Viruses in Horses with Neurologic and Respiratory Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Samples
2.2. Viral Metagenomics
2.3. Generation of Full Genomes of Novel Horse Parvovirus
2.4. Real-Time PCR
2.5. Phylogenetic Analysis
3. Results
3.1. Viral Metagenomics
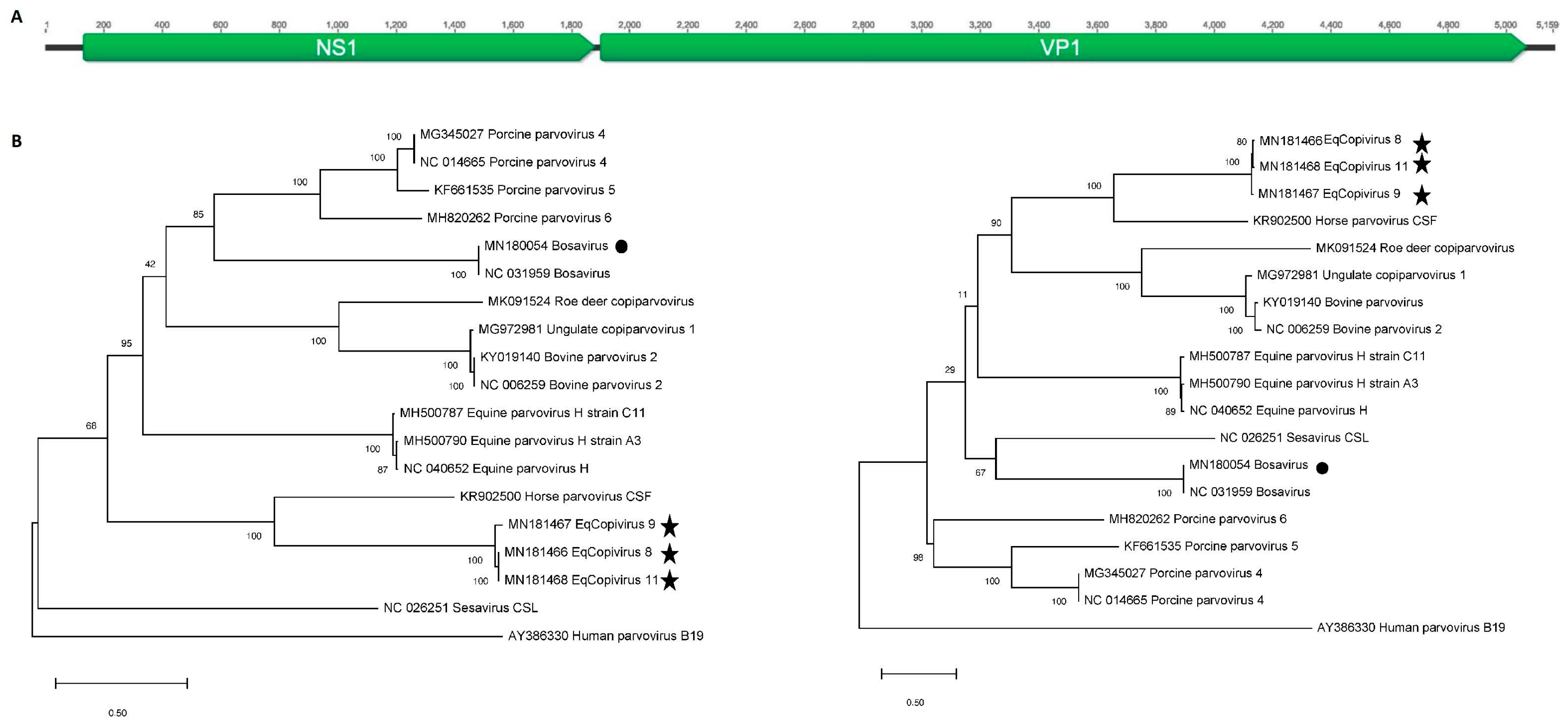
3.2. Generation of Near-Full Length Genomes of Novel Equine Copiparvovirus (Eqcopivirus)
3.3. Real-Time PCR Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Horse Population Report. Food and Agriculture Organization (FAO) of the United Nations. Available online: http://faostat.fao.org/fastat/serviet (accessed on 28 August 2019).
