Insight into the Contribution and Disruption of Host Processes during HDV Replication
Abstract
1. Introduction
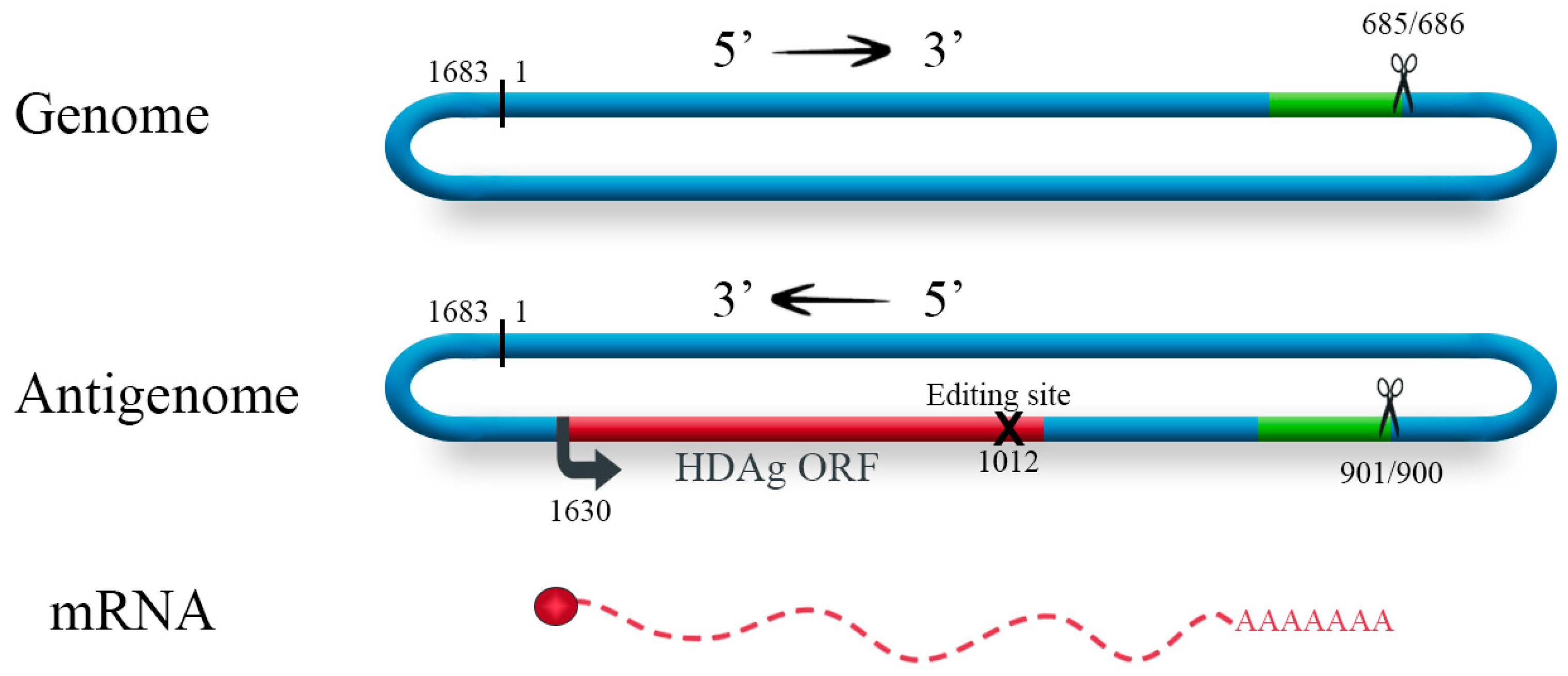
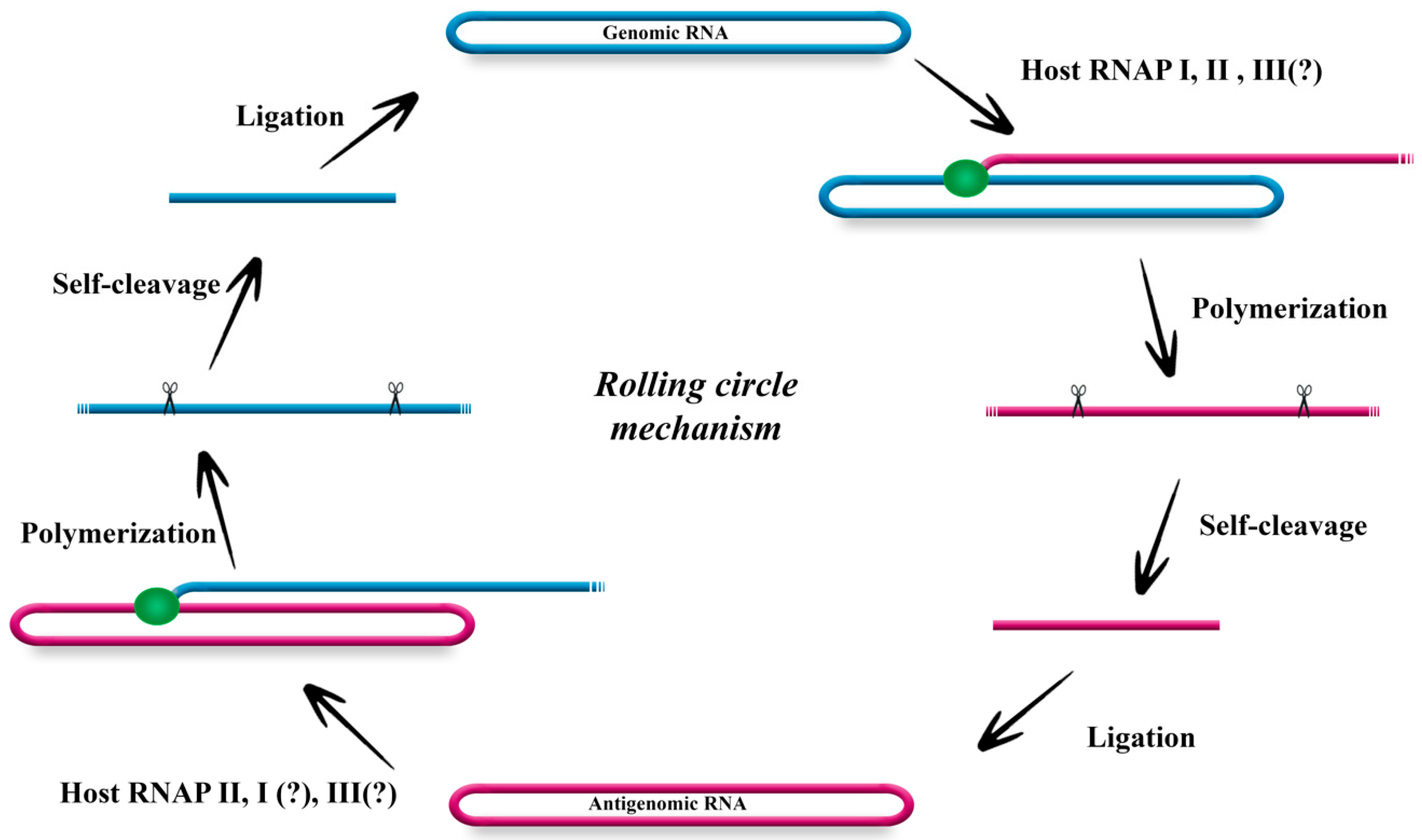
2. The Genome of HDV and Its Replication
3. Interaction with Host Cellular Proteins
4. How HDV Affects Its Host Cell
5. Challenges and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rizzetto, M.; Canese, M.G.; Aricò, S.; Crivelli, O.; Trepo, C.; Bonino, F.; Verme, G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 1977, 18, 997–1003. [Google Scholar] [CrossRef]
- Magnius, L.; Taylor, J.; Mason, W.S.; Sureau, C.; Dény, P.; Norder, H. Ictv Report Consortium ICTV Virus Taxonomy Profile: Deltavirus. J. Gen. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lempp, F.A.; Ni, Y.; Urban, S. Hepatitis delta virus: Insights into a peculiar pathogen and novel treatment options. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, B.; Yurdaydin, C.; Kabaçam, G.; Ratsch, B.A.; Zachou, K.; Bremer, B.; Dalekos, G.N.; Erhardt, A.; Tabak, F.; Yalcin, K.; et al. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology 2014. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Pelchat, M. Origin of hepatitis delta virus. Future Microbiol. 2010, 5, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Navabakhsh, B.; Mehrabi, N.; Estakhri, A.; Mohamadnejad, M.; Poustchi, H. Hepatitis B Virus Infection during Pregnancy: Transmission and Prevention. Middle East J. Dig. Dis. 2011, 3, 92–102. [Google Scholar]
- Wu, J.C.; Chen, C.M.; Sheen, I.J.; Lee, S.D.; Tzeng, H.M.; Choo, K.B. Evidence of transmission of hepatitis D virus to spouses from sequence analysis of the viral genome. Hepatology 1995, 22, 1656–1660. [Google Scholar]
- Wu, J.C.; Wang, Y.J.; Hwang, S.J.; Chen, T.Z.; Wang, Y.S.; Lin, H.C.; Lee, S.D.; Sheng, W.Y. Hepatitis D virus infection among prostitutes in Taiwan. J. Gastroenterol. Hepatol. 1993, 8, 334–337. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Manns, M.P. Epidemiology, pathogenesis and management of hepatitis D: Update and challenges ahead. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 31–40. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Wang, S.-C.; Hsieh, M.-Y.; Huang, C.-F.; Yeh, M.-L.; Yang, J.-F.; Chang, K.; Lin, W.-R.; Lin, C.-Y.; Chen, T.-C.; et al. Hepatitis D virus infections among injecting drug users with and without human immunodeficiency virus infection in Taiwan. Kaohsiung J. Med. Sci. 2016, 32, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Rizzetto, M.; Ciancio, A. Epidemiology of hepatitis D. Semin. Liver Dis. 2012, 32, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Lazinski, D.W. Replicating hepatitis delta virus RNA is edited in the nucleus by the small form of ADAR1. Proc. Natl. Acad. Sci. USA 2002, 99, 15118–15123. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-R.; Lo, S.J. Evolution and diversity of the human hepatitis d virus genome. Adv. Bioinform. 2010, 323654. [Google Scholar] [CrossRef]
- Hsieh, S.Y.; Chao, M.; Coates, L.; Taylor, J. Hepatitis delta virus genome replication: A polyadenylated mRNA for delta antigen. J. Virol. 1990, 64, 3192–3198. [Google Scholar] [PubMed]
