Characterization of the Humoral Immune Response Induced after Infection with Atypical Porcine Pestivirus (APPV)
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of Samples
2.2. RNA Purification and Real-Time RT-PCR
2.3. Conventional RT-PCR and Nucleotide Sequencing
2.4. Indirect APPV-Specific ELISA Assays
2.5. Cells and APPV Stock Used for Virus Neutralization Tests
2.6. Neutralizing Capacity of APPV-Specific Antibodies
3. Results
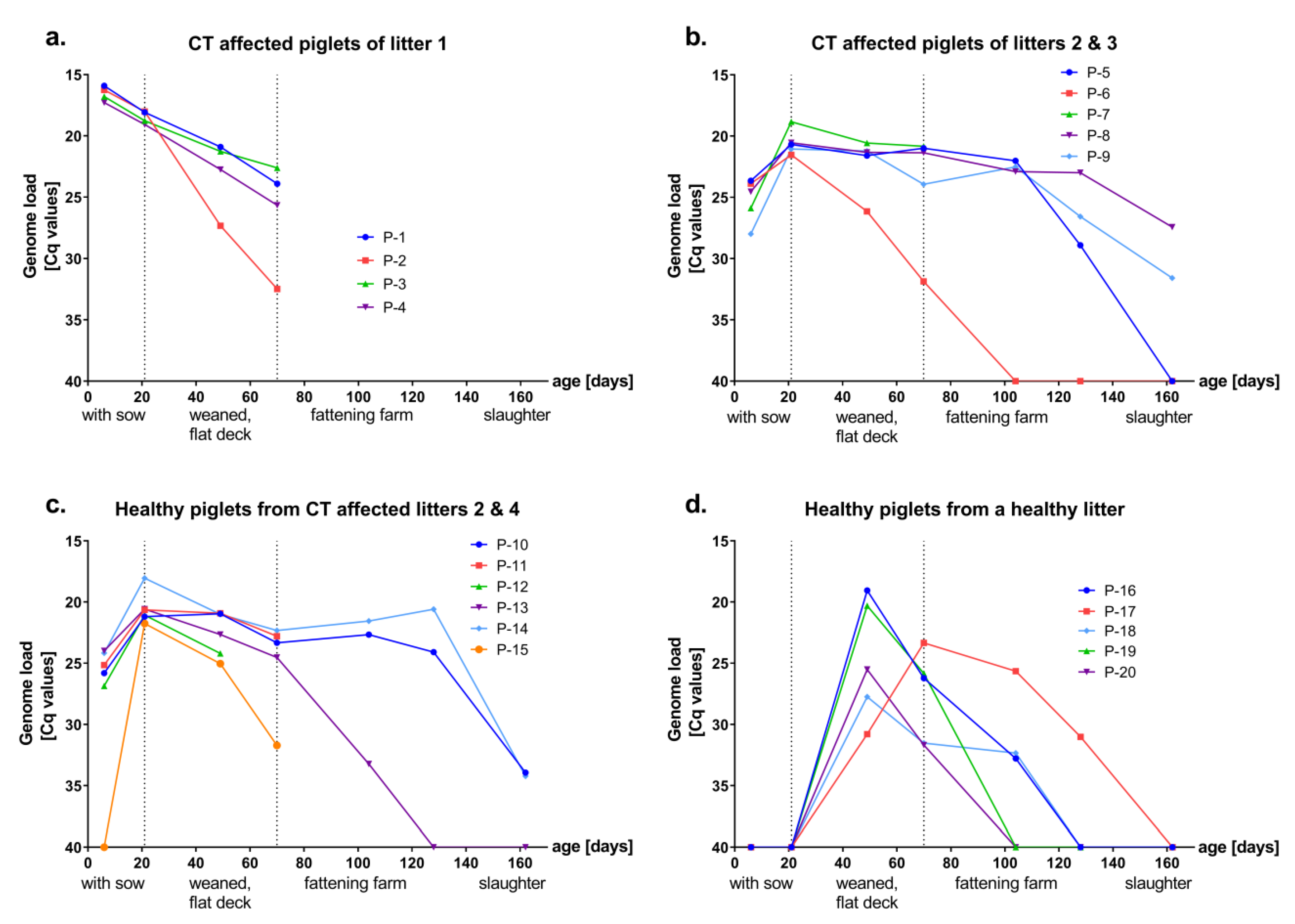
3.1. Presence and Kinetics of APPV Genome in Healthy and CT Affected Piglets
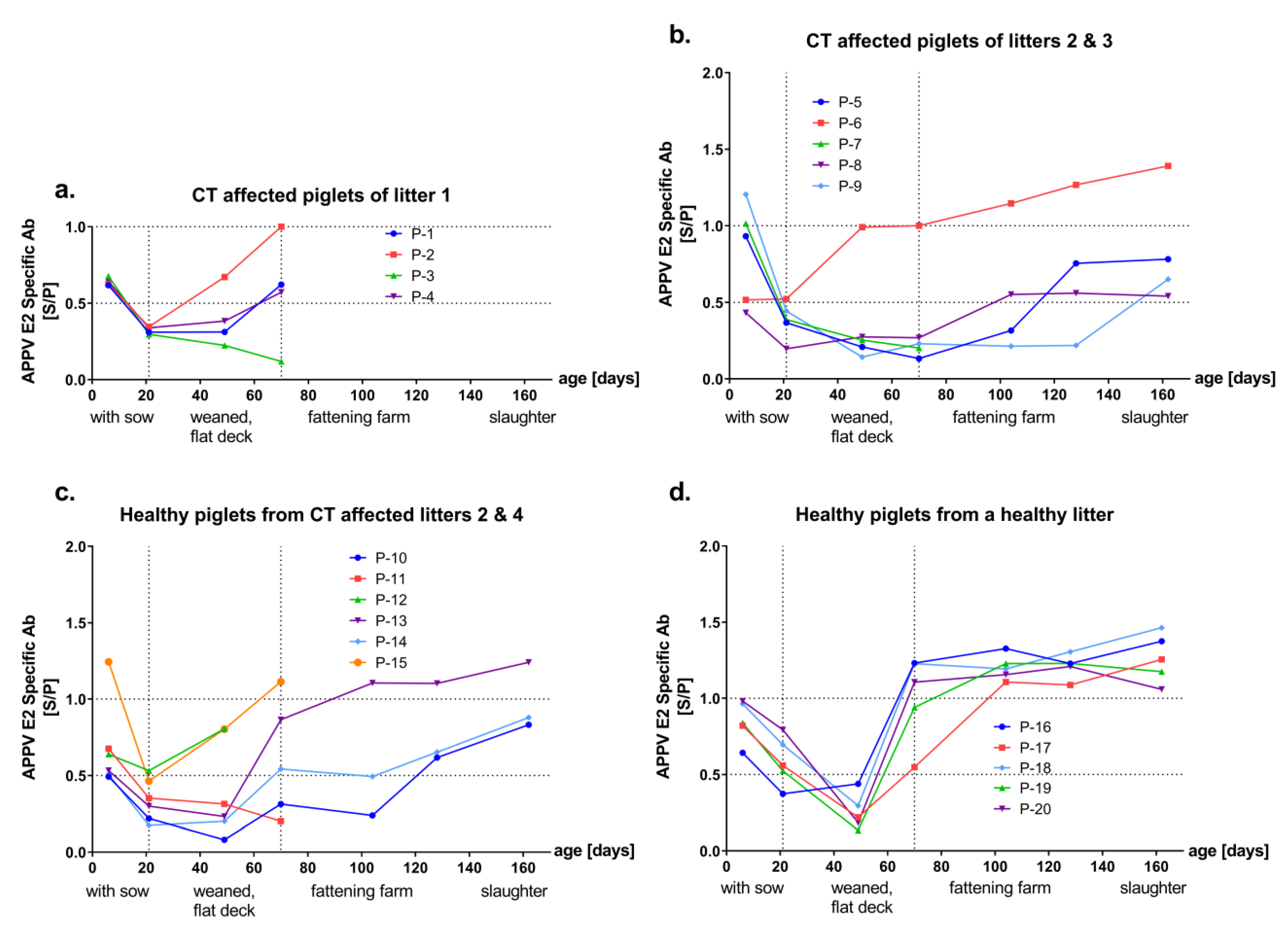
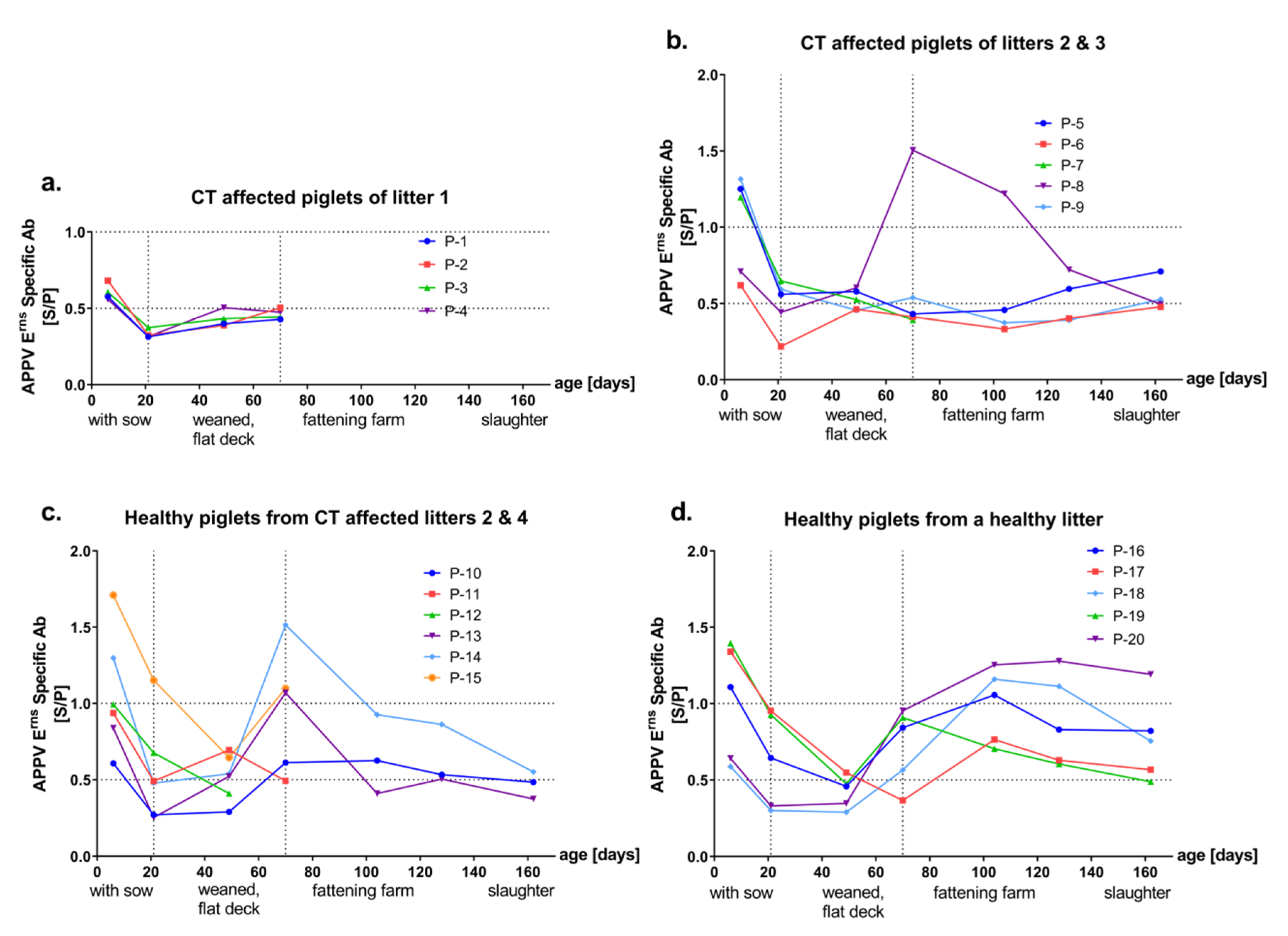
3.2. Detection of Antibodies against the Envelope Proteins Erns and E2 of APPV
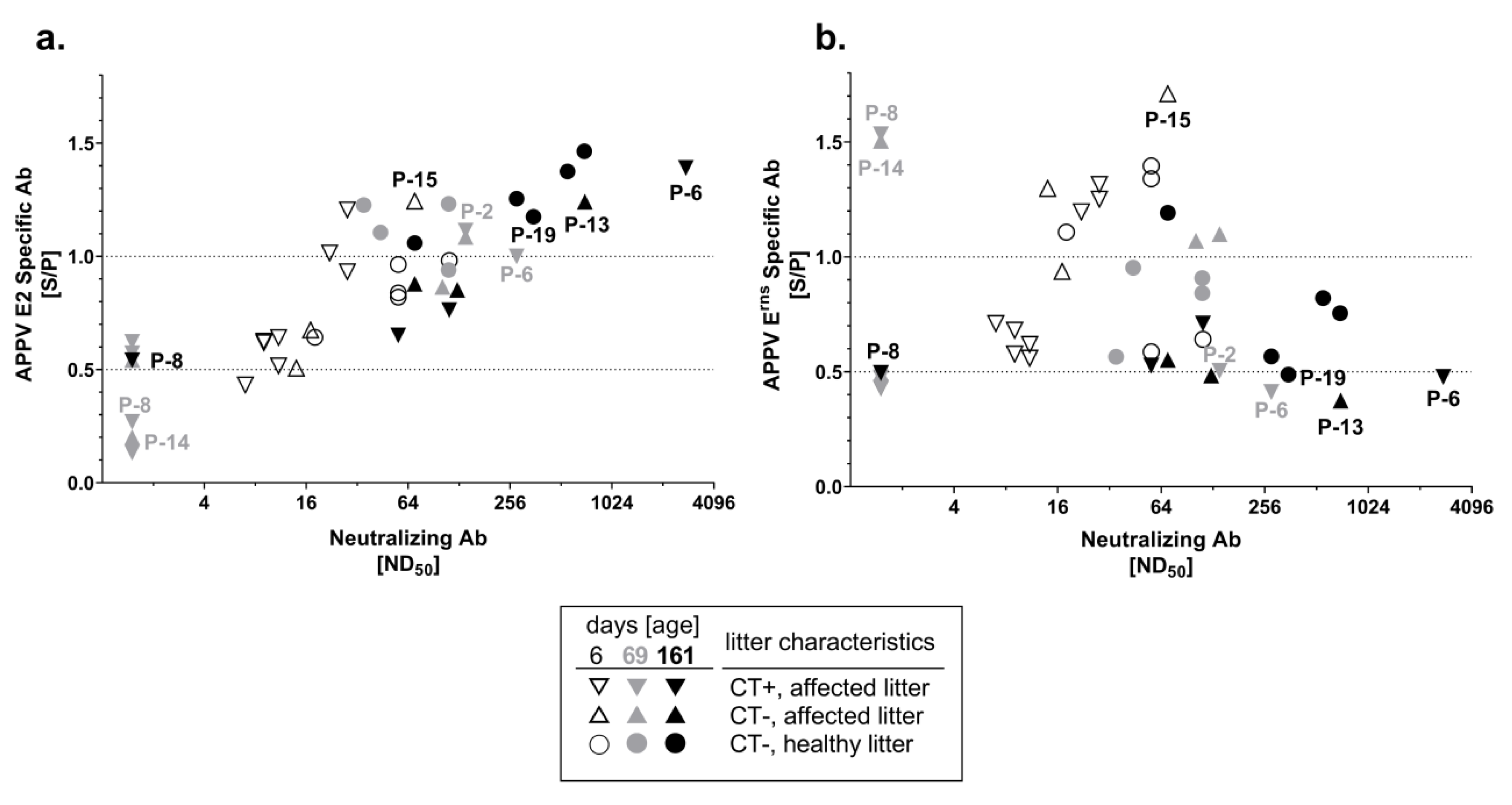
3.3. Neutralizing Capacity of APPV-Specific Antibodies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tautz, N.; Tews, B.A.; Meyers, G. The molecular biology of pestiviruses. Adv. Virus Res. 2015, 93, 47–160. [Google Scholar] [PubMed]
- Smith, D.B.; Meyers, G.; Bukh, J.; Gould, E.A.; Monath, T.; Scott Muerhoff, A.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.T.; Simmonds, P.; et al. Proposed revision to the taxonomy of the genus pestivirus, family flaviviridae. J. Gen. Virol. 2017, 98, 2106–2112. [Google Scholar] [CrossRef]
- Hause, B.M.; Collin, E.A.; Peddireddi, L.; Yuan, F.; Chen, Z.; Hesse, R.A.; Gauger, P.C.; Clement, T.; Fang, Y.; Anderson, G. Discovery of a novel putative atypical porcine pestivirus in pigs in the USA. J. Gen. Virol. 2015, 96, 2994–2998. [Google Scholar] [CrossRef] [PubMed]