- Divers, T.J.; Tennant, B.C.; Kumar, A.; McDonough, S.; Cullen, J.; Bhuva, N.; Jain, K.; Chauhan, L.S.; Scheel, T.K.H.; Lipkin, W.I. , et al. New parvovirus associated with serum hepatitis in horses after inoculation of common biological product. Emerg. Infect. Dis 2018, 24, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Gilkerson, J.R.; Bailey, K.E.; Diaz-Mendez, A.; Hartley, C.A. Update on viral diseases of the equine respiratory tract. Vet. Clin. North. Am. Equine Pract 2015, 31, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Manuja, A.; Gulati, B.R.; Virmani, N.; Tripathi, B.N. Zoonotic viral diseases of equines and their impact on human and animal health. Open Virol. J. 2018, 12, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Middleton, D. Hendra virus. Vet. Clin. North. Am. Equine Pract. 2014, 30, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Vin, R.; Leutenegger, C.M.; Mittel, L.D.; Divers, T.J. Enteric coronavirus infection in adult horses. Vet. J. 2018, 231, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Timoney, P.J.; McCollum, W.H. Equine viral arteritis. Vet. Clin. North. Am. Equine Pract 1993, 9, 295–309. [Google Scholar] [CrossRef]
- Nowotny, N.; Kolodziejek, J.; Jehle, C.O.; Suchy, A.; Staeheli, P.; Schwemmle, M. Isolation and characterization of a new subtype of borna disease virus. J. Virol. 2000, 74, 5655–5658. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.E.; Baylis, M.; Archer, D.; Daly, J.M. The challenges posed by equine arboviruses. Equine Vet. J. 2018, 50, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Chandriani, S.; Skewes-Cox, P.; Zhong, W.; Ganem, D.E.; Divers, T.J.; Van Blaricum, A.J.; Tennant, B.C.; Kistler, A.L. Identification of a previously undescribed divergent virus from the flaviviridae family in an outbreak of equine serum hepatitis. Proc. Nat. Acad. Sci. USA 2013, 110, E1407–E1415. [Google Scholar] [CrossRef]
- Tomlinson, J.E.; Tennant, B.C.; Struzyna, A.; Mrad, D.; Browne, N.; Whelchel, D.; Johnson, P.J.; Jamieson, C.; Lohr, C.V.; Bildfell, R.; et al. Viral testing of 10 cases of theiler’s disease and 37 in-contact horses in the absence of equine biologic product administration: A prospective study (2014-2018). J. Vet. Intern. Med. 2019, 33, 258–265. [Google Scholar] [CrossRef]
- Tomlinson, J.E.; Kapoor, A.; Kumar, A.; Tennant, B.C.; Laverack, M.A.; Beard, L.; Delph, K.; Davis, E.; Schott Ii, H.; Lascola, K.; et al. Viral testing of 18 consecutive cases of equine serum hepatitis: A prospective study (2014-2018). J. Vet. Intern. Med. 2019, 33, 251–257. [Google Scholar] [CrossRef]
- Lu, G.; Sun, L.; Ou, J.; Xu, H.; Wu, L.; Li, S. Identification and genetic characterization of a novel parvovirus associated with serum hepatitis in horses in china. Emerg. Microbes. Infect. 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Simmonds, P.; Gerold, G.; Qaisar, N.; Jain, K.; Henriquez, J.A.; Firth, C.; Hirschberg, D.L.; Rice, C.M.; Shields, S.; et al. Characterization of a canine homolog of hepatitis c virus. Proc. Nat. Acad. Sci. USA 2011, 108, 11608–11613. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.; Kapoor, A.; Schneider, B.S.; Wolfe, N.D.; Culshaw, G.; Corcoran, B.; Durham, A.E.; Burden, F.; McGorum, B.C.; Simmonds, P. Viraemic frequencies and seroprevalence of non-primate hepacivirus and equine pegiviruses in horses and other mammalian species. J. Gen. Virol. 2014, 95, 1701–1711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lyons, S.; Kapoor, A.; Sharp, C.; Schneider, B.S.; Wolfe, N.D.; Culshaw, G.; Corcoran, B.; McGorum, B.C.; Simmonds, P. Nonprimate hepaciviruses in domestic horses, united kingdom. Emerg. Infect. Dis. 2012, 18, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.D.; Dubovi, E.J.; Simmonds, P.; Medina, J.L.; Henriquez, J.A.; Mishra, N.; Wagner, J.; Tokarz, R.; Cullen, J.M.; Iadarola, M.J.; et al. Serology-enabled discovery of genetically diverse hepaciviruses in a new host. J. Virol. 2012, 86, 6171–6178. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.H.; Quan, P.L.; Briese, T.; Street, C.; Jabado, O.; Conlan, S.; Ali Khan, S.; Verdugo, D.; Hossain, M.J.; Hutchison, S.K.; et al. Identification of gbv-d, a novel gb-like flavivirus from old world frugivorous bats (pteropus giganteus) in bangladesh. PLoS Path. 2010, 6, e1000972. [Google Scholar] [CrossRef]
- Kapoor, A.; Simmonds, P.; Cullen, J.M.; Scheel, T.K.; Medina, J.L.; Giannitti, F.; Nishiuchi, E.; Brock, K.V.; Burbelo, P.D.; Rice, C.M.; et al. Identification of a pegivirus (gb virus-like virus) that infects horses. J. Virol. 2013, 87, 7185–7190. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Giannitti, F.; Low, J.; Keyes, C.; Ullmann, L.S.; Deng, X.; Aleman, M.; Pesavento, P.A.; Pusterla, N.; Delwart, E. Exploring the virome of diseased horses. J. Gen. Virol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Paim, W.P.; Weber, M.N.; Cibulski, S.P.; da Silva, M.S.; Puhl, D.E.; Budaszewski, R.F.; Varela, A.P.M.; Mayer, F.Q.; Canal, C.W. Characterization of the viral genomes present in commercial batches of horse serum obtained by high-throughput sequencing. Biologicals 2019. [Google Scholar] [CrossRef]
- Victoria, J.G.; Kapoor, A.; Li, L.; Blinkova, O.; Slikas, B.; Wang, C.; Naeem, A.; Zaidi, S.; Delwart, E. Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis. J. Virol. 2009, 83, 4642–4651. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Deng, X.; Mee, E.T.; Collot-Teixeira, S.; Anderson, R.; Schepelmann, S.; Minor, P.D.; Delwart, E. Comparing viral metagenomics methods using a highly multiplexed human viral pathogens reagent. J. Virol. Methods 2015, 213, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Naccache, S.N.; Ng, T.; Federman, S.; Li, L.; Chiu, C.Y.; Delwart, E.L. An ensemble strategy that significantly improves de novo assembly of microbial genomes from metagenomic next-generation sequencing data. Nucl. Acids Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Le, S.Q.; Gascuel, O. An improved general amino acid replacement matrix. Mol. Biol. Evol. 2008, 25, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Kapusinszky, B.; Yugo, D.M.; Phan, T.G.; Deng, X.; Kanevsky, I.; Opriessnig, T.; Woolums, A.R.; Hurley, D.J.; Meng, X.J.; et al. Virome of us bovine calf serum. Biologicals 2017, 46, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Tattersall, P. Parvoviruses: Small does not mean simple. An. Rev. Virol 2014, 1, 517–537. [Google Scholar] [CrossRef]