- Lai, M.M.C. The molecular biology of hepatitis delta virus. Annu. Rev. Biochem. 1995, 64, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Gudima, S.; Wu, S.Y.; Chiang, C.M.; Moraleda, G.; Taylor, J. Origin of hepatitis delta virus mRNA. J. Virol. 2000, 74, 7204–7210. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Chang, J.; Taylor, J.M. Alternative processing of hepatitis delta virus antigenomic RNA transcripts. J. Virol. 2004, 78, 4517–4524. [Google Scholar] [CrossRef]
- Fu, T.B.; Taylor, J. The RNAs of hepatitis delta virus are copied by RNA polymerase II in nuclear homogenates. J. Virol. 1993, 67, 6965–6972. [Google Scholar]
- Greco-Stewart, V.S.; Miron, P.; Abrahem, A.; Pelchat, M. The human RNA polymerase II interacts with the terminal stem-loop regions of the hepatitis delta virus RNA genome. Virology 2007, 357, 68–78. [Google Scholar] [CrossRef]
- Abrahem, A.; Pelchat, M. Formation of an RNA polymerase II preinitiation complex on an RNA promoter derived from the hepatitis delta virus RNA genome. Nucleic Acids Res. 2008, 36, 5201–5211. [Google Scholar] [CrossRef]
- Modahl, L.E.; Macnaughton, T.B.; Zhu, N.; Johnson, D.L.; Lai, M.M.C. RNA-dependent replication and transcription of hepatitis delta virus RNA involve distinct cellular RNA polymerases. Mol. Cell. Biol. 2000. [Google Scholar] [CrossRef]
- Macnaughton, T.B.; Shi, S.T.; Modahl, L.E.; Lai, M.M.C. Rolling circle replication of hepatitis delta virus RNA is carried out by two different cellular RNA polymerases. J. Virol. 2002. [Google Scholar] [CrossRef]
- Li, Y.-J.; Macnaughton, T.; Gao, L.; Lai, M.M.C. RNA-templated replication of hepatitis delta virus: Genomic and antigenomic RNAs associate with different nuclear bodies. J. Virol. 2006, 80, 6478–6486. [Google Scholar] [CrossRef]
- Greco-Stewart, V.S.; Schissel, E.; Pelchat, M. The hepatitis delta virus RNA genome interacts with the human RNA polymerases I and III. Virology 2009, 386, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Greco-Stewart, V.; Pelchat, M. Interaction of host cellular proteins with components of the hepatitis delta virus. Viruses 2010, 2, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Huang, W.-H.; Hong, S.-Y.; Tsay, Y.-G.; Chen, P.-J. ERK1/2-mediated phosphorylation of small hepatitis delta antigen at serine 177 enhances hepatitis delta virus antigenomic RNA replication. J. Virol. 2008, 82, 9345–9358. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.S.; Lo, S.J.; Chen, P.J.; Lee, Y.-H.W. Casein kinase II and protein kinase C modulate hepatitis delta virus RNA replication but not empty viral particle assembly. J. Virol. 1996, 70, 6190–6198. [Google Scholar]
- Chen, C.W.; Tsay, Y.G.; Wu, H.L.; Lee, C.H.; Chen, D.S.; Chen, P.J. The double-stranded RNA-activated kinase, PKR, can phosphorylate hepatitis D virus small delta antigen at functional serine and threonine residues. J. Biol. Chem. 2002. [Google Scholar] [CrossRef]