- Arruda, B.L.; Arruda, P.H.; Magstadt, D.R.; Schwartz, K.J.; Dohlman, T.; Schleining, J.A.; Patterson, A.R.; Visek, C.A.; Victoria, J.G. Identification of a divergent lineage porcine pestivirus in nursing piglets with congenital tremors and reproduction of disease following experimental inoculation. PLoS ONE 2016, 11, e0150104. [Google Scholar] [CrossRef] [PubMed]
- De Groof, A.; Deijs, M.; Guelen, L.; van Grinsven, L.; van Os-Galdos, L.; Vogels, W.; Derks, C.; Cruijsen, T.; Geurts, V.; Vrijenhoek, M.; et al. Atypical porcine pestivirus: A possible cause of congenital tremor type a-ii in newborn piglets. Viruses 2016, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Hansmann, F.; Baechlein, C.; Fischer, N.; Alawi, M.; Grundhoff, A.; Derking, S.; Tenhundfeld, J.; Pfankuche, V.M.; Herder, V.; et al. Presence of atypical porcine pestivirus (appv) genomes in newborn piglets correlates with congenital tremor. Sci. Rep. 2016, 6, 27735. [Google Scholar] [CrossRef] [PubMed]
- Gatto, I.R.H.; Harmon, K.; Bradner, L.; Silva, P.; Linhares, D.C.L.; Arruda, P.H.; de Oliveira, L.G.; Arruda, B.L. Detection of atypical porcine pestivirus in brazil in the central nervous system of suckling piglets with congenital tremor. Transbound. Emerg. Dis. 2018, 65, 375–380. [Google Scholar] [CrossRef]
- Possatti, F.; Headley, S.A.; Leme, R.A.; Dall Agnol, A.M.; Zotti, E.; de Oliveira, T.E.S.; Alfieri, A.F.; Alfieri, A.A. Viruses associated with congenital tremor and high lethality in piglets. Transbound. Emerg. Dis. 2018, 65, 331–335. [Google Scholar] [CrossRef]
- Schwarz, L.; Riedel, C.; Hogler, S.; Sinn, L.J.; Voglmayr, T.; Wochtl, B.; Dinhopl, N.; Rebel-Bauder, B.; Weissenbock, H.; Ladinig, A.; et al. Congenital infection with atypical porcine pestivirus (appv) is associated with disease and viral persistence. Vet. Res. 2017, 48, 1. [Google Scholar] [CrossRef]
