epidemiological investigation of equine pegivirus and equine hepacivirus coinfection in Brazil. Transbound. Emerg. Dis. 2019, 66, 1737–1751. [Google Scholar] [CrossRef]
- Terrault, N.A.; Dodge, J.L.; Murphy, E.L.; Tavis, J.E.; Kiss, A.; Levin, T.R.; Gish, R.G.; Busch, M.P.; Reingold, A.L.; Alter, M.J. Sexual transmission of hepatitis C virus among monogamous heterosexual couples. The HCV partners study. Hepatology 2013, 57, 881–889. [Google Scholar] [CrossRef]
- Pfaender, S.; Walter, S.; Grabski, E.; Todt, D.; Bruening, J.; Romero-Brey, I.; Gather, T.; Brown, R.J.P.; Hahn, K.; Puff, C.; et al. Immune protection against reinfection with nonprimate hepacivirus. PNAS 2017, 114, E2430–E2439. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Cavalleri, J.-M.V.; Pfaender, S.; Walter, S.; Steinmann, E.; Fischer, N.; Feige, K.; Haas, L.; Becher, P. Frequent presence of hepaci and pegiviruses in commercial equine serum pools. Vet. Microbiol. 2016, 182, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.D.; Evanoff, R.; Wilkinson, T.E.; Divers, T.J.; Knowles, D.P.; Mealey, R.H. Experimental transmission of equine hepacivirus in horses as a model for hepatitis C virus. Hepatology 2015, 61, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-J.S.; Higgs, S.; Horne, K.M.; Vanlandingham, D.L. Flavivirus-mosquito interactions. Viruses 2014, 6, 4703–4730. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Jungbauer, C.; Aberle, S.W.; Kolodziejek, J.; Dimmel, K.; Stiasny, K.; Allerberger, F.; Nowotny, N. Usutu virus infections among blood donors, Austria, July and August 2017—Raising awareness for diagnostic challenges. Euro. Surveill. 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejek, J.; Seidel, B.; Jungbauer, C.; Dimmel, K.; Kolodziejek, M.; Rudolf, I.; Hubálek, Z.; Allerberger, F.; Nowotny, N. West Nile virus positive blood donation and subsequent entomological investigation, Austria, 2014. PLoS ONE 2015, 10, e0126381. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Ching, K.H.; Klimavicz, C.M.; Iadarola, M.J. Antibody profiling by Luciferase Immunoprecipitation Systems (LIPS). J. Vis. Exp. 2009, 32. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Lyons, S.; Kapoor, A.; Schneider, B.S.; Wolfe, N.D.; Culshaw, G.; Corcoran, B.; Durham, A.E.; Burden, F.; McGorum, B.C.; Simmonds, P. Viraemic frequencies and seroprevalence of non-primate hepacivirus and equine pegiviruses in horses and other mammalian species. J. Gen. Virol. 2014, 95, 1701–1711. [Google Scholar] [CrossRef][Green Version]
- Seidel, B.; Nowotny, N.; Bakonyi, T.; Allerberger, F.; Schaffner, F. Spread of Aedes japonicus japonicus (Theobald, 1901) in Austria, 2011-2015, and first records of the subspecies for Hungary, 2012, and the principality of Liechtenstein, 2015. Parasite Vectors 2016, 9, 356. [Google Scholar] [CrossRef]
- Seidel, B.; Montarsi, F.; Huemer, H.P.; Indra, A.; Capelli, G.; Allerberger, F.; Nowotny, N. First record of the Asian bush mosquito, Aedes japonicus japonicus, in Italy: Invasion from an established Austrian population. Parasite Vectors 2016, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Ferenczi, E.; Erdélyi, K.; Kutasi, O.; Csörgő, T.; Seidel, B.; Weissenböck, H.; Brugger, K.; Bán, E.; Nowotny, N. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet. Microbiol. 2013, 165, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.V.; Bakonyi, T.; Soltész, Z.; Zechmeister, T.; Nowotny, N. Uranotaenia unguiculata Edwards, 1913 are attracted to sound, feed on amphibians, and are infected with multiple viruses. Parasite Vectors 2018, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.V.; Kolodziejek, J.; Nowotny, N. Targeted surveillance reveals native and invasive mosquito species infected with Usutu virus. Parasite Vectors 2019, 12, 46. [Google Scholar] [CrossRef]