- Cao, D.; Haussecker, D.; Huang, Y.; Kay, M.A.; Dan, C.; Haussecker, D.; Yong, H.; Kay, M.A.; Cao, D.; Haussecker, D.; et al. Combined proteomic-RNAi screen for host factors involved in human hepatitis delta virus replication. RNA 2009, 15, 1971–1979. [Google Scholar] [CrossRef]
- Huang, C.; Chang, S.C.; Yu, I.-C.; Tsay, Y.-G.; Chang, M.-F. Large hepatitis delta antigen is a novel clathrin adaptor-like protein. J. Virol. 2007, 81, 5985–5994. [Google Scholar] [CrossRef]
- Circle, D.A.; Neel, O.D.; Robertson, H.D.; Clarke, P.A.; Mathews, M.B. Surprising specificity of PKR binding to delta agent genomic RNA. RNA 1997, 3, 438–448. [Google Scholar] [PubMed]
- Lin, S.S.; Chang, S.C.; Wang, Y.H.; Sun, C.Y.; Chang, M.F. Specific interaction between the hepatitis delta virus RNA and glyceraldehyde 3-phosphate dehydrogenase: An enhancement on ribozyme catalysis. Virology 2000. [Google Scholar] [CrossRef] [PubMed]
- Beeharry, Y.; Goodrum, G.; Imperiale, C.J.; Pelchat, M. The Hepatitis Delta Virus accumulation requires paraspeckle components and affects NEAT1 level and PSP1 localization. Sci. Rep. 2018, 8, 6031. [Google Scholar] [CrossRef] [PubMed]
- Sikora, D.; Greco-Stewart, V.S.; Miron, P.; Pelchat, M. The hepatitis delta virus RNA genome interacts with eEF1A1, p54(nrb), hnRNP-L, GAPDH and ASF/SF2. Virology 2009, 390, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Sikora, D.; Zhang, D.; Bojic, T.; Beeharry, Y.; Tanara, A.; Pelchat, M. Identification of a binding site for ASF/SF2 on an RNA fragment derived from the hepatitis delta virus genome. PLoS ONE 2013, 8, e54832. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.; Watson, J.; Havel, C.; White, J. Identification of a prenylation site in delta virus large antigen. Science 1992. [Google Scholar] [CrossRef]
- Otto, J.C.; Casey, P.J. The hepatitis delta virus large antigen is farnesylated both in vitro and in animal cells. J. Biol. Chem. 1996. [Google Scholar] [CrossRef]
- Hwang, S.B.; Lai, M.M.C. Isoprenylation masks a conformational epitope and enhances trans-dominant inhibitory function of the large hepatitis delta antigen. J. Virol. 1994, 68, 2958–2964. [Google Scholar]
- Lee, C.Z.; Chen, P.J.; Lai, M.M.C.; Chen, D.S. Isoprenylation of large hepatitis delta antigan is necessary but not sufficient for hepatitis delta virus assembly. Virology 1994. [Google Scholar] [CrossRef]
- Li, Y.-J.; Stallcup, M.R.; Lai, M.M.C. Hepatitis delta virus antigen is methylated at arginine residues, and methylation regulates subcellular localization and RNA replication. J. Virol. 2004. [Google Scholar] [CrossRef]
- Huang, W.-H.; Mai, R.-T.; Lee, Y.-H.W. Transcription factor YY1 and its associated acetyltransferases CBP and p300 interact with hepatitis delta antigens and modulate hepatitis delta virus RNA replication. J. Virol. 2008, 82, 7313–7324. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.J.; Tsay, Y.G.; Juan, L.J.; Fu, T.F.; Huang, W.H.; Chen, D.S.; Chen, P.J. The small delta antigen of hepatitis delta virus is an acetylated protein and acetylation of lysine 72 may influence its cellular localization and viral RNA synthesis. Virology 2004. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H.; Cheng, T.-S.; Shu, C.-Y.; Jeng, K.-S.; Lai, M.M.C. Modification of small hepatitis delta virus antigen by SUMO protein. J. Virol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Moroianu, J.; Hijikata, M.; Blobel, G.; Radu, A. Mammalian karyopherin alpha 1 beta and alpha 2 beta heterodimers: Alpha 1 or alpha 2 subunit binds nuclear localization signal and beta subunit interacts with peptide repeat-containing nucleoporins. Proc. Natl. Acad. Sci. USA 1995, 92, 6532–6536. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jiang, J.-Y.; Chang, S.C.; Tsay, Y.-G.; Chen, M.-R.; Chang, M.-F. Nuclear export signal-interacting protein forms complexes with lamin A/C-Nups to mediate the CRM1-independent nuclear export of large hepatitis delta antigen. J. Virol. 2013, 87, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Chang, S.C.; Huang, C.; Li, Y.-P.; Lee, C.-H.; Chang, M.-F. Novel nuclear export signal-interacting protein, NESI, critical for the assembly of hepatitis delta virus. J. Virol. 2005, 79, 8113–8120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Huang, C.-R.; Chao, M.; Lo, S.J. The C-terminal sequence of the large hepatitis delta antigen is variable but retains the ability to bind clathrin. Virol. J. 2009, 6, 31. [Google Scholar] [CrossRef]
- Huang, W.H.; Yung, B.Y.; Syu, W.J.; Lee, Y.-H.W. The nucleolar phosphoprotein B23 interacts with hepatitis delta antigens and modulates the hepatitis delta virus RNA replication. J. Biol. Chem. 2001, 276, 25166–25175. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Filipovska, J.; Yano, K.; Furuya, A.; Inukai, N.; Narita, T.; Wada, T.; Sugimoto, S.; Konarska, M.M.; Handa, H. Stimulation of RNA polymerase II elongation by hepatitis delta antigen. Science 2001, 293, 124–127. [Google Scholar] [CrossRef]
- Brazas, R.; Ganem, D. A cellular homolog of hepatitis delta antigen: Implications for viral replication and evolution. Science 1996. [Google Scholar] [CrossRef]
- Lee, C.Z.; Sheu, J.C. Histone H1e interacts with small hepatitis delta antigen and affects hepatitis delta virus replication. Virology 2008. [Google Scholar] [CrossRef]
- Haussecker, D.; Cao, D.; Huang, Y.; Parameswaran, P.; Fire, A.Z.; Kay, M.A. Capped small RNAs and MOV10 in human hepatitis delta virus replication. Nat. Struct. Mol. Biol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Jeong, S.H.; Hwang, S.B. Large hepatitis delta antigen modulates transforming growth factor-β Sagnaling cascades: implication of hepatitis delta virus-induced liver fibrosis. Gastroenterology 2007. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-Y.; Oh, S.-H.; Kang, S.M.; Lim, Y.-S.; Hwang, S.B. Hepatitis delta virus large antigen sensitizes to TNF-alpha-induced NF-kappaB signaling. Mol. Cells 2009, 28, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Bichko, V.V.; Taylor, J.M. Redistribution of the delta antigens in cells replicating the genome of hepatitis delta virus. J. Virol. 1996, 70, 8064–8070. [Google Scholar]