- Mietzsch, M.; Penzes, J.J.; Agbandje-McKenna, M. Twenty-five years of structural parvovirology. Viruses 2019, 11, 362. [Google Scholar] [CrossRef]
- Zadori, Z.; Szelei, J.; Lacoste, M.C.; Li, Y.; Gariepy, S.; Raymond, P.; Allaire, M.; Nabi, I.R.; Tijssen, P. A viral phospholipase a2 is required for parvovirus infectivity. Develop. Cell 2001, 1, 291–302. [Google Scholar] [CrossRef]
- Zhang, L.; Dailey, P.J.; He, T.; Gettie, A.; Bonhoeffer, S.; Perelson, A.S.; Ho, D.D. Rapid clearance of simian immunodeficiency virus particles from plasma of rhesus macaques. J. Virol 1999, 73, 855–860. [Google Scholar]
- Ramratnam, B.; Bonhoeffer, S.; Binley, J.; Hurley, A.; Zhang, L.; Mittler, J.E.; Markowitz, M.; Moore, J.P.; Perelson, A.S.; Ho, D.D. Rapid production and clearance of hiv-1 and hepatitis c virus assessed by large volume plasma apheresis. Lancet 1999, 354, 1782–1785. [Google Scholar] [CrossRef]
- Zhang, L.; Dailey, P.J.; Gettie, A.; Blanchard, J.; Ho, D.D. The liver is a major organ for clearing simian immunodeficiency virus in rhesus monkeys. J. Virol. 2002, 76, 5271–5273. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Penzes, J.J.; et al. Ictv virus taxonomy profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef] [PubMed]
| Disease and Sample Types | Total Reads | Equine Hepacivirus | Equine Pegivirus D | Equid Gamma Herpesvirus2 | Equid Gammaherpes Virus5 | Horse Parvovirus-CSF | Novel Parvovirus: Eqcopivirus | Bosavirus |
|---|---|---|---|---|---|---|---|---|
| Neuro Pool1 Plasma | 2,575,048 | 1023 | 17,058 | |||||
| Neuro Pool4 CSF | 1,106,824 | |||||||
| Neuro Pool2 Plasma | 1,612,796 | 7 | ||||||
| Neuro Pool5 CSF | 1,028,398 | |||||||
| Neuro Pool3 Plasma | 2,080,966 | |||||||
| Neuro Pool6 CSF | 1,057,992 | |||||||
| Respiratory Pool7 Plasma | 1,509,792 | 317 | 36,069 | |||||
| Respiratory Pool10 Swab | 622,376 | 1719 | ||||||
| Respiratory Pool8 Plasma | 603,470 | 1087 | 16,355 | |||||
| Respiratory Pool11 Swab | 949,260 | 120 | 25 | 880 | ||||
| Respiratory Pool9 Plasma | 1,520,736 | |||||||
| Respiratory Pool12 Swab | 629,274 | 395 |
| Sample Type | Pool | Equine Hepacivirus | EqPV-H | Eqcopivirus | Horse Parvovirus-CSF | Bosavirus | |
|---|---|---|---|---|---|---|---|
| Neurological Signs | Plasma 1 | Pool1 | 30.32 | ||||
| Plasma 2 | |||||||
| Plasma 3 | |||||||
| Plasma 4 | 27.7 | 20 | |||||
| Plasma 5 | Pool2 | ||||||
| Plasma 6 | 34.65 | 36.73 | |||||
| Plasma 7 | 23.6 | ||||||
| Plasma 8 | |||||||
| Plasma 9 | Pool3 | ||||||
| Plasma 10 | 33.82 | ||||||
| Plasma 11 | |||||||
| Plasma 12 | |||||||
| Plasma 13 | |||||||
| CSF 1 | Pool4 | ||||||
| CSF 2 | |||||||
| CSF 3 | |||||||
| CSF 4 | |||||||
| CSF 5 | Pool5 | ||||||
| CSF 6 | |||||||
| CSF 7 | |||||||
| CSF 8 | |||||||
| CSF 9 | Pool6 | 30 | |||||
| CSF 10 | |||||||
| CSF 11 | |||||||
| CSF 12 | 36.5 | ||||||
| CSF 13 | |||||||
| Respiratory Signs | Plasma 1 | Pool7 | 37.78 | ||||
| Plasma 2 | |||||||
| Plasma 3 | |||||||
| Plasma 4 | 34 | ||||||
| Plasma 5 | 27.53 | ||||||
| Plasma 6 | Pool8 | ||||||
| Plasma 7 | 30.16 | ||||||
| Plasma 8 | |||||||
| Plasma 9 | 34.6 | 34.01 | 21 | ||||