- Kolodziejek, J.; Jungbauer, C.; Aberle, S.W.; Allerberger, F.; Bagó, Z.; Camp, J.V.; Dimmel, K.; de Heus, P.; Kolodziejek, M.; Schiefer, P.; et al. Integrated analysis of human-animal-vector surveillance: West Nile virus infections in Austria, 2015–2016. Emerg. Microbes Infect. 2018, 7, 25. [Google Scholar] [CrossRef]
- Pachler, K.; Lebl, K.; Berer, D.; Rudolf, I.; Hubalek, Z.; Nowotny, N. Putative new West Nile virus lineage in Uranotaenia unguiculata mosquitoes, Austria, 2013. Emerg. Infect. Dis. 2014, 20, 2119–2122. [Google Scholar] [CrossRef]
- Rudolf, I.; Betášová, L.; Blažejová, H.; Venclíková, K.; Straková, P.; Šebesta, O.; Mendel, J.; Bakonyi, T.; Schaffner, F.; Nowotny, N.; et al. West Nile virus in overwintering mosquitoes, central Europe. Parasite Vectors 2017, 10, 452. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ivanics, E.; Erdélyi, K.; Ursu, K.; Ferenczi, E.; Weissenböck, H.; Nowotny, N. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef]
- Rudolf, I.; Bakonyi, T.; Šebesta, O.; Mendel, J.; Peško, J.; Betášová, L.; Blažejová, H.; Venclíková, K.; Straková, P.; Nowotny, N.; et al. West Nile virus lineage 2 isolated from Culex modestus mosquitoes in the Czech Republic, 2013: Expansion of the European WNV endemic area to the North? Euro. Surveill. 2014, 19, 20867. [Google Scholar] [CrossRef]
- Issel, C.J.; Foil, L.D. Equine infectious anaemia and mechanical transmission. Man and the wee beasties. Rev. Sci. Tech. OIE 2015, 34, 513–523. [Google Scholar] [CrossRef]
- Carn, V.M. The role of dipterous insects in the mechanical transmission of animal viruses. Brit. Vet. J. 1996, 152, 377–393. [Google Scholar] [CrossRef]
- Scoles, G.A.; Miller, J.A.; Foil, L.D. Comparison of the efficiency of biological transmission of Anaplasma marginale (Rickettsiales. Anaplasmataceae) by Dermacentor andersoni Stiles (Acari: Ixodidae) with mechanical transmission by the horse fly, Tabanus fuscicostatus Hine (Diptera: Muscidae). J. Med. Entomol. 2008, 45, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Gather, T.; Walter, S.; Pfaender, S.; Todt, D.; Feige, K.; Steinmann, E.; Cavalleri, J.M.V. Acute and chronic infections with nonprimate hepacivirus in young horses. Vet. Res. 2016, 47, 97. [Google Scholar] [CrossRef] [PubMed]
| Property | EqHV Infection-State | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Abs-/RNA- | Abs+/RNA- | Abs+/RNA+ | Abs-/RNA+ | ||||||
| Property ID | No. of Horses Sampled | n | % | n | % | n | % | n | % |
| 1 | 12 | 4 | 33.33 | 7 | 58.33 | 1 | 8.33 | 0 | 0.00 |
| 2 | 10 | 4 | 40.00 | 5 | 50.00 | 1 | 10.00 | 0 | 0.00 |
| 3 | 20 | 13 | 65.00 | 6 | 30.00 | 1 | 5.00 | 0 | 0.00 |
| 4 | 21 | 7 | 33.33 | 10 | 47.62 | 3 | 14.29 | 1 | 4.76 |
| 5 | 14 | 2 | 14.29 | 11 | 78.57 | 1 | 7.14 | 0 | 0.00 |
| 6 | 32 | 16 | 50.00 | 14 | 43.75 | 1 | 3.13 | 1 | 3.13 |
| 7 | 3 | 1 | 33.33 | 1 | 33.33 | 1 | 33.33 | 0 | 0.00 |