- Greco-Stewart, V.S.; Thibault, C.S.-L.; Pelchat, M. Binding of the polypyrimidine tract-binding protein-associated splicing factor (PSF) to the hepatitis delta virus RNA. Virology 2006, 356, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Mota, S.; Mendes, M.; Penque, D.; Coelho, A.V.; Cunha, C. Changes in the proteome of Huh7 cells induced by transient expression of hepatitis D virus RNA and antigens. J. Proteomics 2008, 71, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Mota, S.; Mendes, M.; Freitas, N.; Penque, D.; Coelho, A.V.; Cunha, C. Proteome analysis of a human liver carcinoma cell line stably expressing hepatitis delta virus ribonucleoproteins. J. Proteomics 2009, 72, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Monjardino, J.; Cheng, D.; Krause, S.; Carmo-Fonseca, M.; Chang, D. Localization of hepatitis delta virus RNA in the nucleus of human cells. RNA 1998, 4, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Yang, A.; Thomas, H.; Monjardino, J. Characterization of stable hepatitis delta expressing hepatoma cell lines: Effect of HDAg on cell growth. Prog. Clin. Biol. Res. 1993, 382, 149–153. [Google Scholar]
- Mendes, M.; Pérez-Hernandez, D.; Vázquez, J.; Coelho, A.V.; Cunha, C. Proteomic changes in HEK-293 cells induced by hepatitis delta virus replication. J. Proteomics 2013, 89, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Gudima, S.O.; Tarn, C.; Nie, X.; Taylor, J.M. Development of a novel system to study hepatitis delta virus genome replication. J. Virol. 2005, 79, 8182–8188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Filzmayer, C.; Ni, Y.; Sültmann, H.; Mutz, P.; Hiet, M.-S.; Vondran, F.W.R.; Bartenschlager, R.; Urban, S. Hepatitis D virus replication is sensed by MDA5 and induces IFN-β/λ responses in hepatocytes. J. Hepatol. 2018, 69, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.H.; Lam, Y.W.; Leung, A.K.L.; Lyon, C.E.; Andersen, J.; Mann, M.; Lamond, A.I. Paraspeckles: A novel nuclear domain. Curr. Biol. 2002, 12, 13–25. [Google Scholar] [CrossRef]
- Hirose, T.; Virnicchi, G.; Tanigawa, A.; Naganuma, T.; Li, R.; Kimura, H.; Yokoi, T.; Nakagawa, S.; Bénard, M.; Fox, A.H.; Pierron, G. NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol. Biol. Cell 2014, 25, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.; Brichler, S.; Radjef, N.; Lebon, P.; Goffard, A.; Hober, D.; Fagard, R.; Kremsdorf, D.; Dény, P.; Gordien, E. Hepatitis delta virus proteins repress hepatitis B virus enhancers and activate the alpha/beta interferon-inducible MxA gene. J. Gen. Virol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.; Brichler, S.; Khan, E.; Chami, M.; Dény, P.; Kremsdorf, D.; Gordien, E. Large hepatitis delta antigen activates STAT-3 and NF-κB via oxidative stress. J. Viral Hepat. 2012, 19, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Waris, G.; Huh, K.W.; Siddiqui, A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol. Cell. Biol. 2001, 21, 7721–7730. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.-T.; Lee, Y.-J.; Ko, J.-L.; Tsai, C.-C.; Tseng, C.-J.; Sheu, G.-T. Hepatitis delta virus epigenetically enhances clusterin expression via histone acetylation in human hepatocellular carcinoma cells. J. Gen. Virol. 