- Shen, H.; Liu, X.; Zhang, P.; Wang, L.; Liu, Y.; Zhang, L.; Liang, P.; Song, C. Identification and characterization of atypical porcine pestivirus genomes in newborn piglets with congenital tremor in china. J. Vet. Sci. 2018, 19, 468–471. [Google Scholar] [CrossRef]
- Pan, S.; Yan, Y.; Shi, K.; Wang, M.; Mou, C.; Chen, Z. Molecular characterization of two novel atypical porcine pestivirus (appv) strains from piglets with congenital tremor in china. Transbound. Emerg. Dis. 2018, 66, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Possatti, F.; de Oliveira, T.E.S.; Leme, R.A.; Zotti, E.; Dall Agnol, A.M.; Alfieri, A.F.; Headley, S.A.; Alfieri, A.A. Pathologic and molecular findings associated with atypical porcine pestivirus infection in newborn piglets. Vet. Microbiol. 2018, 227, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Wernike, K.; Drager, C.; Hoper, D.; Pohlmann, A.; Bergermann, C.; Schroder, C.; Klinkhammer, S.; Blome, S.; Hoffmann, B. High prevalence of highly variable atypical porcine pestiviruses found in germany. Transbound. Emerg. Dis. 2017, 64, e22–e26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wu, K.; Liu, J.; Ge, S.; Xiao, Y.; Shang, Y.; Ning, Z. Identification of atypical porcine pestivirus infection in swine herds in china. Transbound. Emerg. Dis. 2017, 64, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wen, W.; Hao, G.; Hu, Y.; Chen, H.; Qian, P.; Li, X. Phylogenetic and genomic characterization of a novel atypical porcine pestivirus in china. Transbound. Emerg. Dis. 2017, 65, e202–e204. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Gonzalez, S.; Canturri, A.; Perez-Simo, M.; Bohorquez, J.A.; Rosell, R.; Cabezon, O.; Segales, J.; Domingo, M.; Ganges, L. First report of the novel atypical porcine pestivirus in spain and a retrospective study. Transbound. Emerg. Dis. 2017, 64, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Mosena, A.C.S.; Weber, M.N.; da Cruz, R.A.S.; Cibulski, S.P.; da Silva, M.S.; Puhl, D.E.; Hammerschmitt, M.E.; Takeuti, K.L.; Driemeier, D.; de Barcellos, D.; et al. Presence of atypical porcine pestivirus (appv) in brazilian pigs. Transbound. Emerg. Dis. 2017, 65, 22–26. [Google Scholar] [CrossRef]
- Gatto, I.R.H.; Arruda, P.H.; Visek, C.A.; Victoria, J.G.; Patterson, A.R.; Krull, A.C.; Schwartz, K.J.; de Oliveira, L.G.; Arruda, B.L. Detection of atypical porcine pestivirus in semen from commercial boar studs in the united states. Transbound. Emerg. Dis. 2017, 65, e339–e343. [Google Scholar] [CrossRef]
- Postel, A.; Meyer, D.; Cagatay, G.N.; Feliziani, F.; De Mia, G.M.; Fischer, N.; Grundhoff, A.; Milicevic, V.; Deng, M.C.; Chang, C.Y.; et al. High abundance and genetic variability of atypical porcine pestivirus in pigs from europe and asia. Emerg. Infect. Dis. 2017, 23, 2104–2107. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Z.; Zhang, W.; Deng, S. Complete genome sequence of an atypical porcine pestivirus isolated from jiangxi province, china. Genome Announc. 2018, 6, e00439-18. [Google Scholar] [CrossRef]
- Denes, L.; Biksi, I.; Albert, M.; Szeredi, L.; Knapp, D.G.; Szilasi, A.; Balint, A.; Balka, G. Detection and phylogenetic characterization of atypical porcine pestivirus strains in hungary. Transbound. Emerg. Dis. 2018, 65, 2039–2042. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Han, Z.; Li, J.; Huang, Y.; Yang, J.; Ding, H.; Zhang, J.; Zhu, M.; Zhang, Y.; Liao, J.; et al. Atypical porcine pestivirus as a novel type of pestivirus in pigs in china. Front. Microbiol. 