| 8 | 20 | 13 | 65.00 | 6 | 30.00 | 1 | 5.00 | 0 | 0.00 |
| 9 | 13 | 7 | 53.85 | 5 | 38.46 | 1 | 7.69 | 0 | 0.00 |
| 10 | 50 | 34 | 68.00 | 15 | 30.00 | 0 | 0.00 | 1 | 2.00 |
| 11 | 1 | 0 | 0.00 | 0 | 0.00 | 1 | 100 | 0 | 0.00 |
| 12 | 1 | 0 | 0.00 | 0 | 0.00 | 1 | 100 | 0 | 0.00 |
| All other | 189 | 105 | 55.56 | 84 | 44.44 | 0 | 0.00 | 0 | 0.00 |
| Total | 386 | 206 | 53.37 | 164 | 42.49 | 13 | 3.37 | 3 | 0.78 |
| Parameter (Reference Range) | GLDH (<13 U/L) | GGT (<30 U/L) | Bile acids (<20 umol/L) | Albumin (2.4–4.5 g/dl) | ||||
|---|---|---|---|---|---|---|---|---|
| EqHV RT-qPCR Status | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative |
| n | 16 | 45 | 16 | 45 | 16 | 45 | 16 | 45 |
| Range (min–max) | 1.81–67.45 | 1.44–42.54 | 4–61 | 1–42 | 3–19 | 2–11 | 2.69–3.25 | 1.99–4.41 |
| Normal distribution | No | No | No | No | No | No | Yes | Yes |
| Median | 4.84 | 2.78 | 14.5 | 12 | 5 | 5 | 3.04 | 2.98 |
| Mean | 12.98 | 4.83 | 16.13 | 13.33 | 5.81 | 5.53 | 3.03 | 3.04 |
| Standard deviation | 20.27 | 6.83 | 13.27 | 7.92 | 3.94 | 2.16 | 0.16 | 0.52 |
| Parametric/Nonparametric test | The Mann–Whitney U test | The Mann–Whitney U test | The Mann–Whitney U test | Independent samples t-test | ||||
| p-value | p = 0.013 * | p = 0.434 | p = 0.659 | p = 0.855 | ||||
| Isolate | Accession Numbers | ||
|---|---|---|---|
| 5‘UTR | NS3 | NS5B | |
| N40-17 | MN475754 | MN475766 | MN475773 |
| N87-17 | MN475755 | MN475767 | MN475774 |
| N107-17 | MN475756 | MN475768 | MN475775 |
| N147-17 | MN475757 | MN475769 | MN475776 |
| N154-17 | MN475758 | MN475770 | MN475777 |
| N201-17 | MN475759 | n.a. | MN475778 |
| N234-17 | MN475760 | MN475771 | MN475779 |
| N235-17 | MN475761 | MN475772 | MN475780 |
| N265-17 | MN475762 | n.a. | MN475781 |
| N351-17 | MN475763 | n.a. | MN475782 |
| N364-17 | MN475764 | n.a. | MN475783 |
| N403-17 | MN475765 | n.a. | n.a. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badenhorst, M.; de Heus, P.; Auer, A.; Rümenapf, T.; Tegtmeyer, B.; Kolodziejek, J.; Nowotny, N.; Steinmann, E.; Cavalleri, J.-M.V. No Evidence of Mosquito Involvement in the Transmission of Equine Hepacivirus (Flaviviridae) in an Epidemiological Survey of Austrian Horses. Viruses 2019, 11, 1014. https://doi.org/10.3390/v11111014
Badenhorst M, de Heus P, Auer A, Rümenapf T, Tegtmeyer B, Kolodziejek J, Nowotny N, Steinmann E, Cavalleri J-MV. No Evidence of Mosquito Involvement in the Transmission of Equine Hepacivirus (Flaviviridae) in an Epidemiological Survey of Austrian Horses. Viruses. 2019; 11(11):1014. https://doi.org/10.3390/v11111014
Chicago/Turabian StyleBadenhorst, Marcha, Phebe de Heus, Angelika Auer, Till Rümenapf, Birthe Tegtmeyer, Jolanta Kolodziejek, Norbert Nowotny, Eike Steinmann, and Jessika-M.V. Cavalleri. 2019. "No Evidence of Mosquito Involvement in the Transmission of Equine Hepacivirus (Flaviviridae) in an Epidemiological Survey of Austrian Horses" Viruses 11, no. 11: 1014. https://doi.org/10.3390/v11111014
APA StyleBadenhorst, M., de Heus, P., Auer, A., Rümenapf, T., Tegtmeyer, B., Kolodziejek, J., Nowotny, N., Steinmann, E., & Cavalleri, J.-M. V. (2019). No Evidence of Mosquito Involvement in the Transmission of Equine Hepacivirus (Flaviviridae) in an Epidemiological Survey of Austrian Horses. Viruses, 11(11), 1014. https://doi.org/10.3390/v11111014