2009, 90, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Minoia, S.; Carbonell, A.; Gisel, A.; Delgado, S.; López-Carrasco, A.; Navarro, B.; Di Serio, F. Viroids, the simplest RNA replicons: How they manipulate their hosts for being propagated and how their hosts react for containing the infection. Virus Res. 2015, 209, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Gago-Zachert, S. Viroids, infectious long non-coding RNAs with autonomous replication. Virus Res. 2016, 212, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Grubb, D.; Elleuch, A.; Nohales, M.A.; Delgado, S.; Gago, S. Rolling-circle replication of viroids, viroid-like satellite RNAs and hepatitis delta virus: variations on a theme. RNA Biol. 2011, 8, 200–206. [Google Scholar] [CrossRef] [PubMed]
| Host Protein (Cell Types Used) | Function | Interaction | Reference |
|---|---|---|---|
| Double-stranded RNA-activated protein kinase R (PKR) (HepG2, HeLa) | Phosphorylation (S117, S180, T182) | HDAg-S, RNA | [28] |
| Casein Kinase II (CKII) (HuH-7) | Phosphorylation (S2, S213) | HDAg-S | [27] |
| Protein Kinase C (PKC) (HuH-7) | Phosphorylation (S210) | HDAg-L | [27] |
| Extracellular signal-related kinases 1 and 2 (ERK1/2) (HEK-293T) | Phosphorylation (S177) | HDAg-S | [26] |
| Protein farnesyltransferase (FTase) (Cos-7, d H189, HuH-7, NIH3T3) | Isoprenylation with farnesyl (C211) | HDAg-L | [36,37,38,39] |
| Protein arginine methyltransferase 1 (PRMT1) (HuH-7) | Methylation (R13) | HDAgs | [40] |
| P300 cellular acetyltransferase (HeLa, HuH-7, and HepG2) | Acetylation (K72) | HDAgs | [41,42] |
| Small ubiquitin-related modifier | Sumoylation of lysine residues | HDAg-S | [43] |
| Isoform 1 (SUMO1) (HuH-7) | |||
| Ubc9 (HuH-7) | Sumoylation of lysine residues | HDAg-S | [43] |
| Karyopherin (importin) 2α (BRL) | Nuclear import | HDAg-S | [44] |
| Nuclear export signal-interacting protein (NESI) (HuH-7, HepG2, COS7) | Nuclear import | HDAg-L | [45,46] |
| Lamin A/C (HuH-7) | Nuclear stability, chromatin structure and gene expression | HDAg-L | [45] |
| Clathrin heavy chain (HepG2, COS7, HuH-7) | Exocytosis | HDAg-L | [30,47] |
| Nucleophosmin (B23) (HuH-7 HepG2) | Nucleolar localization, shuttling, RNA synthesis/accumulation | HDAgs | [48] |
| DRB sensitivity-inducing factor (DSIF) (HeLa) | Relieves transcriptional repression; stimulates elongation by RNAP II | HDAgs | [49] |
| Delta interacting protein A (HEK-293) | Transcriptional regulation | HDAgs | [50] |
| Yin Yang 1 (YY1) (HeLa, HuH-7, HepG2) | RNA synthesis/accumulation | HDAgs | [41] |
| Histone H1e (COS7, HuH-7) | RNA synthesis/accumulation | HDAg-S | [51] |
| MOV10 (HuH-7, HEK-293) | RNA remodeling | HDAgs | [52] |
| Smad3 (HuH-7, Cos7) | Host gene expression | HDAgs | [53] |
| c-Jun (HuH-7, Cos7) | Host gene expression | HDAgs | [53] |
| TRAF2 (HEK-293, HuH-7) | Host gene expression | HDAgs | [54] |
| ZNF326 (HEK-293) | Transcription elongation | HDAg-S | [29] |
| CCAR1(HEK-293) | Helicase | HDAg-S | [29] |
| CDC5L (HEK-293) | Helicase | HDAg-S | [29] |