2017, 8, 862. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yue, H.; Tang, C.; Ruan, W.; Zhou, Q.; Zhang, B. Prevalence and genome characteristics of atypical porcine pestivirus in southwest china. J. Gen. Virol. 2018, 100, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Cagatay, G.N.; Antos, A.; Meyer, D.; Maistrelli, C.; Keuling, O.; Becher, P.; Postel, A. Frequent infection of wild boar with atypical porcine pestivirus (appv). Transbound. Emerg. Dis. 2018, 65, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Colom-Cadena, A.; Ganges, L.; Munoz-Gonzalez, S.; Castillo-Contreras, R.; Bohorquez, J.A.; Rosell, R.; Segales, J.; Marco, I.; Cabezon, O. Atypical porcine pestivirus in wild boar (sus scrofa), spain. Vet. Rec. 2018, 183, 569. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.I.; Deng, M.C.; Huang, Y.L.; Chang, C.Y. Structures and functions of pestivirus glycoproteins: Not simply surface matters. Viruses 2015, 7, 3506–3529. [Google Scholar] [CrossRef] [PubMed]
- Konig, M.; Lengsfeld, T.; Pauly, T.; Stark, R.; Thiel, H.J. Classical swine fever virus: Independent induction of protective immunity by two structural glycoproteins. J. Virol. 1995, 69, 6479–6486. [Google Scholar]
- Weiland, E.; Stark, R.; Haas, B.; Rumenapf, T.; Meyers, G.; Thiel, H.J. Pestivirus glycoprotein which induces neutralizing antibodies forms part of a disulfide-linked heterodimer. J. Virol. 1990, 64, 3563–3569. [Google Scholar]
- Weiland, E.; Ahl, R.; Stark, R.; Weiland, F.; Thiel, H.J. A second envelope glycoprotein mediates neutralization of a pestivirus, hog cholera virus. J. Virol. 1992, 66, 3677–3682. [Google Scholar]