| Plasma 10 | Pool9 | ||||||
| Plasma 11 | |||||||
| Plasma 12 | |||||||
| Plasma 13 | |||||||
| Plasma 14 | |||||||
| Swab 1 | Pool10 | 26.85 | |||||
| Swab 2 | 25.65 | ||||||
| Swab 3 | 25.28 | 26.4 | |||||
| Swab 4 | 36.7 | 26.82 | |||||
| Swab 6 | Pool11 | 25.02 | |||||
| Swab 7 | 32.62 | 27.75 | |||||
| Swab 8 | 25.57 | ||||||
| Swab 9 | 26.24 | ||||||
| Swab 10 | Pool12 | 21.2 | 24.92 | ||||
| Swab 11 | 23.1 | 23.37 | |||||
| Swab 12 | 26.63 | ||||||
| Swab 13 | 32.41 | 23.29 | |||||
| Swab 14 | 37.2 | 26 | 25.3 | ||||
| Healthy Control Group | Plasma 1 | ||||||
| Plasma 2 | 16.8 | ||||||
| Plasma 3 | 21.8 | 37.3 | |||||
| Plasma 4 | 26.5 | ||||||
| Plasma 5 | 36.5 | ||||||
| Plasma 6 | |||||||
| Plasma 7 | |||||||
| Plasma 8 | |||||||
| Plasma 9 | |||||||
| Plasma 10 | |||||||
| Plasma 11 | 28.08 | ||||||
| Plasma 12 | |||||||
| Plasma 13 | |||||||
| Plasma 14 | 30.21 | ||||||
| Plasma 15 | |||||||
| Plasma 16 | |||||||
| Plasma 17 | 26.08 | ||||||
| Plasma 18 | |||||||
| Plasma 19 | 23.02 | 36.9 | |||||
| Plasma 20 | |||||||
| Plasma 21 | |||||||
| Plasma 22 | |||||||
| Plasma 23 | 23.53 | ||||||
| Plasma 24 | |||||||
| Plasma 25 | 25.11 | ||||||
| Plasma 26 | |||||||
| Plasma 27 | 33.83 | ||||||
| Plasma 28 | |||||||
| Plasma 29 | |||||||
| Plasma 30 | |||||||
| Plasma 31 | 33.99 | 30.09 | |||||
| Plasma 32 | 37.93 | 26.34 | 30.1 | ||||
| Plasma 33 | |||||||
| Plasma 34 | |||||||
| Plasma 35 | |||||||
| Plasma 36 | 33.22 | ||||||
| Plasma 37 | |||||||
| Plasma 38 | |||||||
| Plasma 39 | |||||||
| Plasma 40 | 34.69 | 28.63 | |||||
| Plasma 41 | 30.12 |
| Neuro Plasma n = 13 | Neuro CSF n = 13 | Respiratory Plasma n = 14 | Respiratory Swab n = 13 | Healthy Control Plasma n = 41 | Total Samples n = 94 | P Value = Plasma Neurological Versus Healthy | P Value = Plasma Respiratory Versus Healthy | |
|---|---|---|---|---|---|---|---|---|
| Equine flavivirus | 3 (23%) | 0 | 0 | 2 (15%) | 6 (15%) | 11 (12%) | 0.32 | 0.67 |
| EqPV-H | 2 (15%) | 0 | 2 (15%) | 0 | 7 (17%) | 11 (12%) | 1 | 1 |
| Eqcopivirus | 1 (7%) | 1 (7%) | 4 (30%) | 3 (23%) | 7 (17%) | 15 (16%) | 0.443 | 0.663 |
| Horse parvovirus-CSF | 1 (7%) | 1 (7%) | 1 (7%) | 3 (23%) | 2 (5%) | 8 (8.5%) | 1 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altan, E.; Li, Y.; Sabino-Santos Jr, G.; Sawaswong, V.; Barnum, S.; Pusterla, N.; Deng, X.; Delwart, E. Viruses in Horses with Neurologic and Respiratory Diseases. Viruses 2019, 11, 942. https://doi.org/10.3390/v11100942
Altan E, Li Y, Sabino-Santos Jr G, Sawaswong V, Barnum S, Pusterla N, Deng X, Delwart E. Viruses in Horses with Neurologic and Respiratory Diseases. Viruses. 2019; 11(10):942. https://doi.org/10.3390/v11100942
Chicago/Turabian StyleAltan, Eda, Yanpeng Li, Gilberto Sabino-Santos Jr, Vorthon Sawaswong, Samantha Barnum, Nicola Pusterla, Xutao Deng, and Eric Delwart. 2019. "Viruses in Horses with Neurologic and Respiratory Diseases" Viruses 11, no. 10: 942. https://doi.org/10.3390/v11100942
APA StyleAltan, E., Li, Y., Sabino-Santos Jr, G., Sawaswong, V., Barnum, S., Pusterla, N., Deng, X., & Delwart, E. (2019). Viruses in Horses with Neurologic and Respiratory Diseases. Viruses, 11(10), 942. https://doi.org/10.3390/v11100942