| Chromodomain helicase-DNA-binding protein 4 (CHD4) (HEK-293) | Remodeling of chromatin | HDAg-S | [29] |
| Centrosome-associated protein 350 (CEP350) (HEK-293) | Microtubule-organization at the centrosome | HDAg-S | [29] |
| Centrosomal protein 170kDa isoform alpha (HEK-293) | Microtubule organization | HDAg-S | [29] |
| H2A and H4 Histones (HEK-293) | Histone components | HDAg-S | [29] |
| Probable G-protein coupled receptor 179 precursor (HEK-293) | Signal transduction | HDAg-S | [29] |
| SC35 (HuH-7) | Splicing factor | HDAg-S, gRNA | [55] |
| Adenosine deaminase acting on RNA (ADAR 1) (HuH-7, HEK-293) | Post-transcriptional modification of HDV antigenome | agRNA | [12] |
| Glyceraldehydes 3-phosphate dehydrogenase (GAPDH) (HeLa) | Enhances delta ribozyme activity | agRNA | [32,34] |
| RNAP I (HeLa) | Antigenome synthesis | RNA | [24] |
| RNAP II (HeLa) | Genome synthesis, mRNA synthesis, Antigenome synthesis | HDAg-S, RNA | [19,20] |
| RNAP III (HeLa) | RNA | [24] | |
| Polypyrimidine tract-binding protein associated splicing factor (PSF) (HEK-293, HuH-7) | Nuclear processes | RNA | [33,56] |
| 54 kDa nuclear RNA-binding protein (p54nrb) (HEK-293, HeLa) | Nuclear processes | RNA | [33,34] |
| Paraspeckle protein 1 (PSP1) (HEK-293) | RNA | [33] | |
| Heterogeneous nuclear ribonucleoprotein L (hnRNPL) (HeLa) | mRNA processing | RNA | [34] |
| Arginine/serine-rich splicing factor (ASF) (HeLa, HEK-293) | Splicing | RNA | [34,35] |
| Eukaryotic elongation factor 1A1 (eEF1A1) (HeLa) | Ribosomal aa-tRNA transport, gene expression | RNA | [34] |
| Host Protein (Cell Types Used) | Biological Function | Reference |
|---|---|---|
| P53 (HEK-293) | Tumor suppressor and the regulation of cell cycle | [61] |
| Heat shock 10 kDa protein (HSPE) (HEK-293) | Chaperone, efficient protein folding | [61] |
| ELAV-like protein 1(HEK-293) | c-myc stabilization | [61] |
| Transportin 1 (HEK-293) | Receptor for nuclear localization signals | [61] |
| Eukaryotic Translation Initiation Factor 3 Subunit D (EIF3D) (HEK-293) | Translation initiation factor activity | [61] |
| Cofilin 1 (HEK-293) | ILK signaling pathway | [61] |
| 14-3-3 σ (HEK-293) | Signal transduction | [61] |
| FAM136A (HEK-293) | Nuclear-encoded mitochondrial gene | [61] |
| BRI3BP (HEK-293) | Tumorigenesis, p53/TP53 stabilization | [61] |
| Histone H1 binding protein (NASP) (HEK-293) | Signal transduction; Cell communication | [29] |
| Triose phosphate isomerase (TPI) (HEK-293) | Metabolism; Energy pathways | [29] |
| Polyadenylate binding protein (PABP) (HEK-293) | RNA metabolism | [29] |
| Rho GDP dissociation inhibitor (GDI) (HEK-293) | GTPase activator | [29] |
| Guanine nucleotide-binding protein (HEK-293) | Signal transduction pathway | [29] |
| Brebrin 1 (HEK-293) | Cell growth and/or maintenance | [29] |
| Keratine 8 (HEK-293) | Cell growth and/or maintenance | [29] |
| Vinculin (HEK-293) | Cell growth and/or maintenance | [29] |
| Lamin C (HEK-293) | Cell growth and/or maintenance | [29] |
| Acetyl-CoA acetyltransferase (HEK-293) | Metabolism; Energy pathways | [29] |