- Zhang, H.; Wen, W.; Hao, G.; Chen, H.; Qian, P.; Li, X. A subunit vaccine based on e2 protein of atypical porcine pestivirus induces th2-type immune response in mice. Viruses 2018, 10, 673. [Google Scholar] [CrossRef]
- Postel, A.; Meyer, D.; Petrov, A.; Becher, P. Recent emergence of a novel porcine pestivirus: Interference with classical swine fever diagnosis? Emerg. Microbes Infect. 2017, 6, e19. [Google Scholar] [CrossRef] [PubMed]
- European Commission, Commission Decision 2002/106/ec Approving a Diagnostic Manual Establishing Diagnostic Procedures, Sampling Methods and Criteria for Evaluation of the Laboratory Tests for the Confirmation of Classical Swine Fever. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:32002D0106 (accessed on 9 February 2002).
- Bourne, F.J. The immunoglobulin system of the suckling pig. Proc. Nutr. Soc. 1973, 32, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Moennig, V. Introduction to classical swine fever: Virus, disease and control policy. Vet. Microbiol. 2000, 73, 93–102. [Google Scholar] [CrossRef]
- Postel, A.; Austermann-Busch, S.; Petrov, A.; Moennig, V.; Becher, P. Epidemiology, diagnosis and control of classical swine fever: Recent developments and future challenges. Transbound. Emerg. Dis. 2018, 65, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Moennig, V.; Floegel-Niesmann, G.; Greiser-Wilke, I. Clinical signs and epidemiology of classical swine fever: A review of new knowledge. Vet. J. (Lond. Eng. 1997) 2003, 165, 11–20. [Google Scholar] [CrossRef]