| Zinc finger protein 326 (HEK-293) | Regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | [29] |
| High mobility group box 1 (HEK-293) | Regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | [29] |
| Guanine nucleotide binding protein (HEK-293) | Signal transduction; Cell communication | [29] |
| Serum albumin (HEK-293) | Transport | [29] |
| Heterogeneous nuclear ribonuclearprotein D (hnRNP D) (HuH-7) | mRNA metabolism and transport | [57] |
| Heat shock protein 105 (HSP105) (HuH-7) | Prevents the aggregation of misfolded proteins | [57] |
| Annexin IV (HuH-7) | Regulation of early stages of apoptosis | [57] |
| Proteasome activator (HuH-7) | Metabolism; energy pathways | [57] |
| NADH2 dehydrogenase (ubiquinone) flavoprotein 1 precursor (HuH-7) | Metabolism; energy pathways | [57] |
| Adenylate kinase 2B (HuH-7) | Metabolism; energy pathways | [57] |
| Eukaryotic translation initiation factor 2 subunit 1 (HuH-7) | Protein metabolism | [57] |
| Serine (or cysteine) proteinase inhibitor (HuH-7) | Protein metabolism | [57] |
| Heat shock 60 kDa protein | Protein metabolism | [57] |
| CKAP4 protein (HuH-7) | Cell growth and/ or maintenance | [57] |
| Tubulin alpha 6 (HuH-7) | Cell growth and/ or maintenance | [57] |
| Keratin 8 & Keratin, type I cytoskeletal 19 (HuH-7) | Cell growth and/ or maintenance | [57] |
| Dihydropyrimidinase related | Neurogenesis | [57] |
| Protein 2 (HuH-7) | ||
| TRIM 28 protein (HuH-7) | Regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | [57] |
| DNA structure specific endonuclease FEN1 (HuH-7) | Regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | [57] |
| Ribonuclearprotein La (HuH-7) | Regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | [57] |
| High density lipoprotein binding protein (vigilin) (HEK-293, HuH-7) | Transport | [29,57] |
| N-ethylmaleimide-sensitive factor attachment protein (HEK-293, HuH-7) | Transport | [29,57] |
| Sorting nexin 5 (HEK-293, HuH-7) | Transport | [29,57] |
| Dopamine receptor interacting protein 4 (HuH-7) | Apoptosis | [57] |
| Interferon β/λ (HepG2, HuH-7, HepaRG) | Signaling proteins | [63] |
| Interleukine 8 (IL8) (HEK-293) | Antiviral protein | [33] |
| Nuclear Enriched Associated Transcript 1 (Neat1) (HEK-293) | Scaffold protein | [33] |
| Clusterin (HuH-7) | Role in tumorigenesis | [69] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodrum, G.; Pelchat, M. Insight into the Contribution and Disruption of Host Processes during HDV Replication. Viruses 2019, 11, 21. https://doi.org/10.3390/v11010021
Goodrum G, Pelchat M. Insight into the Contribution and Disruption of Host Processes during HDV Replication. Viruses. 2019; 11(1):21. https://doi.org/10.3390/v11010021
Chicago/Turabian StyleGoodrum, Gabrielle, and Martin Pelchat. 2019. "Insight into the Contribution and Disruption of Host Processes during HDV Replication" Viruses 11, no. 1: 21. https://doi.org/10.3390/v11010021
APA StyleGoodrum, G., & Pelchat, M. (2019). Insight into the Contribution and Disruption of Host Processes during HDV Replication. Viruses, 11(1), 21. https://doi.org/10.3390/v11010021