- Meyer, D.; Loeffen, W.; Postel, A.; Fritsche, S.; Becher, P. Reduced specificity of e(rns) antibody elisas for samples from piglets with maternally derived antibodies induced by vaccination of sows with classical swine fever marker vaccine cp7_e2alf. Transbound. Emerg. Dis. 2018, 65, e505–e508. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Deng, M.C.; Wang, F.I.; Huang, C.C.; Chang, C.Y. The challenges of classical swine fever control: Modified live and e2 subunit vaccines. Virus Res. 2014, 179, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.; Peterhans, E. Pestiviruses. Ann. Rev. Anim. Biosci. 2014, 2, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Moennig, V.; Becher, P. Pestivirus control programs: How far have we come and where are we going? Anim. Health Res. Rev. 2015, 16, 83–87. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cagatay, G.N.; Meyer, D.; Wendt, M.; Becher, P.; Postel, A. Characterization of the Humoral Immune Response Induced after Infection with Atypical Porcine Pestivirus (APPV). Viruses 2019, 11, 880. https://doi.org/10.3390/v11100880
Cagatay GN, Meyer D, Wendt M, Becher P, Postel A. Characterization of the Humoral Immune Response Induced after Infection with Atypical Porcine Pestivirus (APPV). Viruses. 2019; 11(10):880. https://doi.org/10.3390/v11100880
Chicago/Turabian StyleCagatay, Gökce Nur, Denise Meyer, Michael Wendt, Paul Becher, and Alexander Postel. 2019. "Characterization of the Humoral Immune Response Induced after Infection with Atypical Porcine Pestivirus (APPV)" Viruses 11, no. 10: 880. https://doi.org/10.3390/v11100880
APA StyleCagatay, G. N., Meyer, D., Wendt, M., Becher, P., & Postel, A. (2019). Characterization of the Humoral Immune Response Induced after Infection with Atypical Porcine Pestivirus (APPV). Viruses, 11(10), 880. https://doi.org/10.3390/v11100880




